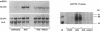Abstract
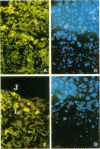
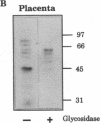
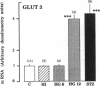
The localization of the two major placental glucose transporter isoforms, GLUT1 and GLUT3 was studied in 20-d pregnant rats. Immunocytochemical studies revealed that GLUT1 protein is expressed ubiquitously in the junctional zone (maternal side) and the labyrinthine zone (fetal side) of the placenta. In contrast, expression of GLUT3 protein is restricted to the labyrinthine zone, specialized in nutrient transfer. After 19-d maternal insulinopenic diabetes (streptozotocin), placental GLUT3 mRNA and protein levels were increased four-to-fivefold compared to nondiabetic rats, whereas GLUT1 mRNA and protein levels remained unmodified. Placental 2-deoxyglucose uptake and glycogen concentration were also increased fivefold in diabetic rats. These data suggest that GLUT3 plays a major role in placental glucose uptake and metabolism. The role of hyperglycemia in the regulation of GLUT3 expression was assessed by lowering the glycemia of diabetic pregnant rats. After a 5-d phlorizin infusion to pregnant diabetic rats, placental GLUT3 mRNA and protein levels returned to levels similar to those observed in nondiabetic rats. Furthermore, a short-term hyperglycemia (12 h), achieved by performing hyperglycemic clamps induced a fourfold increase in placental GLUT3 mRNA and protein with no concomitant change in GLUT1 expression. This study provides the first evidence that placental GLUT3 mRNA and protein expression can be stimulated in vivo under hyperglycemic conditions. Thus, GLUT3 transporter isoform appears to be highly sensitive to ambient glucose levels and may play a pivotal role in the severe alterations of placental function observed in diabetic pregnancies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins V., Flozak A. S., Ogata E. S., Simmons R. A. The effects of severe maternal diabetes on glucose transport in the fetal rat. Endocrinology. 1994 Jul;135(1):409–415. doi: 10.1210/endo.135.1.8013378. [DOI] [PubMed] [Google Scholar]
- Barash V., Gutman A., Shafrir E. Mechanism of placental glycogen deposition in diabetes in the rat. Diabetologia. 1983 Jan;24(1):63–68. doi: 10.1007/BF00275950. [DOI] [PubMed] [Google Scholar]
- Battaglia F. C., Meschia G. Principal substrates of fetal metabolism. Physiol Rev. 1978 Apr;58(2):499–527. doi: 10.1152/physrev.1978.58.2.499. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Davidson N. O. GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellitus, and comparison to human testis. Am J Physiol. 1994 Dec;267(6 Pt 2):R1488–R1495. doi: 10.1152/ajpregu.1994.267.6.R1488. [DOI] [PubMed] [Google Scholar]
- Challier J. C., Hauguel S., Desmaizieres V. Effect of insulin on glucose uptake and metabolism in the human placenta. J Clin Endocrinol Metab. 1986 May;62(5):803–807. doi: 10.1210/jcem-62-5-803. [DOI] [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem. 1976 Mar;71(1):96–105. doi: 10.1016/0003-2697(76)90014-2. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daniels M. C., Kansal P., Smith T. M., Paterson A. J., Kudlow J. E., McClain D. A. Glucose regulation of transforming growth factor-alpha expression is mediated by products of the hexosamine biosynthesis pathway. Mol Endocrinol. 1993 Aug;7(8):1041–1048. doi: 10.1210/mend.7.8.8232303. [DOI] [PubMed] [Google Scholar]
- Desoye G., Hofmann H. H., Weiss P. A. Insulin binding to trophoblast plasma membranes and placental glycogen content in well-controlled gestational diabetic women treated with diet or insulin, in well-controlled overt diabetic patients and in healthy control subjects. Diabetologia. 1992 Jan;35(1):45–55. doi: 10.1007/BF00400851. [DOI] [PubMed] [Google Scholar]
- Devaskar S. U., Devaskar U. P., Schroeder R. E., deMello D., Fiedorek F. T., Jr, Mueckler M. Expression of genes involved in placental glucose uptake and transport in the nonobese diabetic mouse pregnancy. Am J Obstet Gynecol. 1994 Nov;171(5):1316–1323. doi: 10.1016/0002-9378(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Eriksson U. J., Borg L. A. Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia. 1991 May;34(5):325–331. doi: 10.1007/BF00405004. [DOI] [PubMed] [Google Scholar]
- Farrell C. L., Pardridge W. M. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart D. Z., Broderius M. A., Borson N. D., Drewes L. R. Neurons and microvessels express the brain glucose transporter protein GLUT3. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):733–737. doi: 10.1073/pnas.89.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewolb I. H., Merdian W., Warshaw J. B., Enders A. C. Fine structural abnormalities of the placenta in diabetic rats. Diabetes. 1986 Nov;35(11):1254–1261. doi: 10.2337/diab.35.11.1254. [DOI] [PubMed] [Google Scholar]
- Girard J., Ferré P., Pégorier J. P., Duée P. H. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev. 1992 Apr;72(2):507–562. doi: 10.1152/physrev.1992.72.2.507. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Brant A. M., Kahn B. B., Shepherd P. R., McCoid S. C., Gibbs E. M. Expression of the brain-type glucose transporter is restricted to brain and neuronal cells in mice. Diabetologia. 1992 Apr;35(4):304–309. doi: 10.1007/BF00401196. [DOI] [PubMed] [Google Scholar]
- Haber R. S., Weinstein S. P., O'Boyle E., Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993 Jun;132(6):2538–2543. doi: 10.1210/endo.132.6.8504756. [DOI] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S., Leturque A., Alsat E., Loizeau M., Evain-Brion D., Girard J. Developmental expression of Glut1 glucose transporter and c-fos genes in human placental cells. Placenta. 1994 Jan;15(1):35–46. doi: 10.1016/s0143-4004(05)80234-6. [DOI] [PubMed] [Google Scholar]
- Hauguel S., Desmaizieres V., Challier J. C. Glucose uptake, utilization, and transfer by the human placenta as functions of maternal glucose concentration. Pediatr Res. 1986 Mar;20(3):269–273. doi: 10.1203/00006450-198603000-00015. [DOI] [PubMed] [Google Scholar]
- Jansson T., Wennergren M., Illsley N. P. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993 Dec;77(6):1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Hartmann M., Blaschitz A., Desoye G. Ultrastructural localization of insulin receptors in human placenta. Am J Reprod Immunol. 1993 Sep-Oct;30(2-3):136–145. doi: 10.1111/j.1600-0897.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Rossetti L., Lodish H. F., Charron M. J. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest. 1991 Jun;87(6):2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto U. M., Martinez-Valdez H., Bilan P. J., Burdett E., Ramlal T., Klip A. Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem. 1991 Feb 5;266(4):2615–2621. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leturque A., Burnol A. F., Ferré P., Girard J. Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol. 1984 Jan;246(1 Pt 1):E25–E31. doi: 10.1152/ajpendo.1984.246.1.E25. [DOI] [PubMed] [Google Scholar]
- Leturque A., Hauguel S., Kande J., Girard J. Glucose utilization by the placenta of anesthetized rats: effect of insulin, glucose, and ketone bodies. Pediatr Res. 1987 Oct;22(4):483–487. doi: 10.1203/00006450-198710000-00025. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Crabb J. H., Ransome K. J. Endoglycosidase f cleaves the oligosaccharides from the glucose transporter of the human erythrocyte. Biochim Biophys Acta. 1984 Jan 25;769(2):404–410. doi: 10.1016/0005-2736(84)90324-9. [DOI] [PubMed] [Google Scholar]
- Maher F., Vannucci S., Takeda J., Simpson I. A. Expression of mouse-GLUT3 and human-GLUT3 glucose transporter proteins in brain. Biochem Biophys Res Commun. 1992 Jan 31;182(2):703–711. doi: 10.1016/0006-291x(92)91789-s. [DOI] [PubMed] [Google Scholar]
- Marshall S., Bacote V., Traxinger R. R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991 Mar 15;266(8):4706–4712. [PubMed] [Google Scholar]
- Mills J. L., Baker L., Goldman A. S. Malformations in infants of diabetic mothers occur before the seventh gestational week. Implications for treatment. Diabetes. 1979 Apr;28(4):292–293. doi: 10.2337/diab.28.4.292. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Kornhauser J. M., Burant C. F., Seino S., Mayo K. E., Bell G. I. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992 Jan 5;267(1):467–472. [PubMed] [Google Scholar]
- Nagamatsu S., Sawa H., Inoue N., Nakamichi Y., Takeshima H., Hoshino T. Gene expression of GLUT3 glucose transporter regulated by glucose in vivo in mouse brain and in vitro in neuronal cell cultures from rat embryos. Biochem J. 1994 May 15;300(Pt 1):125–131. doi: 10.1042/bj3000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Boado R. J., Farrell C. R. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990 Oct 15;265(29):18035–18040. [PubMed] [Google Scholar]
- Postic C., Burcelin R., Rencurel F., Pegorier J. P., Loizeau M., Girard J., Leturque A. Evidence for a transient inhibitory effect of insulin on GLUT2 expression in the liver: studies in vivo and in vitro. Biochem J. 1993 Jul 1;293(Pt 1):119–124. doi: 10.1042/bj2930119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J. H., Jodarski G., Shanahan M. R. Maternal insulin and placental 3-O-methyl glucose transport. J Dev Physiol. 1986 Aug;8(4):247–253. [PubMed] [Google Scholar]
- Schroeder R. E., Devaskar U. P., Trail S. E., Demello D. E., Cole D. P., Devaskar S. U. Effect of maternal diabetes on the expression of genes regulating fetal brain glucose uptake. Diabetes. 1993 Oct;42(10):1487–1496. doi: 10.2337/diab.42.10.1487. [DOI] [PubMed] [Google Scholar]
- Thomas C. R., Eriksson G. L., Eriksson U. J. Effects of maternal diabetes on placental transfer of glucose in rats. Diabetes. 1990 Mar;39(3):276–282. doi: 10.2337/diab.39.3.276. [DOI] [PubMed] [Google Scholar]
- Thomas C. R., Lowy C. Placental transfer and uptake of 2-deoxyglucose in control and diabetic rats. Metabolism. 1992 Nov;41(11):1199–1203. doi: 10.1016/0026-0495(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Thorens B., Flier J. S., Lodish H. F., Kahn B. B. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. doi: 10.2337/diab.39.6.712. [DOI] [PubMed] [Google Scholar]
- Tordjman K. M., Leingang K. A., Mueckler M. Differential regulation of the HepG2 and adipocyte/muscle glucose transporters in 3T3L1 adipocytes. Effect of chronic glucose deprivation. Biochem J. 1990 Oct 1;271(1):201–207. doi: 10.1042/bj2710201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxinger R. R., Marshall S. Insulin regulation of pyruvate kinase activity in isolated adipocytes. Crucial role of glucose and the hexosamine biosynthesis pathway in the expression of insulin action. J Biol Chem. 1992 May 15;267(14):9718–9723. [PubMed] [Google Scholar]
- Vaulont S., Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994 Jan;8(1):28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- Willman S. P., Leveno K. J., Guzick D. S., Williams M. L., Whalley P. J. Glucose threshold for macrosomia in pregnancy complicated by diabetes. Am J Obstet Gynecol. 1986 Feb;154(2):470–475. doi: 10.1016/0002-9378(86)90692-7. [DOI] [PubMed] [Google Scholar]
- Wolf H. J., Desoye G. Immunohistochemical localization of glucose transporters and insulin receptors in human fetal membranes at term. Histochemistry. 1993 Nov;100(5):379–385. doi: 10.1007/BF00268936. [DOI] [PubMed] [Google Scholar]
- Zhou J., Bondy C. A. Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest. 1993 Mar;91(3):845–852. doi: 10.1172/JCI116305. [DOI] [PMC free article] [PubMed] [Google Scholar]