Abstract
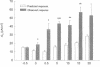
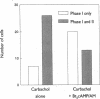
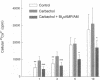
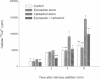
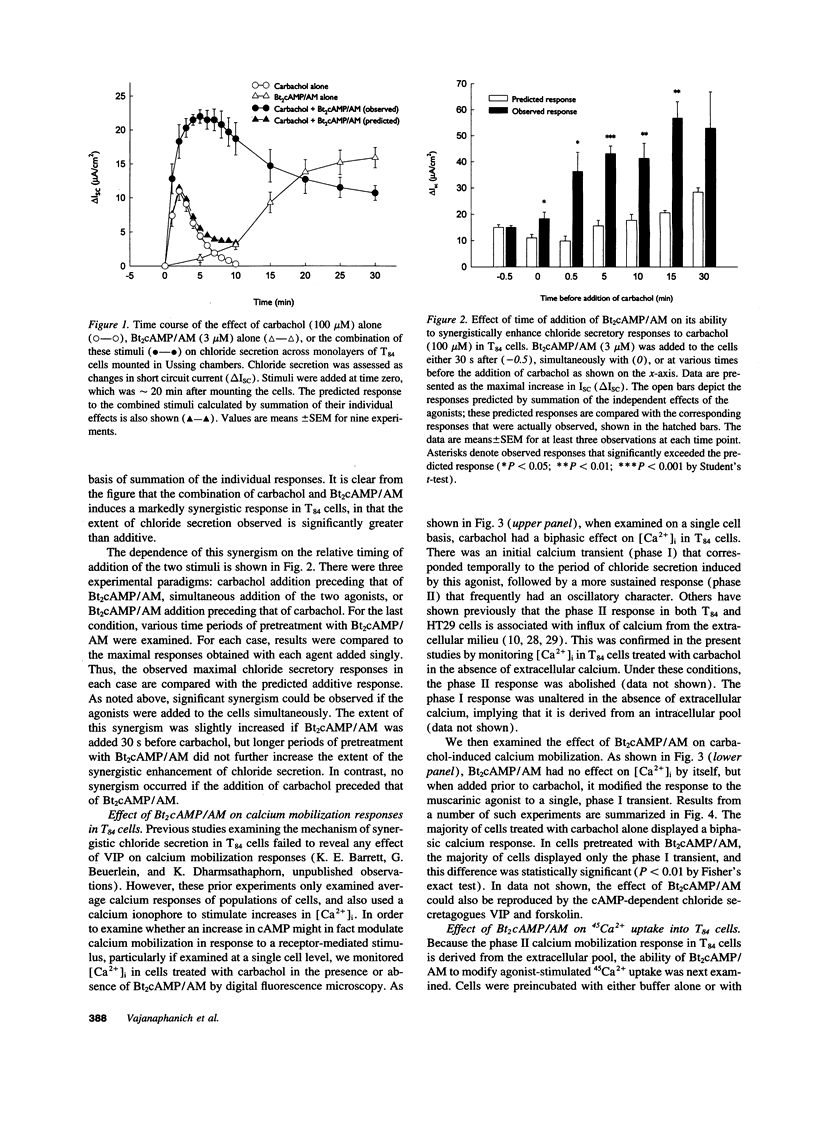
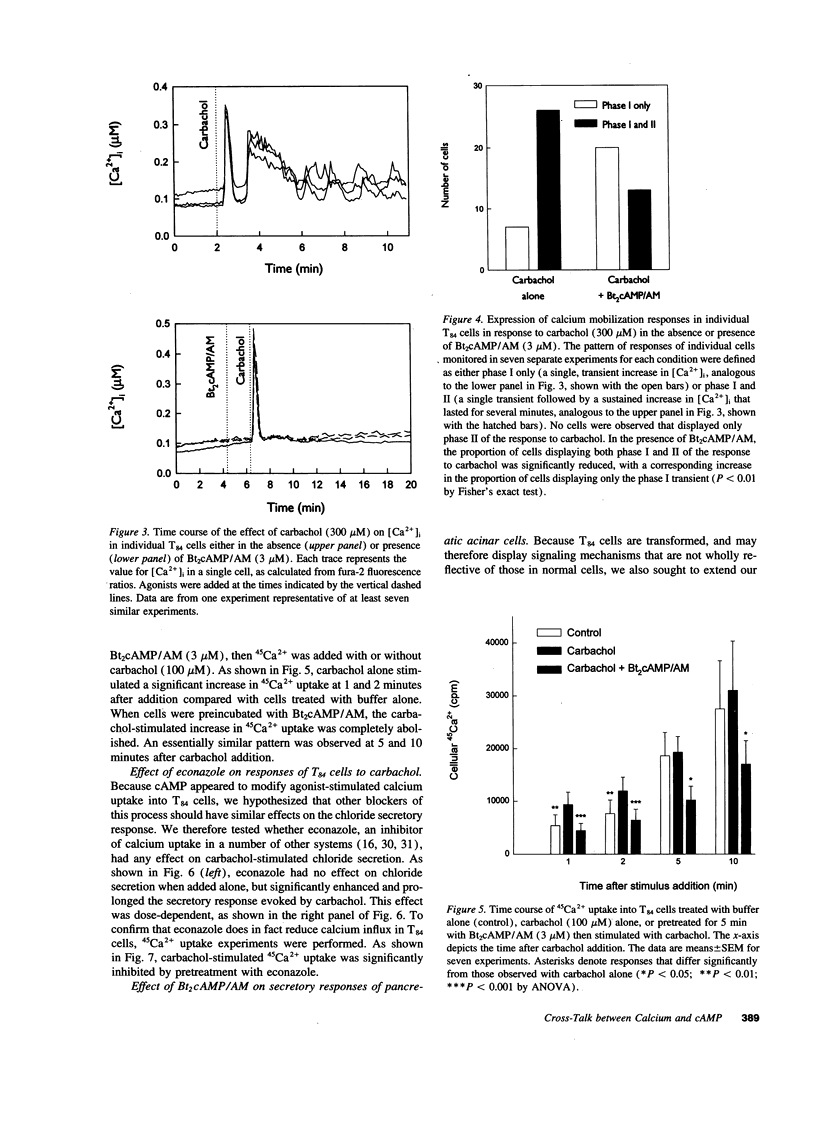
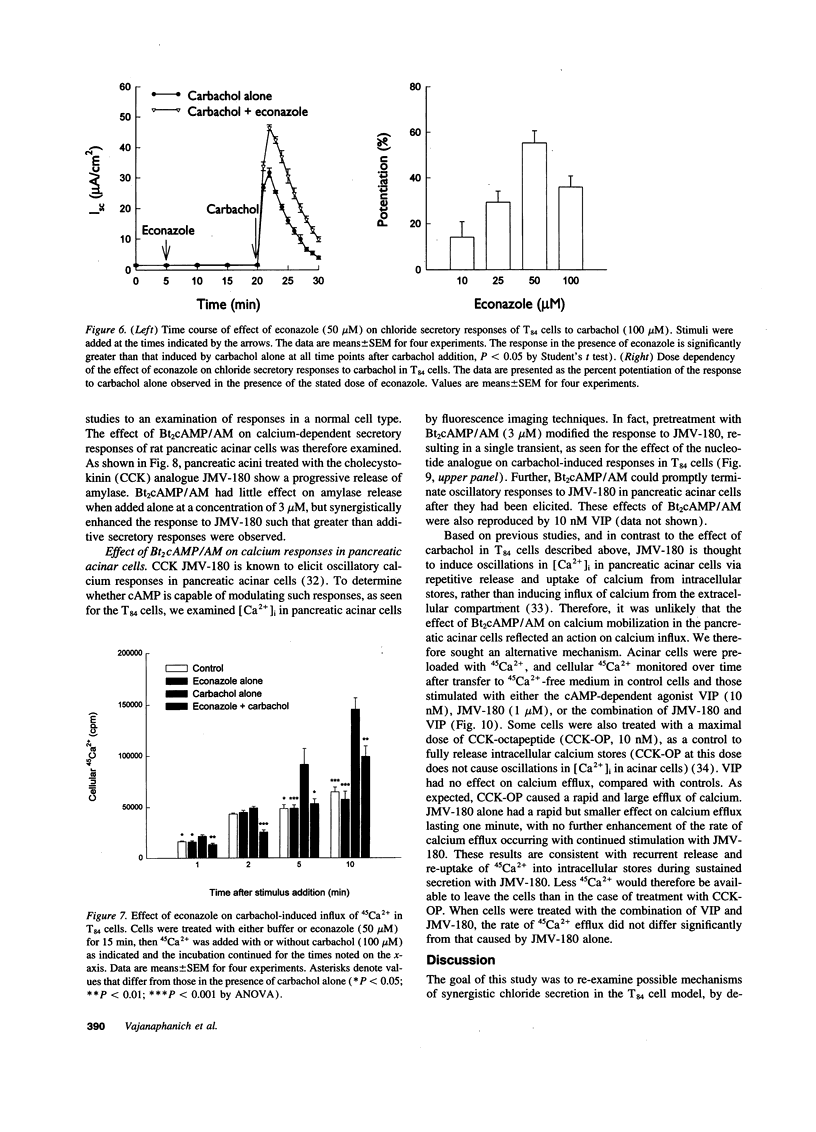
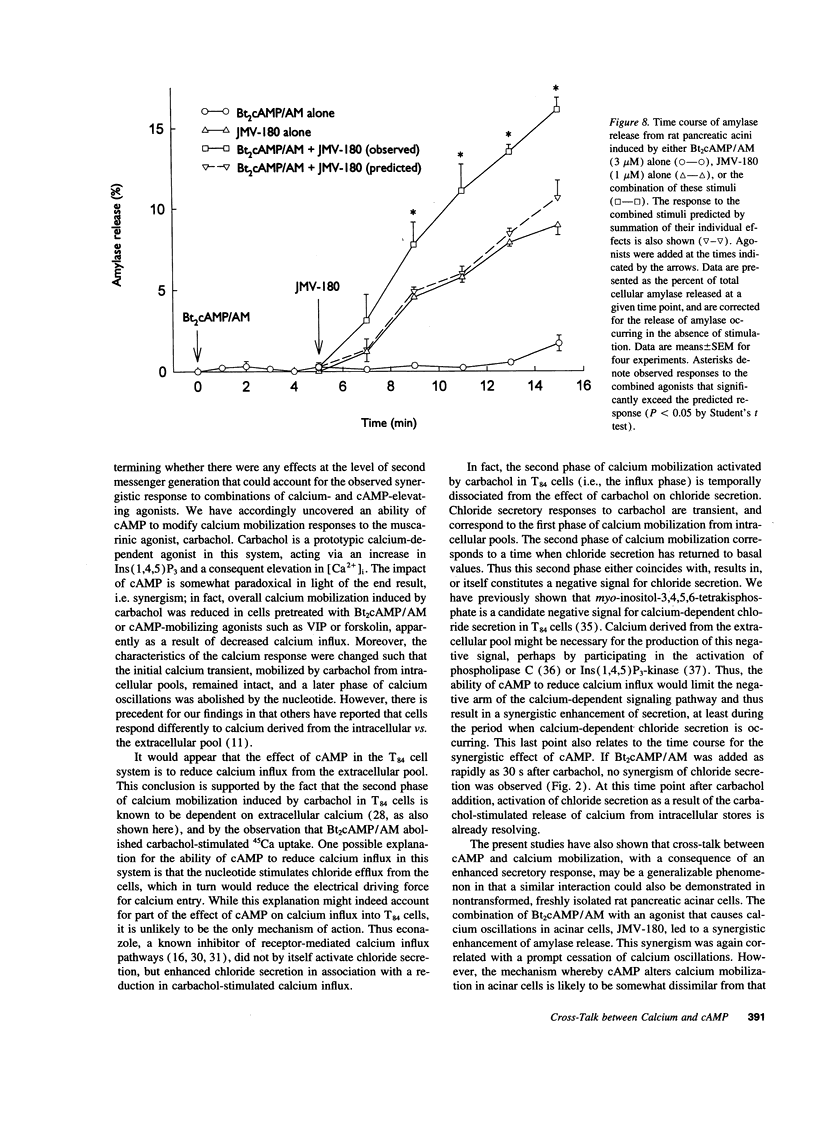
Treatment of various cells with combinations of agents that increase either cAMP or cytosolic calcium can lead to synergistic responses. This study examined interactions, or cross-talk, between these two intracellular messengers and its implication for signaling in two secretory cell types, T84 human colonic epithelial cells and rat pancreatic acinar cells. T84 cell chloride secretion was measured in Ussing chambers. Acinar cell activation was monitored as amylase secretion. Cytosolic calcium was assessed via fura-2 microfluorimetry. A cell-permeant analogue of cAMP synergistically enhanced secretory responses to calcium-mobilizing hormones in both cell types, but paradoxically reduced overall calcium mobilization. The reduction in calcium mobilization could be attributed to an inhibition of calcium influx in T84 cells, although a different mechanism likely operates in acinar cells. The effects of the cAMP analogue were reproduced by other agents that increase cAMP. Furthermore, econazole, an inhibitor of calcium influx, potentiated secretory responses to calcium-dependent stimulation in T84 cells without itself inducing secretion. We conclude that there is cross-talk between calcium and cAMP-dependent signaling pathways at the level of second messenger generation in two secretory cell types. This cross-talk appears to regulate the extent of secretory responses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allert N., Leipziger J., Greger R. cAMP and Ca2+ act co-operatively on the Cl- conductance of HT29 cells. Pflugers Arch. 1992 Jul;421(4):403–405. doi: 10.1007/BF00374233. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Montero M., García-Sancho J. Cytochrome P-450 may link intracellular Ca2+ stores with plasma membrane Ca2+ influx. Biochem J. 1991 Feb 15;274(Pt 1):193–197. doi: 10.1042/bj2740193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. E. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C859–C868. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Bird G. S., Obie J. F., Putney J. W., Jr The mechanism for synergism between phospholipase C- and adenylylcyclase-linked hormones in liver. Cyclic AMP-dependent kinase augments inositol trisphosphate-mediated Ca2+ mobilization without increasing the cellular levels of inositol polyphosphates. J Biol Chem. 1991 Mar 15;266(8):4772–4781. [PubMed] [Google Scholar]
- Cartwright C. A., McRoberts J. A., Mandel K. G., Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor D. C., Ahmed Z., Duffey M. E. Cholinergic stimulation produces oscillations of cytosolic Ca2+ in a secretory epithelial cell line, T84. Am J Physiol. 1991 Mar;260(3 Pt 1):C598–C608. doi: 10.1152/ajpcell.1991.260.3.C598. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Cohn J., Beuerlein G. Multiple calcium-mediated effector mechanisms regulate chloride secretory responses in T84-cells. Am J Physiol. 1989 Jun;256(6 Pt 1):C1224–C1230. doi: 10.1152/ajpcell.1989.256.6.C1224. [DOI] [PubMed] [Google Scholar]
- Erneux C., Moreau C., Vandermeers A., Takazawa K. Interaction of calmodulin with a putative calmodulin-binding domain of inositol 1,4,5-triphosphate 3-kinase. Effects of synthetic peptides and site-directed mutagenesis of Trp165. Eur J Biochem. 1993 Jun 1;214(2):497–501. doi: 10.1111/j.1432-1033.1993.tb17947.x. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Fischer H., Illek B., Negulescu P. A., Clauss W., Machen T. E. Carbachol-activated calcium entry into HT-29 cells is regulated by both membrane potential and cell volume. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1438–1442. doi: 10.1073/pnas.89.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo J. F., Gudas L. J. The effect of dibutyryl cyclic AMP and butyrate on F9 teratocarcinoma cellular retinoic acid-binding protein activity. J Biol Chem. 1987 Apr 5;262(10):4492–4500. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gusovsky F., Lueders J. E., Kohn E. C., Felder C. C. Muscarinic receptor-mediated tyrosine phosphorylation of phospholipase C-gamma. An alternative mechanism for cholinergic-induced phosphoinositide breakdown. J Biol Chem. 1993 Apr 15;268(11):7768–7772. [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Kim K. T., Westhead E. W. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleineke J. W., Janssens P. A. Hormone-induced rise in cytosolic Ca2+ in axolotl hepatocytes: extracellular origin and control by cAMP. Am J Physiol. 1993 Nov;265(5 Pt 1):C1281–C1288. doi: 10.1152/ajpcell.1993.265.5.C1281. [DOI] [PubMed] [Google Scholar]
- Loessberg P. A., Zhao H., Muallem S. Synchronized oscillation of Ca2+ entry and Ca2+ release in agonist-stimulated AR42J cells. J Biol Chem. 1991 Jan 25;266(3):1363–1366. [PubMed] [Google Scholar]
- MacVinish L. J., Pickles R. J., Cuthbert A. W. Cyclic AMP and Ca2+ interactions affecting epithelial chloride secretion in human cultured colonic epithelia. Br J Pharmacol. 1993 Feb;108(2):462–468. doi: 10.1111/j.1476-5381.1993.tb12826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. J., Mayer B., Hymel L. J. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. Am J Physiol. 1993 Mar;264(3 Pt 1):C654–C662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M. S., Fimmel C. J., Pandol S. J. Agonist-sensitive calcium pool in the pancreatic acinar cell. II. Characterization of reloading. Am J Physiol. 1988 Aug;255(2 Pt 1):G229–G235. doi: 10.1152/ajpgi.1988.255.2.G229. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Jensen R. T., Gardner J. D. Mechanism of [Tyr4]bombesin-induced desensitization in dispersed acini from guinea pig pancreas. J Biol Chem. 1982 Oct 25;257(20):12024–12029. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The cycling of calcium as an intracellular messenger. Sci Am. 1989 Oct;261(4):66–73. doi: 10.1038/scientificamerican1089-66. [DOI] [PubMed] [Google Scholar]
- Reinlib L., Mikkelsen R., Zahniser D., Dharmsathaphorn K., Donowitz M. Carbachol-induced cytosolic free Ca2+ increases in T84 colonic cells seen by microfluorimetry. Am J Physiol. 1989 Dec;257(6 Pt 1):G950–G960. doi: 10.1152/ajpgi.1989.257.6.G950. [DOI] [PubMed] [Google Scholar]
- Rephaeli A., Rabizadeh E., Aviram A., Shaklai M., Ruse M., Nudelman A. Derivatives of butyric acid as potential anti-neoplastic agents. Int J Cancer. 1991 Aug 19;49(1):66–72. doi: 10.1002/ijc.2910490113. [DOI] [PubMed] [Google Scholar]
- Schulman H., Hanson P. I., Meyer T. Decoding calcium signals by multifunctional CaM kinase. Cell Calcium. 1992 Jun-Jul;13(6-7):401–411. doi: 10.1016/0143-4160(92)90053-u. [DOI] [PubMed] [Google Scholar]
- Schultz C., Vajanaphanich M., Harootunian A. T., Sammak P. J., Barrett K. E., Tsien R. Y. Acetoxymethyl esters of phosphates, enhancement of the permeability and potency of cAMP. J Biol Chem. 1993 Mar 25;268(9):6316–6322. [PubMed] [Google Scholar]
- Vajanaphanich M., Schultz C., Rudolf M. T., Wasserman M., Enyedi P., Craxton A., Shears S. B., Tsien R. Y., Barrett K. E., Traynor-Kaplan A. Long-term uncoupling of chloride secretion from intracellular calcium levels by Ins(3,4,5,6)P4. Nature. 1994 Oct 20;371(6499):711–714. doi: 10.1038/371711a0. [DOI] [PubMed] [Google Scholar]
- Weymer A., Huott P., Liu W., McRoberts J. A., Dharmsathaphorn K. Chloride secretory mechanism induced by prostaglandin E1 in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1828–1836. doi: 10.1172/JCI112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule D. I., Essington T. E., Williams J. A. Pilocarpine and carbachol exhibit markedly different patterns of Ca2+ signaling in rat pancreatic acinar cells. Am J Physiol. 1993 Apr;264(4 Pt 1):G786–G791. doi: 10.1152/ajpgi.1993.264.4.G786. [DOI] [PubMed] [Google Scholar]
- Yule D. I., Williams J. A. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol but not to JMV-180 in rat pancreatic acinar cells. J Biol Chem. 1992 Jul 15;267(20):13830–13835. [PubMed] [Google Scholar]
- van Tits L. J., Michel M. C., Motulsky H. J., Maisel A. S., Brodde O. E. Cyclic AMP counteracts mitogen-induced inositol phosphate generation and increases in intracellular Ca2+ concentrations in human lymphocytes. Br J Pharmacol. 1991 Jun;103(2):1288–1294. doi: 10.1111/j.1476-5381.1991.tb09782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]