Abstract
Objective To review the literature on the use of inhaled nitric oxide to treat acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and to summarise the effects of nitric oxide, compared with placebo or usual care without nitric oxide, in adults and children with ALI or ARDS.
Design Systematic review and meta-analysis.
Data sources Medline, CINAHL, Embase, and CENTRAL (to October 2006), proceedings from four conferences, and additional information from authors of 10 trials.
Review methods Two reviewers independently selected parallel group randomised controlled trials comparing nitric oxide with control and extracted data related to study methods, clinical and physiological outcomes, and adverse events.
Main outcome measures Mortality, duration of ventilation, oxygenation, pulmonary arterial pressure, adverse events.
Results 12 trials randomly assigning 1237 patients met inclusion criteria. Overall methodological quality was good. Using random effects models, we found no significant effect of nitric oxide on hospital mortality (risk ratio 1.10, 95% confidence interval 0.94 to 1.30), duration of ventilation, or ventilator-free days. On day one of treatment, nitric oxide increased the ratio of partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2 ratio) (13%, 4% to 23%) and decreased the oxygenation index (14%, 2% to 25%). Some evidence suggested that improvements in oxygenation persisted until day four. There was no effect on mean pulmonary arterial pressure. Patients receiving nitric oxide had an increased risk of developing renal dysfunction (1.50, 1.11 to 2.02).
Conclusions Nitric oxide is associated with limited improvement in oxygenation in patients with ALI or ARDS but confers no mortality benefit and may cause harm. We do not recommend its routine use in these severely ill patients.
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), defined by acute hypoxaemia and bilateral lung infiltrates on radiography without left atrial hypertension,1 are characterised by inflammation of the alveolar-capillary membrane triggered by various insults.2 Because the pathophysiology involves mismatching of ventilation and perfusion and pulmonary hypertension, the possibility of using inhaled nitric oxide (NO) generated considerable interest.3 Nitric oxide is a selective pulmonary vasodilator and has anti-inflammatory properties.4 5 Based on limited data on efficacy, clinicians rapidly adopted this therapy; 63% of European intensive care specialists surveyed in 1997 reported using it, primarily for ALI or ARDS.6 A more recent survey of specialists in Ontario, Canada, found that a substantial proportion (39%) reported using nitric oxide at least sometimes in selected patients with ARDS.7
A systematic review and meta-analysis of nitric oxide published in 20038 9 that included five randomised controlled trialsw3-w7 found no effect on mortality or ventilator-free days; one trial showed improved oxygenation.w3 Because confidence intervals were wide, the authors concluded that the effects of nitric oxide on morbidity and mortality were uncertain. We have incorporated data from new randomised controlled trials to evaluate the effects of nitric oxide on pulmonary physiology (oxygenation and pulmonary arterial pressure) and important clinical outcomes (mortality, duration of ventilation, and adverse effects) in patients with established ALI or ARDS.
Methods
Search strategy
We electronically searched Medline, CINAHL, Embase, and CENTRAL (to October 2006), limiting citations to randomised controlled trials. We also searched proceedings of four conferences (1994-2006), screened bibliographies of retrieved studies and recent review articles,10 11 12 13 14 15 16 17 18 and contacted content experts to identify additional trials. There were no language restrictions. Further details of the search strategy and other aspects of study methods are on bmj.com.
Study selection
Two reviewers independently screened studies for inclusion, retrieved potentially relevant studies, and decided on study eligibility. We selected parallel group trials that enrolled adults or children (excluding neonates), with ≥80% of patients or a separately reported subgroup having ALI or ARDS (using authors' definitions). Included trials compared nitric oxide with placebo or usual treatment (not prevention) for ALI or ARDS and reported mortality (at any time), duration of ventilation, ventilator-free days, or pulmonary physiological parameters on days one to four of treatment (PaO2 (partial pressure of oxygen)/FiO2 (fraction of inspired oxygen); oxygenation index, defined as 100 × mean airway pressure/(PaO2/FiO2); mean pulmonary arterial pressure). We included trials with cointerventions applied equally in both groups. We assessed agreement between reviewers for trial eligibility using Cohen's κ.19
Data abstraction and validity assessment
Two reviewers independently abstracted data and methods from included trials. We resolved by consensus any disagreements that remained after contacting trial authors. From included studies we abstracted method of randomisation and allocation concealment, blinding of caregivers and outcomes assessors, and number of withdrawals after randomisation and determined whether mechanical ventilation, weaning, and sedation were standardised or applied equally in treatment groups.
We attempted to contact authors of all included trials to request additional data and clarify data and methods if necessary.
Quantitative data synthesis
Our primary outcome was mortality in hospital (or, if not available, mortality in the intensive care unit or at 28 or 30 days). We decided a priori to combine trials with less than half of patients crossing over from control to nitric oxide arms in analyses of clinical outcomes. Our analyses adhered to the intention to treat principle. In studies with two or more nitric oxide groups receiving different doses, we combined data to determine an overall effect for the nitric oxide group.
Secondary outcomes included duration of ventilation, ventilator-free days to 28 or 30 days, and pulmonary physiology. We decided post hoc to combine data on renal dysfunction after obtaining outcomes for most randomised patients, but we describe other adverse events qualitatively.
We used random effects models20 implemented in Review Manager 4.2.7 (Cochrane Collaboration, Oxford) for all analyses and considered P≤0.05 (two sided) as significant. We report binary outcomes as risk ratios and continuous outcomes as weighted mean differences (measure of absolute change) and ratios of means (measure of relative change).21 Summary effect estimates are presented with 95% confidence intervals.
We assessed homogeneity between studies for each outcome using the Cochran Q statistic,22 with P≤0.10 indicating significant heterogeneity,23 and I2 24 25 with suggested thresholds for low (25%-49%), moderate (50%-74%), and high (≥75%) values. We developed several a priori hypotheses to explain significant heterogeneity (excluding duration of ventilation and ventilator-free days), including dose and duration of nitric oxide therapy and whether therapy was restricted to patients whose oxygenation improved acutely (“nitric oxide responders”) or to those with ARDS (the more hypoxaemic subset of ALI).
Results
Trial flow
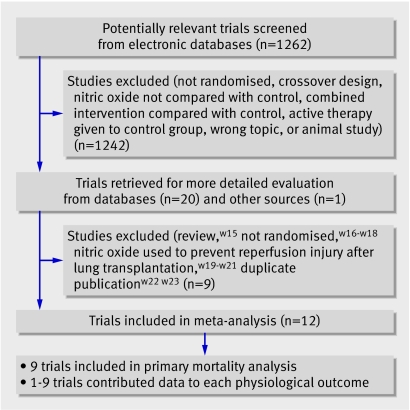
Electronic database searches yielded 1262 citations. After evaluating these citations, conference abstracts, review articles, and bibliographies of included trials, we included 12 parallel group randomised controlled trialsw1-w12 (fig 1). The two reviewers completely agreed (κ=1) on the selection of included studies. We obtained additional information from 10 authors (new clinicalw1 w2w5w7 w8w11 or physiological dataw1 w2w7w11; clarifications of dataw6w9 or methodsw1-w3w5-w11).
Fig 1 Number of trials evaluated at each stage of the systematic review
Study characteristics and methodological quality
Table 1 describes the included studies, two of which were published as abstracts only.w2w7 Data from one trial were distributed in two abstracts,w8w13 and data from another trial were distributed in two articles.w6w14 Trials randomised 1237 patients (median 40; range 14-385) with ALI or ARDS. Two trials enrolled only children,w1w6 one trial included a few children,w4 and the remaining trials enrolled only adults. All patients met American-European Consensus Conference1 oxygenation criteria for ARDS except for one trial that included some patients with ALI.w12 Seven trials used a fixed dose of nitric oxide (median 10 ppm; range 5-10 ppm),w1 w2w6w8w10-w12 and five used the lowest dose to achieve an oxygenation responsew4 w5w7w9 or randomised patients to different doses.w3 One trial enrolled only patients whose oxygenation improved after a nitric oxide challenge (“nitric oxide responders”),w7 and one used a cointervention (a recruitment manoeuvre) in both groups.w11 Trials continued nitric oxide until prespecified gas exchange end pointsw1w3w5w7-w10w12 or for a fixed period of time after which nitric oxide was tapered by using gas exchange criteriaw4w6 or managed at clinicians' discretion.w2 One trial did not report on criteria for stopping nitric oxide.w11 The median duration of administration was 6.5 days (range 3.5-9.0 days; data available from five trialsw5w7-w9w11). One trial randomised patients to nitric oxide or control for 24 hours, after which all patients received nitric oxide.w1 In five other trials, control patients received nitric oxide as rescue therapy after randomisation if they met prespecified criteria (<50% of controls in three trialsw4w7 w8 and ≥50% in two trialsw2w6). Not for profit agencies funded five trials,w1w4-w6w10 industry funded two trials,w3w12 both sources funded or supported four trials,w2w7-w9 and one trial did not report this information.w11
Table 1.
Details of randomised trials of inhaled nitric oxide (NO) in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
| Author (funding*) | Population | Details of NO administration | Control group and crossovers |
|---|---|---|---|
| Day,w1 1997 (not for profit) | 24 children, 1 centre. Acute bilateral CXR infiltrates, PEEP >6 cm H2O, FiO2 >0.5 for >12 hours. Enrolment ≤48 h after meeting study criteria | 10 ppm until oxygenation and PEEP criteria met | Usual care. All patients randomised to control received NO 10 ppm after 24 hour; no crossovers before 24 hour |
| Schwebel,w2 1997 (not for profit; industry supplied gas) | 19 adults, 17 centres. Any CXR infiltrates, P/F <200 mm Hg, 10<PAOP<18 mm Hg, 6<PEEP<10 cm H2O. Duration of ARDS ≤24 hours | 10 ppm for 17 hours, then at clinician's discretion; mean 4.6 days (range 1.25 to 11) | Placebo gas (nitrogen). Crossovers mandated before 17 hours if P/F ≤100 mm Hg and permitted thereafter (at least 5/10 patients randomised to control received NO) |
| Dellinger,w3 1998 (industry) | 177 adults, 30 centres. AECC criteria for ARDS and FiO2 ≥0.5, PEEP ≥8 cm H2O. Duration of ARDS ≤72 hours | 1.25, 5, 20, 40 or 80 ppm until 28 days or oxygenation and PEEP criteria met. Protocol for weaning NO | Placebo gas (nitrogen). No crossovers |
| Michael,w4 1998 (not for profit) | 40 patients, 1 centre; 37/40 ≥18 years. AECC criteria for ARDS except P/F ≤150 mm Hg and FiO2 ≥0.8 for ≥12 hours or ≥0.65 for ≥24 hours | 5, 10, 15, 20 ppm every 6 hours for 24 hours then clinically adjusted; mean dose ∼13 ppm, tapered if oxygenation not improved by 72 hours | Usual care. Patients with oxygenation failure received NO (2 patients before 72 hours and 7 patients after 72 hours, of 20 randomised to control) |
| Troncy,w5 1998 (not for profit) | 30 adults, 1 centre. Lung injury score30 ≥2.5 | Initial titration (2.5, 5, 10, 20, 30, 40 ppm every 10 min) and daily re-titration; mean dose 5.3 ppm. Duration: until oxygenation and PEEP criteria met; mean 8 (SD 5) days | Usual care. No crossovers |
| Dobyns,w6 1999 (not for profit) | 108 children (>1 month old, median age 2.5 years), 7 centres. Any CXR infiltrates, OI ≥15 on 2 arterial blood gases within 6 hours (mean duration of ventilation before randomisation 3.5 days in NO group, 3.7 days in control group) | 10 ppm for 72 hours, then weaned if failure criteria not met | Usual care. Patients meeting failure criteria could receive NO (27/55 patients randomised to control met failure criteria and 2 other patients withdrawn from control group; 29 patients likely received NO) |
| Lundin,w7 1999 (not for profit and industry) | 180 adults, 43 centres. Any CXR infiltrates, P/F ≤165 mm Hg, PEEP ≥5 cm H2O, mean airway pressure >10 cm H2O. Duration of ventilation 0.75-4 days. NO responder† | 1-40 ppm (“lowest effective dose”); mean dose 9 (SD 8) ppm, until end point met (reversal of ALI or severe respiratory failure), up to 30 days; mean 9 (SD 6) days | Usual care. Patients meeting severe respiratory failure criteria could receive NO (6/87 patients randomised to control received NO) |
| Payen,w8 1999 (not for profit; industry supplied gas) | 203 adults, 23 centres. AECC criteria for ARDS, lung injury score30 2-3 after 24 hours of “therapeutic optimisation” (mean duration of ventilation before randomisation: 5.3 days in NO group, 5.9 days in control group) | 10 ppm, until oxygenation and PEEP criteria met; median 5 days | Placebo gas (nitrogen). Patients meeting failure criteria crossed to other group (19/105 patients randomised to control and 12/98 patients randomised to NO crossed over) |
| Mehta,w9 2001 (not for profit and industry) | 14 adults, 1 centre. Bilateral CXR infiltrates, P/F <200 mm Hg, PEEP ≥8 cm H2O, PAOP <18 cmH2O. Duration of ARDS ≤5 days | Daily titration (5, 10, 20 ppm every 30 min) for 4 days. Most received 5-10 ppm on day 2-4; continued until oxygenation criteria met; mean 8 (SD 9) days | Usual care. No crossovers |
| Gerlach,w10 2003 (not for profit) | 40 adults, 1 centre. Bilateral CXR infiltrates, P/F ≤150 mm Hg, PEEP ≥10 cm H2O, PAOP ≤18 mm Hg. Duration of ventilation ≥48 hours with FiO2 ≥0.6 (median duration of ventilation before randomisation: 14 days in NO group, 11.5 days in control group) | 10 ppm (with daily dose response analysis) until weaning initiated | Usual care. No crossovers |
| Park,w11 2003 (not reported) | 17 adults, 1 centre. AECC criteria for ARDS. Duration of ARDS ≤2 days | 5 ppm for mean 3.5 (SD 1.5) days (stopping criteria not reported). Patients also received one lung recruitment manoeuvre (same as control group). Third group (n=6) received NO 5 ppm alone for 8.2 (SD 4.7) days | One lung recruitment manoeuvre (inflation pressure of 30-35 cm H2O for 30 seconds). No crossovers |
| Taylor,w12 2004 (industry) | 385 adults, 46 centres. AECC criteria for ALI except P/F ≤250 mm Hg, 0.5 ≤ FiO2 ≤0.95 on PEEP ≥8 cm H2O. Duration of ALI ≤3 days | 5 ppm for 28 days or until oxygenation and PEEP criteria met | Placebo gas (nitrogen). No crossovers |
AECC=American-European Consensus Conference1; CXR=chest radiograph; LIS=lung injury score,30 OI=oxygenation index (100×mean airway pressure/(PaO2/FiO2)), PAOP=pulmonary artery occlusion pressure; PEEP=positive end expiratory pressure; P/F ratio=partial pressure of inspired oxygen (PaO2)/fraction of inspired oxygen (FiO2).
*Funding refers to data collection and analysis and supply of study gas (where information available).
†Patients given NO 0, 2, 10, 40 ppm every 10 min and response defined as relative increase in PaO2 of 25% (n=140) or 20% (n=40). Responders were randomised.
The 12 trials had good scientific quality (table 2). Ten concealed randomisation,w1-w3w5-w8w10-w12 and five blinded clinicians.w2 w3w6w8w12 Mechanical ventilation was delivered according to protocol in three unblinded trialsw5w10 w11 and one blinded trialw2 and according to guidelines in three blinded trials.w3w6w12 Six trials described or standardised at least one other cointervention, such as corticosteroids,w3 sedation,w5 prone ventilation,w5w9-w12 and ventilator weaning.w11 w12 All trials had complete follow-up, analysed patients by assigned group, and withdrew no one from clinical outcomes analyses. One trial stopped early because of slow enrolment (achieving 45% of the planned sample sizew7), and another trial enrolled 75% of the planned sample, for unclear reasons.w12
Table 2.
Scientific quality of trials of inhaled nitric oxide (NO) in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)*
| Author | Allocation concealment | Blinding | Ventilation | Other cointerventions |
|---|---|---|---|---|
| Day,w1 1997 | Blinded draw of 1 lot per eligible patient | None | Clinician discretion | Not described |
| Schwebel,w2 1997 | Table of gas cylinder codes (revealed in sequence) | Clinicians, outcomes assessors | Protocol (no details) for 17 hours; clinician's discretion thereafter | Not described |
| Dellinger,w3 1998 | Sealed, opaque envelopes | Clinicians†, outcomes assessors | Guideline (Pplat≤35 cm H2O; PEEP to optimise compliance; FiO2 minimised) | More patients in NO group received corticosteroids after day 6 (20/112 v 6/57) |
| Michael,w4 1998 | Not reported | None | Clinician discretion; mode unchanged for 72 hours; mean PEEP similar between groups for 72 hours | Not described |
| Troncy, w5 1998 | Envelopes‡ | None | Protocol (VT 10 ml/kg and goal PaCO2 35-45 mm Hg; maximum PEEP 15 cm H2O and goal PaO2 >85 mm Hg) | Sedation, blood transfusion, and nutrition protocols. No prone ventilation in any patient |
| Dobyns,w6 1999 | Envelopes‡ | Clinicians†, outcomes assessors | Guideline (“open lung approach” with Ppk ≤35-40 cm H2O, VT limitation, titrated PEEP; HFOV by clinician discretion) | Not described |
| Lundin,w7 1999 | Central | None | Clinician discretion | Not described |
| Payen,w8 1999 | Central | Clinicians, outcomes assessors | Guideline before randomisation; unclear if applied afterwards (VT, Pplat, Ppk limitation; various recruitment strategies) | Not described |
| Mehta,w9 2001 | No§ | None | Clinician discretion | No prone ventilation in any patient |
| Gerlach,w10 2003 | Envelopes‡ | None | Protocol (no details) | Protocol for prone ventilation and extracorporeal membrane oxygenation |
| Park,w11 2003 | One random number generated when patient eligible | None | Protocol (VT 6 ml/kg; Pplat ≤30 cmH2O; PEEP to optimise PaO2; FiO2 minimised) | Weaning protocol. No prone ventilation in any patient |
| Taylor,w12 2004 | Central | Clinicians†, outcomes assessors | Guideline (Pplat ≤ 35 cm H2O; PEEP to optimise compliance; FiO2 minimised) | Prone ventilation similar (NO: 10/192 and control: 14/193), weaning protocol |
FiO2=fraction of inspired oxygen; HFOV=high frequency oscillatory ventilation; PaO2=partial pressure of arterial oxygen; PaCO2=partial pressure of arterial carbon dioxide; Pplat=plateau airway pressure; Ppk=peak airway pressure; PEEP=positive end expiratory pressure; VT=tidal volume.
*No study reported withdrawals of patients or loss to follow-up for mortality; all patients analysed according to assigned group.
†Unblinded investigator at each site.
‡Envelopes sealed, sequentially numbered, and opaque.
§Computer generated random numbers. Investigator had access to entire randomisation list at time of randomisation.
Data synthesis
Effect of nitric oxide on clinical outcomes
We combined nine trialsw3-w5w7-w12 in the mortality analysis (three were placebo controlledw3w8w12; five used “usual care” controlsw4 w5w7w9 w10; one used recruitment manoeuvres in both armsw11). We combined three trials that reported duration of ventilation (including all patientsw10 w11 or only survivorsw7) and five trials reporting ventilator-free days.w3w5w8w11 w12
Meta-analyses (table 3) showed that nitric oxide did not affect mortality (risk ratio 1.10; 95% confidence interval 0.94 to 1.30; fig 2), duration of ventilation (17% increase, −20% to 70%; 3.6 additional days, −4.0 to 11.1 days), or ventilator-free days (6% decrease, −16% to 6%; 0.6 fewer days, −1.8 to 0.7 days). There was moderate to high heterogeneity between studies for duration of ventilation only.
Table 3.
Effects of inhaled nitric oxide (NO) on clinical and physiological outcomes in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
| Outcome | No of trials (patients) | Treatment effect (95% CI); P value | P value for homogeneity; I2 | |||
|---|---|---|---|---|---|---|
| Ratio of means | Weighted mean difference | Ratio of means | Weighted mean difference | |||
| Mortality* | 9 (1086) | — | — | — | — | |
| Duration of ventilation (days) | 3 (237) | 1.17 (0.80 to 1.70); 0.41 | 3.6 (−4.0 to 11.1); 0.36 | 0.02; 76% | 0.07; 63% | |
| Days without ventilation† | 5 (804) | 0.94 (0.84 to 1.06); 0.33 | −0.6 (−1.8 to 0.7); 0.37 | 0.71; 0% | 0.66; 0% | |
| PaO2/FiO2 (mm Hg): | ||||||
| Day 1 | 9 (553) | 1.13 (1.04 to 1.23); 0.003 | 16 (4 to 27); 0.007 | 0.19; 29% | 0.11; 39% | |
| Day 2 | 5 (416) | 1.07 (1.02 to 1.13); 0.006 | 9 (−3 to 20); 0.14 | 0.43; 0% | 0.18; 37% | |
| Day 3 | 5 (450) | 1.05 (0.98 to 1.13); 0.17 | 7 (−4 to 18); 0.21 | 0.54; 0% | 0.49; 0% | |
| Day 4 | 4 (334) | 1.07 (1.02 to 1.12); 0.01 | 15 (4 to 25); 0.009 | 0.85; 0% | 0.90; 0% | |
| Oxygenation index (100)×mean airway pressure/(PaO2/FiO2) (cm H2O/mm Hg); | ||||||
| Day 1 | 3 (296) | 0.86 (0.75 to 0.98); 0.02 | −3 (−5 to −0.5); 0.02 | 0.46; 0% | 0.72; 0% | |
| Day 2 | 1 (164) | 0.81 (0.67 to 0.98); 0.03 | −3 (−6 to −0.04); 0.05‡ | — | — | |
| Day 3 | 2 (245) | 0.82 (0.64 to 1.06); 0.13 | −3 (−7 to −0.2); 0.04 | 0.28; 16% | 0.34; 0% | |
| Day 4 | 1 (134) | 0.78 (0.63 to 0.96); 0.02 | −4 (−8 to −0.3); 0.03‡ | — | — | |
| Mean pulmonary arterial pressure (mm Hg): | ||||||
| Day 1 | 4 (165) | 0.95 (0.88 to 1.03); 0.24 | −2 (−4 to 1); 0.22 | 0.27; 23% | 0.27; 23% | |
| Day 2 | 3 (167) | 0.96 (0.89 to 1.02); 0.19 | −1 (−3 to 0.6); 0.18 | 0.64; 0% | 0.68; 0% | |
| Day 3 | 2 (111) | 0.94 (0.87 to 1.02); 0.12 | −2 (−4 to 0.5); 0.12 | 0.95; 0% | 0.97; 0% | |
| Day 4 | 3 (130) | 0.94 (0.88 to 1.01); 0.08 | −2 (−4 to 0.3); 0.10 | 0.81; 0% | 0.72; 0% | |
| Renal dysfunction§ | 4 (895) | — | — | — | — | |
PaO2/FiO2=partial pressure of arterial oxygen/fraction of inspired oxygen, ratio of means=nitric oxide relative to control. We used random effects models for all analyses and assessed heterogeneity using Cochran's Q test22 (P value for homogeneity shown) and I2.24
*Risk ratio 1.10 (0.94 to 1.30); P=0.23, homogeneity P=1.00, I2=0%. Two trials with ≥50% of control patients crossing over to NO also reported mortality data.w2w6 Inclusion of these trials did not alter summary mortality estimate (risk ratio 1.09, 0.94 to 1.27).
†Combined trials reporting days without ventilation to day 28 and day 30.
‡Mean difference because only one trial contributed data.
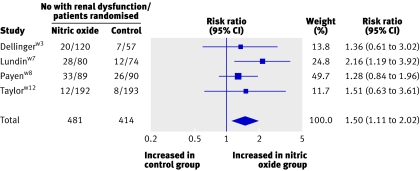
§Risk ratio 1.50 (1.11 to 2.02); P=0.008; homogeneity P=0.57, I2=0%. Renal dysfunction was defined as new renal replacement therapy,w8 new renal replacement therapy or new raised creatinine concentration (>300 µmol/lw7), or raised creatinine concentration (>177 µmol/lw3 or ≥265 µmol/lw12). Denominator includes only patients without baseline renal dysfunction,w7 w8 w12 except possibly for one trial.w3 Use of a different definition of renal dysfunction (“adverse event”) in one trialw3 did not alter summary estimate (risk ratio 1.49, 1.10 to 2.03).
Fig 2 Effect of nitric oxide on mortality. Weight is the relative contribution of each study to the overall estimate of treatment effect on a log scale assuming a random effects model. Two trials with ≥50% of control patients crossing over to nitric oxide also reported mortality data.w2w6 Inclusion of these trials did not alter summary mortality estimate (risk ratio 1.09, 0.94 to 1.27)
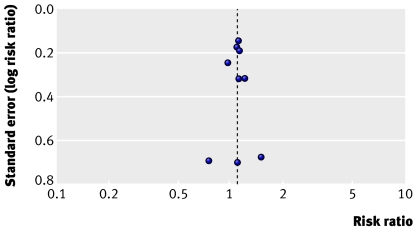
A funnel plot of standard error versus risk ratio for mortality did not suggest publication bias (fig 3).
Fig 3 Funnel plot for outcome of mortality in trials of nitric oxide. Each point represents one trial
Effect of nitric oxide on physiological outcomes
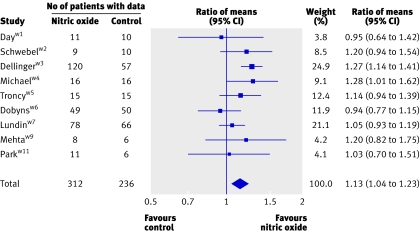
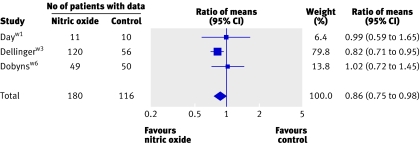
On the first day of therapy, NO was associated with small improvements in the PaO2/FiO2 ratio (nine trials; 13% higher, 4% to 23%; 16 mm Hg higher, 4 mm Hg to 27 mm Hg; fig 4) and oxygenation index (three trials; 14% lower, 2% to 25%; 3 cm H2O/mm Hg lower, 0.5 cm H2O/mm Hg to 5 cm H2O/mm Hg; fig 5). Some evidence suggested that improvements in oxygenation in the nitric oxide group persisted beyond day one. The PaO2/FiO2 ratio was higher on day two and four (but not on day three, and only in the ratio of means analysis on day two). The oxygenation index remained lower on days two, three, and four (only in the weighted mean difference analysis on day three), but only onew3 (days two and four) or twow3w6 (day three) trials contributed data. Differences in mean pulmonary arterial pressure were not significant on any day.
Fig 4 Effect of nitric oxide on PaO2/FiO2 ratio at 24 hours. Weight is relative contribution of each study to overall estimate of treatment effect (ratio of means, nitric oxide relative to control) on log scale assuming a random effects model. For some trials, number of patients with data is less than number randomised
Fig 5 Effect of nitric oxide on oxygenation index at 24 hours. Weight is relative contribution of each study to overall estimate of treatment effect (ratio of means, nitric oxide relative to control) on log scale assuming a random effects model. For each trial, number of patients with data is less than number randomised
There was no evidence of important statistical heterogeneity in the physiological outcomes.
Adverse effects
Table 4 gives details of adverse effects. All 12 trials gave information about methaemoglobin concentrations. Four nitric oxide patients (of 651 randomised) and three control patients (of 586 randomised) developed >5% methaemoglobinaemia.w3w7w12 One trial reported three patients developing raised nitrogen dioxide concentrations; all had received 80 ppm nitric oxide .w3 Nitric oxide increased the risk of renal dysfunction in one unblindedw7 and three blindedw3w8w12 trials that enrolled 72% of patients in all included trials (risk ratio 1.50, 1.11 to 2.02; fig 6). Other adverse events were variably reported, and we did not combine these data.
Table 4.
Adverse effects of inhaled nitric oxide
| Author, year | Methaemoglobin and nitrogen dioxide concentrations | Other adverse effects |
|---|---|---|
| Day,w1 1997 | No known raised concentrations | None |
| Schwebel,w2 1997 | No methaemoglobinaemia | None |
| Dellinger,w3 1998 | Methaemoglobin concentration >5% (none >7%): NO 2.5% (3/120; 40 ppm, n=1; 80 ppm, n=2), control 2% (1/57); nitrogen dioxide level >3 ppm: NO 2.5% (3/120; all received 80 ppm), control: none | Renal function (“defined by adverse events”): NO 11% (13/120), control 9% (5/57); creatinine >177 µmol/l: NO 17% (20/120), control 13% (7/57). Adverse events “possibly” related to study gas: NO 3% (4/120: myopathy, agitation; abnormal liver enzymes; apnoea, lung haemorrhage, coagulopathy; renal dysfunction), control 2% (1/57: hypertension). All adverse events: no significant differences |
| Michael,w4 1998 | No methaemoglobinaemia | Bleeding. Blood transfusion: NO 5% (1/20), control 0/20. Intracranial hemorrhage after thrombolytic therapy: NO 5% (1/20), control 0/20 |
| Troncy,w5 1998 | No methaemoglobinaemia | Not reported |
| Dobyns,w6 1999 | Methaemoglobin concentration >5%: none; nitrogen dioxide concentration >2 ppm: none | No difference in “intensive care unit-dependent therapies”31 |
| Lundin,w7 1999 | Methaemoglobin concentration >5%: NO 1% (1/93), control 1% (1/87); median methaemoglobin concentrations over 30 days: NO 0.5%-1.2%, control 0.2%-1.0% (“overall lower” than in NO group) | Adverse events related to study gas: NO 1% (1/93: gastrointestinal bleeding), control 2% (2/87: coagulopathy, intracranial bleed). Renal function “abnormal”: NO 13% (12/93), control 5% (4/87). Renal replacement (incident cases): NO 27% (23/84), control 13% (10/79); risk ratio 2.16, 1.10 to 4.25. Creatinine >300 µmol/l without renal replacement (incident cases): NO 6% (5/80), control 3% (2/74). Other serious adverse events more common in NO group: circulatory failure: NO 31% (29/93), control 20% (17/87); encephalopathy: NO 3% (3/93), control none; sepsis: NO 8% (7/93), control, 3% (3/87). Other adverse events: no difference in incidence of raised total bilirubin, pneumothorax, or platelet, bleeding or clotting disorders or haemodynamic failure (definitions of haemodynamic v circulatory failure not given) |
| Payen,w8 1999 | Methaemoglobin concentrations reported as always acceptable and no different between groups | Renal replacement (incident cases): NO 37% (33/89), control 29% (26/90); risk ratio 1.28, 0.84 to 1.96. Bleeding: NO 6% (6/105), control 3% (3/98) |
| Mehta,w9 2001 | Methaemoglobin concentration >3%: none (concentration in 1/8 NO patients was 3.8% before therapy); nitrogen dioxide concentration >2 ppm: none | None |
| Gerlach,w10 2003 | No methaemoglobinaemia; no patients with increased nitrogen dioxide concentrations | No bleeding. No difference between groups in number of additional organ dysfunctions32 |
| Park,w11 2003 | No methaemoglobinaemia | None |
| Taylor,w12 2004 | Methaemoglobin concentration >5%: NO 0/192, control 0.005% (1/193); nitrogen dioxide concentration >2 ppm: none | All adverse events: no difference (NO, 630 events; control, 666 events). No difference in cardiovascular, gastrointestinal, endocrine, haematological, metabolic and nutritional, and neurological adverse events. Adverse events with different frequencies. Infections: NO 66 infections, control 41 infections. Respiratory: NO 51% (98/192), control 61% (118/193); pneumonia, pneumothorax, apnoea more common in control group. Renal function: creatinine ≥265 µmol/l: NO 6% (12/192), control 4% (8/193); creatinine ≥309 µmol/l: NO 5% (10/192), control 3% (6/193) |
Fig 6 Effect of nitric oxide on renal dysfunction (defined as new renal replacement therapy,w8 new renal replacement therapy or new raised creatinine concentration (>300 µmol/lw7), or raised creatinine concentration (>177 µmol/lw3 or ≥265 µmol/lw12)). The denominator includes only patients without baseline renal dysfunction,w7 w8 w12 except possibly for one trial.w3 Use of a different definition of renal dysfunction (“adverse event”) in one trialw3 did not alter the summary estimate (risk ratio 1.49, 1.10 to 2.03). Weight is relative contribution of each study to overall estimate of treatment effect on log scale assuming a random effects model
Discussion
The routine use of inhaled nitric oxide is not beneficial for patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Our meta-analysis included 12 trials that randomly assigned 1237 patients and investigated the effects of inhaled nitric oxide in such patients. We found no benefit of nitric oxide on survival and an increased risk of renal dysfunction. Oxygenation improved over the first 24 hours (13% relative increase in PaO2/FiO2 ratio; 14% decrease in oxygenation index), with some data suggesting improvements to 96 hours. Given the limited physiological improvements and possible harm, we cannot recommend routine use of nitric oxide in these patients.
The trend towards increased mortality in patients receiving nitric oxide was highly consistent across trials, with no trial dominating the meta-analysis. Given the strength and magnitude of this trend, consistency across trials, biological plausibility,18w10 and the finding of other potential adverse effects of nitric oxide (for example, renal failure), our analysis raises concerns about its nitric oxide in this setting.
Adverse events
Descriptive analyses suggest that methemoglobinaemia and raised nitrogen dioxide concentration are not common or clinically important consequences, except possibly in patients receiving high doses (at least 80 ppm) of nitric oxide for several days. Data from four large trials representing nearly three quarters of all randomised patients showed an increased risk of renal dysfunction in patients receiving nitric oxide. Cautious interpretation is warranted, however, as this result was a post hoc analysis and is potentially subject to publication bias (we were unable to obtain explicit data on renal outcomes in eight of 12 smaller trials, in which this relation may not have been measured or observed). In addition, the potential physiological mechanisms linking administration of inhaled nitric oxide to acute renal dysfunction—inhibition of mitochondrial and enzymatic function and damage to deoxyribonucleic acid and membranes—are controversial because of its simultaneous protective effects on renal blood flow and leukocyte adhesion.26
Why nitric oxide may not be beneficial
There are several possible explanations for the lack of benefit of routine administration of nitric oxide in patients with ALI/ARDS. Firstly, short term physiological improvements in oxygenation seem to have no impact on patients' survival,27 possibly because oxygenation is not necessarily related to severity of lung injury. Secondly, as most patients with ARDS die of multiple organ failure rather than refractory hypoxaemia,28 small changes in oxygenation might not lead to improvements in outcome. Thirdly, the prolonged fixed dosing regimen in most trials may have attenuated benefit over time because of increased sensitisation, dampening the oxygenation benefit while continuing to expose patients to toxic effects such as oxidative damage.18w10 Fourthly, the benefits of nitric oxide may have been overwhelmed by a harmful mechanical ventilation strategy, which perpetuated multiple organ failure.29 This, however, would not account for our finding of potential harm. Finally, trials restricting enrolment to patients with an acute oxygenation response to nitric oxide may have found a positive effect on mortality, although this hypothesis was not supported in one trial.w7
Strengths and limitations
We used several methods to reduce bias (comprehensive literature search, duplicate data abstraction, prespecified criteria for methodological assessment and analysis) and analysed a comprehensive set of clinical and physiological outcomes. We were unable to obtain anyw4w12 or completew3 additional information from three trials. Considering secondary clinical outcomes, we expected to find variation between trials in duration of ventilation and ventilator-free days related to different populations of patients. We analysed these outcomes, while acknowledging the limited interpretability of this analysis. Finally, given the small number of trials contributing to analyses of many physiological outcomes, the tests for heterogeneity were underpowered.
Although our results do not exclude the possibility that some subgroups of patients may benefit from nitric oxide, the consistent lack of a mortality benefit across trials mitigates this possibility. The included trials did not specifically study the issue of nitric oxide as rescue therapy for patients with critically low oxygenation. With nitric oxide, short term improved oxygenation in these patients may create a window for other strategies to improve lung function, such as treatment of the underlying cause of ARDS.
Previous research
A previous systematic review and meta-analysis of inhaled nitric oxide for acute hypoxaemic respiratory failure8 9 included fewer randomised controlled trialsw3-w7 and found no effect on mortality (risk ratio 0.98, 95% confidence interval 0.66 to 1.44; two trials, 204 patients). Our report is consistent with this work and extends it by including more trials, thus narrowing the confidence limits around the estimate of mortality. We also provide new estimates of the impact of nitric oxide on other clinical and physiological end points and raise the possibility of harm induced by nitric oxide.
In conclusion, our systematic review and meta-analysis found that inhaled nitric oxide improved oxygenation in patients with ALI and ARDS at 24 hours of therapy, with some evidence for a more prolonged effect. Given that the best available evidence suggests no survival advantage and possible increased mortality and renal dysfunction with nitric oxide, we do not recommend its routine use. Despite a lack of evidence for benefit, some clinicians may still consider nitric oxide for life threatening hypoxaemia, in conjunction with other supportive therapies. Given the challenges of enrolling such severely ill patients into large trials, definitive data supporting or refuting a role for nitric oxide in such desperate situations may not be forthcoming, leaving clinicians to rely on their judgment and the current evidence.
What is already known on this topic
Inhaled nitric oxide continues to be used to improve oxygenation in patients with acute lung injury, despite no clear supporting evidence and several discouraging reviews and editorials
A previous meta-analysis in 2003 included five randomised trials of nitric oxide; there are now 12 trials
What this study adds
Nitric oxide improves oxygenation temporarily but does not improve survival and may cause harm
We do not recommend routine use of nitric oxide in patients with acute lung injury
Supplementary Material
We thank Phil Dellinger, Emily Dobyns, Herwig Gerlach, and Sangeeta Mehta for providing additional information about their trials; Pascal Beuret, Gilbert Blaise, Ronald Day, Stefan Lundin, Kwang Joo Park, Didier Payen, and Benoît Vallet for providing additional outcomes data; Natasha Stankovic for assistance in translation; and Jim Julian for constructive comments on an earlier draft of the manuscript.
Contributors: NKJA conceived and designed the study, acquired data, analysed and interpreted data, and drafted the manuscript. KEAB contributed to study design and acquired and interpreted data. JOF acquired and interpreted data. JTG interpreted data. DJC and MOM contributed to study design and interpreted data. All authors revised the manuscript for important intellectual content and approved the final version. NKJA is guarantor.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:819-24. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [DOI] [PubMed] [Google Scholar]
- 3.Furchgott RF. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA 1996;276:1186-8. [PubMed] [Google Scholar]
- 4.Gries A, Bode C, Peter K, Herr A, Bohrer H, Motsch J, et al. Inhaled nitric oxide inhibits human platelet aggregation, P-selectin expression, and fibrinogen binding in vitro and in vivo. Circulation 1998;97:1481-7. [DOI] [PubMed] [Google Scholar]
- 5.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 1991;88:4651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beloucif S, Payen D. A European survey of the use of inhaled nitric oxide in the ICU. Working Group on Inhaled NO in the ICU of the European Society of Intensive Care Medicine. Intensive Care Med 1998;24:864-77. [DOI] [PubMed] [Google Scholar]
- 7.Meade MO, Jacka MJ, Cook DJ, Dodek P, Griffith L, Guyatt GH, et al. Survey of interventions for the prevention and treatment of acute respiratory distress syndrome. Crit Care Med 2004;32:946-54. [DOI] [PubMed] [Google Scholar]
- 8.Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxic respiratory failure in children and adults: a meta-analysis. Anesth Analg 2003;97:989-98. [DOI] [PubMed] [Google Scholar]
- 9.Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev 2003;(1):CD002787. [DOI] [PubMed]
- 10.McIntyre RC Jr, Pulido EJ, Bensard DD, Shames BD, Abraham E. Thirty years of clinical trials in acute respiratory distress syndrome. Crit Care Med 2000;28:3314-31. [DOI] [PubMed] [Google Scholar]
- 11.Conner BD, Bernard GR. Acute respiratory distress syndrome. Potential pharmacologic interventions. Clin Chest Med 2000;21:563-87. [DOI] [PubMed] [Google Scholar]
- 12.Brower RG, Ware LB, Berthiaume Y, Matthay MA. Treatment of ARDS. Chest 2001;120:1347-67. [DOI] [PubMed] [Google Scholar]
- 13.Dos Santos CC, Chant C, Slutsky AS. Pharmacotherapy of acute respiratory distress syndrome. Expert Opin Pharmacother 2002;3:875-88. [DOI] [PubMed] [Google Scholar]
- 14.Cranshaw J, Griffiths MJ, Evans TW. The pulmonary physician in critical care—part 9: non-ventilatory strategies in ARDS. Thorax 2002;57:823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasaka S, Hasegawa N, Ishizaka A. Pharmacology of acute lung injury. Pulm Pharmacol Ther 2002;15:83-95. [DOI] [PubMed] [Google Scholar]
- 16.Wiedemann HP, Arroliga AC, Komara Jr JJ. Emerging systemic pharmocologic approaches in acute respiratory distress syndrome. Respir Care Clin N Am 2003;9:419-35. [DOI] [PubMed] [Google Scholar]
- 17.Fan E, Mehta S. High-frequency oscillatory ventilation and adjunctive therapies: inhaled nitric oxide and prone positioning. Crit Care Med 2005;33:S182-7. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths MJD, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med 2005;353:2683-95. [DOI] [PubMed] [Google Scholar]
- 19.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 1973;33:613-9. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trial s 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med 2005;142:510-24. [DOI] [PubMed] [Google Scholar]
- 22.Cochran W. The combination of estimates from different experiments. Biometrics 1954;10:101-29. [Google Scholar]
- 23.Berlin JA, Laird NM, Sacks HS, Chalmers TC. A comparison of statistical methods for combining event rates from clinical trials. Stat Med 1989;8:141-51. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdivielso JM, Blantz RC. Acute renal failure: is nitric oxide the bad guy? Antioxid Redox Signal 2002;4:925-34. [DOI] [PubMed] [Google Scholar]
- 27.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1985;132:485-9. [DOI] [PubMed] [Google Scholar]
- 29.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54-61. [DOI] [PubMed] [Google Scholar]
- 30.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome [Erratum, Am Rev Respir Dis Am Rev Respir Dis 1989;139:1065]. [DOI] [PubMed] [Google Scholar]
- 31.Pollack MM, Getson PR, Ruttimann UE, Steinhart CM, Kanter RK, Katz RW, et al. Efficiency of intensive care. A comparative analysis of eight pediatric intensive care units. JAMA 1987;258:1481-6. [PubMed] [Google Scholar]
- 32.Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrere JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg 1985;120:1109-15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.