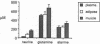Abstract
To determine the relationship between circulating metabolic fuels and their local concentrations in peripheral tissues we measured glycerol, glucose, and amino acids by microdialysis in muscle and adipose interstitium of 10 fasted, nonobese human subjects during (a) baseline, (b) euglycemic hyperinsulinemia (3 mU/kg per min for 3 h) and, (c) local norepinephrine reuptake blockade (NOR). At baseline, interstitial glycerol was strikingly higher (P < 0.0001) in muscle (3710 microM) and adipose tissue (2760 microM) compared with plasma (87 microM), whereas interstitial glucose (muscle 3.3, fat 3.6 mM) was lower (P < 0.01) than plasma levels (4.8 mM). Taurine, glutamine, and alanine levels were higher in muscle than in adipose or plasma (P < 0.05). Euglycemic hyperinsulinemia did not affect interstitial glucose, but induced a fall in plasma glycerol and amino acids paralleled by similar changes in the interstitium of both tissues. Local NOR provoked a fivefold increase in glycerol (P < 0.001) and twofold increase in norepinephrine (P < 0.01) in both muscle and adipose tissues. To conclude, interstitial substrate levels in human skeletal muscle and adipose tissue differ substantially from those in the circulation and this disparity is most pronounced for glycerol which is raised in muscle as well as adipose tissue. In muscle, insulin suppressed and NOR increased interstitial glycerol concentrations. Our data suggest unexpectedly high rates of intramuscular lipolysis in humans that may play an important role in fuel metabolism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfvidsson B., Zachrisson H., Möller-Loswick A. C., Hyltander A., Sandström R., Lundholm K. Effect of systemic hyperinsulinemia on amino acid flux across human legs in postabsorptive state. Am J Physiol. 1991 Jan;260(1 Pt 1):E46–E52. doi: 10.1152/ajpendo.1991.260.1.E46. [DOI] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P., Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990 Mar;85(3):893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In situ studies of catecholamine-induced lipolysis in human adipose tissue using microdialysis. J Pharmacol Exp Ther. 1990 Jul;254(1):284–288. [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In vivo interactions between beta-1 and beta-2 adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J Pharmacol Exp Ther. 1991 Oct;259(1):317–322. [PubMed] [Google Scholar]
- Bolinder J., Hagström E., Ungerstedt U., Arner P. Microdialysis of subcutaneous adipose tissue in vivo for continuous glucose monitoring in man. Scand J Clin Lab Invest. 1989 Sep;49(5):465–474. doi: 10.1080/00365518909089123. [DOI] [PubMed] [Google Scholar]
- Bolinder J., Ungerstedt U., Arner P. Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia. 1992 Dec;35(12):1177–1180. doi: 10.1007/BF00401374. [DOI] [PubMed] [Google Scholar]
- Brooks B. J., Arch J. R., Newsholme E. A. Effect of some hormones on the rate of the triacylglycerol/fatty-acid substrate cycle in adipose tissue of the mouse in vivo. Biosci Rep. 1983 Mar;3(3):263–267. doi: 10.1007/BF01122458. [DOI] [PubMed] [Google Scholar]
- Coppack S. W., Fisher R. M., Gibbons G. F., Humphreys S. M., McDonough M. J., Potts J. L., Frayn K. N. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 1990 Oct;79(4):339–348. doi: 10.1042/cs0790339. [DOI] [PubMed] [Google Scholar]
- Coppack S. W., Frayn K. N., Humphreys S. M., Whyte P. L., Hockaday T. D. Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism. 1990 Apr;39(4):384–390. doi: 10.1016/0026-0495(90)90253-9. [DOI] [PubMed] [Google Scholar]
- Dagenais G. R., Tancredi R. G., Zierler K. L. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976 Aug;58(2):421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- During M. J., Spencer D. D. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992 Nov;32(5):618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- Elia M., Khan K., Calder G., Kurpad A. Glycerol exchange across the human forearm assessed by a combination of tracer and arteriovenous exchange techniques. Clin Sci (Lond) 1993 Jan;84(1):99–104. doi: 10.1042/cs0840099. [DOI] [PubMed] [Google Scholar]
- Elia M., Neale G., Livesey G. Alanine and glutamine release from the human forearm: effects of glucose administration. Clin Sci (Lond) 1985 Aug;69(2):123–133. doi: 10.1042/cs0690123. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971 Dec;50(12):2703–2714. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., Jacob R., Walesky M., Sherwin R. S., DeFronzo R. A. Effect of free fatty acids on blood amino acid levels in human. Am J Physiol. 1986 Jun;250(6 Pt 1):E686–E694. doi: 10.1152/ajpendo.1986.250.6.E686. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Khan K., Coppack S. W., Elia M. Amino acid metabolism in human subcutaneous adipose tissue in vivo. Clin Sci (Lond) 1991 May;80(5):471–474. doi: 10.1042/cs0800471. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest. 1968;21(3):263–276. doi: 10.3109/00365516809076994. [DOI] [PubMed] [Google Scholar]
- Hagström-Toft E., Arner P., Johansson U., Eriksson L. S., Ungerstedt U., Bolinder J. Effect of insulin on human adipose tissue metabolism in situ. Interactions with beta-adrenoceptors. Diabetologia. 1992 Jul;35(7):664–670. doi: 10.1007/BF00400260. [DOI] [PubMed] [Google Scholar]
- Hagström-Toft E., Arner P., Näslund B., Ungerstedt U., Bolinder J. Effects of insulin deprivation and replacement on in vivo subcutaneous adipose tissue substrate metabolism in humans. Diabetes. 1991 Jun;40(6):666–672. doi: 10.2337/diab.40.6.666. [DOI] [PubMed] [Google Scholar]
- Hagström-Toft E., Arner P., Wahrenberg H., Wennlund A., Ungerstedt U., Bolinder J. Adrenergic regulation of human adipose tissue metabolism in situ during mental stress. J Clin Endocrinol Metab. 1993 Feb;76(2):392–398. doi: 10.1210/jcem.76.2.8381801. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. Ethanol may be used with the microdialysis technique to monitor blood flow changes in skeletal muscle: dialysate glucose concentration is blood-flow-dependent. Acta Physiol Scand. 1991 Nov;143(3):355–356. doi: 10.1111/j.1748-1716.1991.tb09243.x. [DOI] [PubMed] [Google Scholar]
- Holm C., Belfrage P., Fredrikson G. Immunological evidence for the presence of hormone-sensitive lipase in rat tissues other than adipose tissue. Biochem Biophys Res Commun. 1987 Oct 14;148(1):99–105. doi: 10.1016/0006-291x(87)91081-3. [DOI] [PubMed] [Google Scholar]
- Jacobson I., Sandberg M., Hamberger A. Mass transfer in brain dialysis devices--a new method for the estimation of extracellular amino acids concentration. J Neurosci Methods. 1985 Nov-Dec;15(3):263–268. doi: 10.1016/0165-0270(85)90107-4. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Fowelin J., Smith U., Lönnroth P. Characterization by microdialysis of intracellular glucose level in subcutaneous tissue in humans. Am J Physiol. 1988 Aug;255(2 Pt 1):E218–E220. doi: 10.1152/ajpendo.1988.255.2.E218. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Larsson A., Smith U., Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992 May;89(5):1610–1617. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. A., Smith U., Lönnroth P. Interstitial glycerol concentration measured by microdialysis in two subcutaneous regions in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E918–E922. doi: 10.1152/ajpendo.1990.258.6.E918. [DOI] [PubMed] [Google Scholar]
- Johansson U., Arner P., Bolinder J., Hagström-Toft E., Ungerstedt U., Eriksson L. S. Influence of insulin on glucose metabolism and lipolysis in adipose tissue in situ in patients with liver cirrhosis. Eur J Clin Invest. 1993 Dec;23(12):837–844. doi: 10.1111/j.1365-2362.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Reilly J. P., Veneman T., Mandarino L. J. Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E923–E929. doi: 10.1152/ajpendo.1990.258.6.E923. [DOI] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. Effects of microdialysis-perfusion with anisoosmotic media on extracellular amino acids in the rat hippocampus and skeletal muscle. J Neurochem. 1989 Aug;53(2):525–535. doi: 10.1111/j.1471-4159.1989.tb07365.x. [DOI] [PubMed] [Google Scholar]
- Liu D., Moberg E., Kollind M., Lins P. E., Adamson U., Macdonald I. A. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992 Mar;35(3):287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- Londos C., Honnor R. C., Dhillon G. S. cAMP-dependent protein kinase and lipolysis in rat adipocytes. III. Multiple modes of insulin regulation of lipolysis and regulation of insulin responses by adenylate cyclase regulators. J Biol Chem. 1985 Dec 5;260(28):15139–15145. [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987 Aug;253(2 Pt 1):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Madsen J., Malchow-Møller A. Effects of glucose, insulin and nicotinic acid on adipose tissue blood flow in rats. Acta Physiol Scand. 1983 Jun;118(2):175–180. doi: 10.1111/j.1748-1716.1983.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Natali A., Buzzigoli G., Taddei S., Santoro D., Cerri M., Pedrinelli R., Ferrannini E. Effects of insulin on hemodynamics and metabolism in human forearm. Diabetes. 1990 Apr;39(4):490–500. doi: 10.2337/diab.39.4.490. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969 May;112(4):465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A. The regulation of intracellular and extracellular fuel supply during sustained exercise. Ann N Y Acad Sci. 1977;301:81–91. doi: 10.1111/j.1749-6632.1977.tb38188.x. [DOI] [PubMed] [Google Scholar]
- Nieminen M. L., Tuomisto L., Solatunturi E., Eriksson L., Paasonen M. K. Taurine in the osmoregulation of the Brattleboro rat. Life Sci. 1988;42(21):2137–2143. doi: 10.1016/0024-3205(88)90128-2. [DOI] [PubMed] [Google Scholar]
- Nilsson N. O., Strålfors P., Fredrikson G., Belfrage P. Regulation of adipose tissue lipolysis: effects of noradrenaline and insulin on phosphorylation of hormone-sensitive lipase and on lipolysis in intact rat adipocytes. FEBS Lett. 1980 Feb 25;111(1):125–130. doi: 10.1016/0014-5793(80)80776-9. [DOI] [PubMed] [Google Scholar]
- Oscai L. B., Essig D. A., Palmer W. K. Lipase regulation of muscle triglyceride hydrolysis. J Appl Physiol (1985) 1990 Nov;69(5):1571–1577. doi: 10.1152/jappl.1990.69.5.1571. [DOI] [PubMed] [Google Scholar]
- Palmer W. K., Caruso R. A., Oscai L. B. Possible role of lipoprotein lipase in the regulation of endogenous triacylglycerols in the rat heart. Biochem J. 1981 Jul 15;198(1):159–166. doi: 10.1042/bj1980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer W. K., Kane T. A. Hormone-stimulated lipolysis in cardiac myocytes. Biochem J. 1983 Oct 15;216(1):241–243. doi: 10.1042/bj2160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rosdahl H., Ungerstedt U., Jorfeldt L., Henriksson J. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. J Physiol. 1993 Nov;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. J., Sluiter W. J., Schoonen A. J. Glucose concentration in subcutaneous extracellular space. Diabetes Care. 1993 May;16(5):695–700. doi: 10.2337/diacare.16.5.695. [DOI] [PubMed] [Google Scholar]
- Simonsen L., Bülow J., Madsen J., Hermansen F., Astrup A. Local forearm and whole-body respiratory quotient in humans after an oral glucose load: methodological problems. Acta Physiol Scand. 1993 Jan;147(1):69–75. doi: 10.1111/j.1748-1716.1993.tb09473.x. [DOI] [PubMed] [Google Scholar]
- Small C. A., Garton A. J., Yeaman S. J. The presence and role of hormone-sensitive lipase in heart muscle. Biochem J. 1989 Feb 15;258(1):67–72. doi: 10.1042/bj2580067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staten M. A., Totty W. G., Kohrt W. M. Measurement of fat distribution by magnetic resonance imaging. Invest Radiol. 1989 May;24(5):345–349. doi: 10.1097/00004424-198905000-00002. [DOI] [PubMed] [Google Scholar]
- Tagliaferro A. R., Dobbin S., Curi R., Leighton B., Meeker L. D., Newsholme E. A. Effects of diet and exercise on the in vivo rates of the triglyceride-fatty acid cycle in adipose tissue and muscle of the rat. Int J Obes. 1990 Nov;14(11):957–971. [PubMed] [Google Scholar]
- Wolfe R. R., Peters E. J., Klein S., Holland O. B., Rosenblatt J., Gary H., Jr Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am J Physiol. 1987 Feb;252(2 Pt 1):E189–E196. doi: 10.1152/ajpendo.1987.252.2.E189. [DOI] [PubMed] [Google Scholar]
- Zierler K. L. Fatty acids as substrates for heart and skeletal muscle. Circ Res. 1976 Jun;38(6):459–463. doi: 10.1161/01.res.38.6.459. [DOI] [PubMed] [Google Scholar]