Abstract
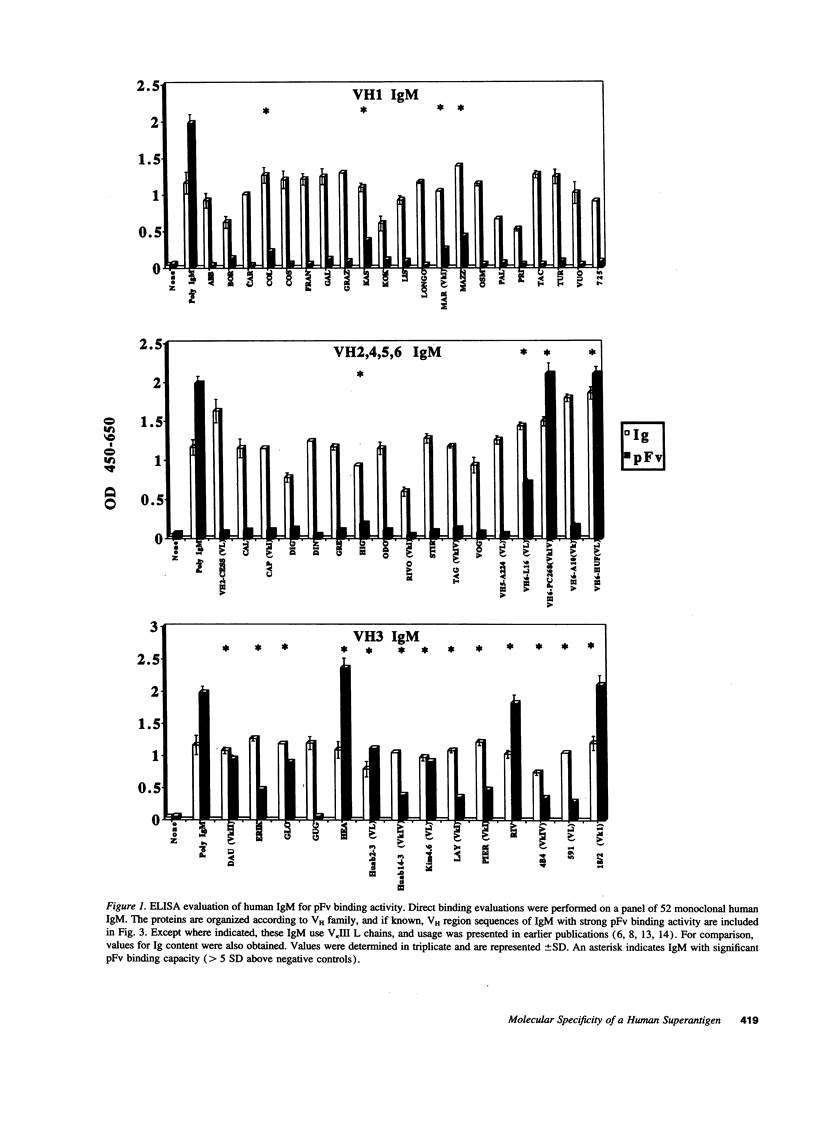
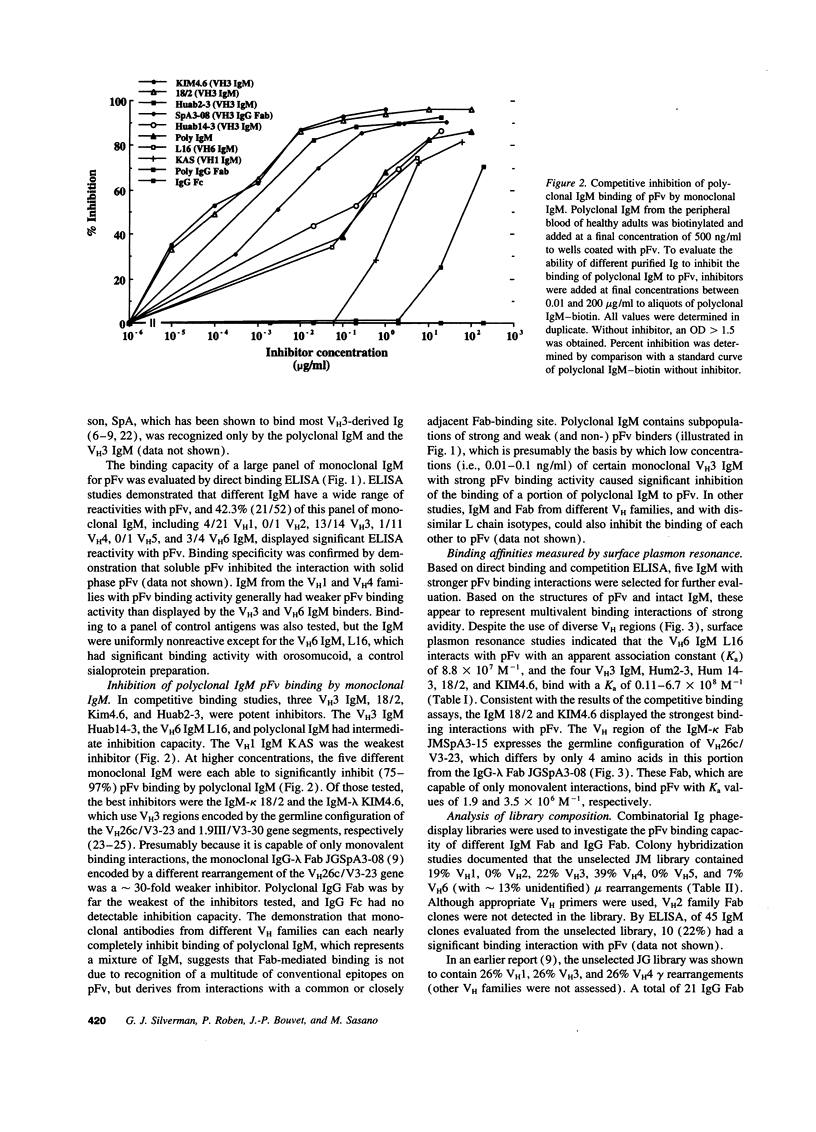
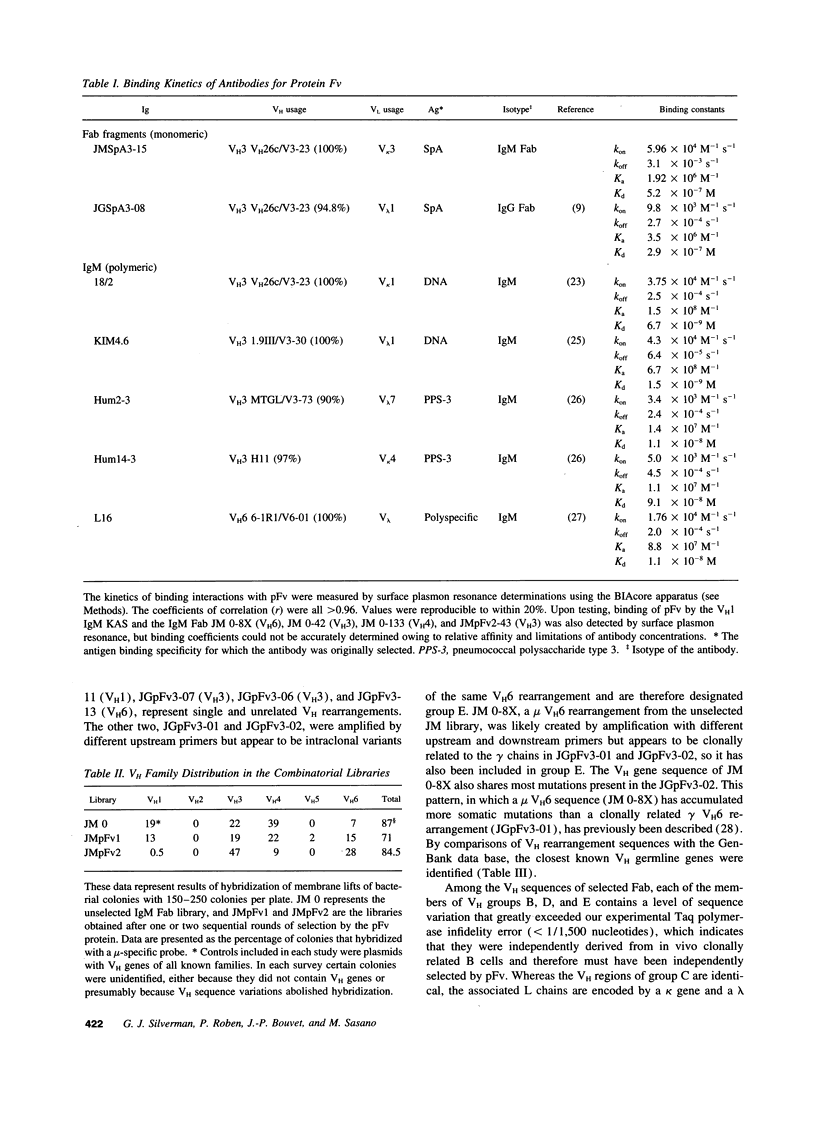
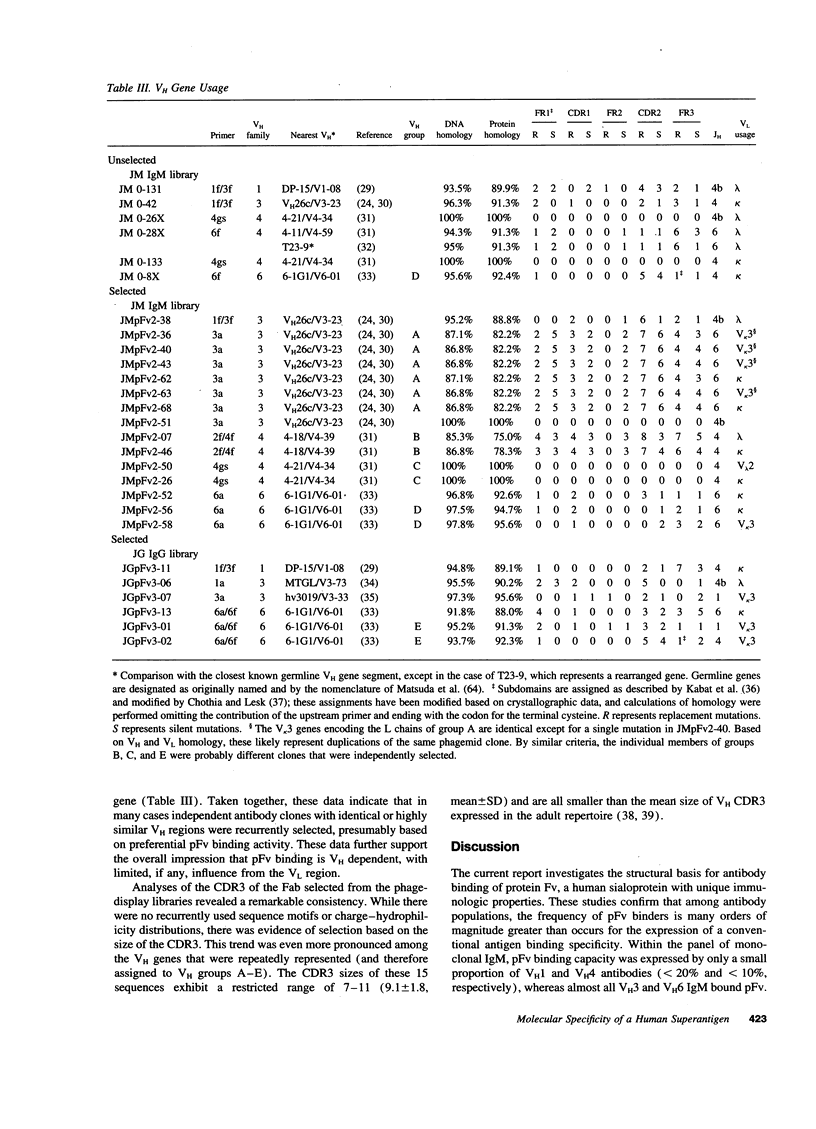
Protein Fv (pFv) is a recently described 175-kD gut-associated sialoprotein with a potent capacity for augmentation of antibody-dependent immune functions. To investigate the molecular basis for Fab-mediated binding of pFv, we evaluated a panel of 52 monoclonal IgM and found that approximately 40% bound pFv. Whereas the majority (> or = 75%) of V H3 and V H6 IgM strongly bound pFv, only a small minority (< 20%) of IgM from other V H families bound pFv, and these antibodies had weaker binding interactions. Inhibition studies suggested that all binding occurred at the same (or overlapping) site(s) on pFv. Surface plasmon resonance studies demonstrated binding affinity constants up to 6.7 x 10(8) M-1 for pFv. Biopanning of IgM and IgG Fab phage-display libraries with pFv preferentially selected for V H3 and V H6 antibodies, but also obtained certain V H4 IgM. V H sequence analyses of 36 pFv-binding antibodies revealed that binding did not correlate with CDR sequence, JH, or L chain usage. However, there was preferential selection of pFv binders with V H CDR3 of small size. These studies demonstrate that a protein which enhances immune defense in the gut has structural and functional properties similar to known superantigens.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berberian L., Goodglick L., Kipps T. J., Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 1993 Sep 17;261(5128):1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. E., Nickerson K. G., Pollock R. R., Barth J. E., Schuurman R. K., Knowles D. M., Chess L., Alt F. W. VH gene usage in humans: biased usage of the VH6 gene in immature B lymphoid cells. Eur J Immunol. 1991 May;21(5):1311–1314. doi: 10.1002/eji.1830210532. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Pires R., Charlemagne J., Pillot J., Iscaki S. Non-immune binding of human protein Fv to immunoglobulins from various mammalian and non-mammalian species. Scand J Immunol. 1991 Oct;34(4):491–496. doi: 10.1111/j.1365-3083.1991.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Pires R., Quan C., Iscaki S., Pillot J. Non-immune VH-binding specificity of human protein Fv. Scand J Immunol. 1991 Apr;33(4):381–386. doi: 10.1111/j.1365-3083.1991.tb01785.x. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Pirès R., Iscaki S., Pillot J. Nonimmune macromolecular complexes of Ig in human gut lumen. Probable enhancement of antibody functions. J Immunol. 1993 Sep 1;151(5):2562–2571. [PubMed] [Google Scholar]
- Bouvet J. P., Pirès R., Lunel-Fabiani F., Crescenzo-Chaigne B., Maillard P., Valla D., Opolon P., Pillot J. Protein F. A novel F(ab)-binding factor, present in normal liver, and largely released in the digestive tract during hepatitis. J Immunol. 1990 Aug 15;145(4):1176–1180. [PubMed] [Google Scholar]
- Buluwela L., Rabbitts T. H. A VH gene is located within 95 Kb of the human immunoglobulin heavy chain constant region genes. Eur J Immunol. 1988 Nov;18(11):1843–1845. doi: 10.1002/eji.1830181130. [DOI] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd, Persson M. A., Koenig S., Chanock R. M., Lerner R. A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns E., Kwong P. C., Misener V., Ip P., Bell D. A., Siminovitch K. A. Analysis of variable region genes encoding a human anti-DNA antibody of normal origin. Implications for the molecular basis of human autoimmune responses. J Immunol. 1989 Jul 15;143(2):685–691. [PubMed] [Google Scholar]
- Chen P. P., Liu M. F., Sinha S., Carson D. A. A 16/6 idiotype-positive anti-DNA antibody is encoded by a conserved VH gene with no somatic mutation. Arthritis Rheum. 1988 Nov;31(11):1429–1431. doi: 10.1002/art.1780311113. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Cuisinier A. M., Gauthier L., Boubli L., Fougereau M., Tonnelle C. Mechanisms that generate human immunoglobulin diversity operate from the 8th week of gestation in fetal liver. Eur J Immunol. 1993 Jan;23(1):110–118. doi: 10.1002/eji.1830230118. [DOI] [PubMed] [Google Scholar]
- Demaison C., Chastagner P., Thèze J., Zouali M. Somatic diversification in the heavy chain variable region genes expressed by human autoantibodies bearing a lupus-associated nephritogenic anti-DNA idiotype. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):514–518. doi: 10.1073/pnas.91.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow R., Reed R. Interactions in the fourth dimension. Biotechnology (N Y) 1992 Apr;10(4):390–393. doi: 10.1038/nbt0492-390. [DOI] [PubMed] [Google Scholar]
- Hillson J. L., Karr N. S., Oppliger I. R., Mannik M., Sasso E. H. The structural basis of germline-encoded VH3 immunoglobulin binding to staphylococcal protein A. J Exp Med. 1993 Jul 1;178(1):331–336. doi: 10.1084/jem.178.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg D., Freitas A. A., Portnoï D., Jacquemart F., Avrameas S., Coutinho A. Antibody repertoires of normal BALB/c mice: B lymphocyte populations defined by state of activation. Immunol Rev. 1986 Oct;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Huang C., Stewart A. K., Schwartz R. S., Stollar B. D. Immunoglobulin heavy chain gene expression in peripheral blood B lymphocytes. J Clin Invest. 1992 Apr;89(4):1331–1343. doi: 10.1172/JCI115719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Stollar B. D. A majority of Ig H chain cDNA of normal human adult blood lymphocytes resembles cDNA for fetal Ig and natural autoantibodies. J Immunol. 1993 Nov 15;151(10):5290–5300. [PubMed] [Google Scholar]
- Husby S., Mestecky J., Moldoveanu Z., Holland S., Elson C. O. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol. 1994 May 1;152(9):4663–4670. [PubMed] [Google Scholar]
- Kang A. S., Barbas C. F., Janda K. D., Benkovic S. J., Lerner R. A. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M., Solvason N. The role of idiotypic interactions and B-cell subsets in development of the B-cell repertoire. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):203–207. doi: 10.1101/sqb.1989.054.01.025. [DOI] [PubMed] [Google Scholar]
- Logtenberg T., Young F. M., Van Es J. H., Gmelig-Meyling F. H., Alt F. W. Autoantibodies encoded by the most Jh-proximal human immunoglobulin heavy chain variable region gene. J Exp Med. 1989 Oct 1;170(4):1347–1355. doi: 10.1084/jem.170.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist M. Surface plasmon resonance for detection and measurement of antibody-antigen affinity and kinetics. Curr Opin Immunol. 1993 Apr;5(2):282–286. doi: 10.1016/0952-7915(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Shin E. K., Nagaoka H., Matsumura R., Haino M., Fukita Y., Taka-ishi S., Imai T., Riley J. H., Anand R. Structure and physical map of 64 variable segments in the 3'0.8-megabase region of the human immunoglobulin heavy-chain locus. Nat Genet. 1993 Jan;3(1):88–94. doi: 10.1038/ng0193-88. [DOI] [PubMed] [Google Scholar]
- Matthyssens G., Rabbitts T. H. Structure and multiplicity of genes for the human immunoglobulin heavy chain variable region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6561–6565. doi: 10.1073/pnas.77.11.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olee T., Yang P. M., Siminovitch K. A., Olsen N. J., Hillson J., Wu J., Kozin F., Carson D. A., Chen P. P. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J Clin Invest. 1991 Jul;88(1):193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Piazza A. J., Ermak T. H. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991 Jan;190(1):10–18. doi: 10.1002/aja.1001900103. [DOI] [PubMed] [Google Scholar]
- Padlan E. A. Anatomy of the antibody molecule. Mol Immunol. 1994 Feb;31(3):169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Parmley S. F., Smith G. P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988 Dec 20;73(2):305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Patella V., Bouvet J. P., Marone G. Protein Fv produced during vital hepatitis is a novel activator of human basophils and mast cells. J Immunol. 1993 Nov 15;151(10):5685–5698. [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Chen B. L., Phizackerley R. P., Saul F. The three-dimensional structure of the fab' fragment of a human myeloma immunoglobulin at 2.0-angstrom resolution. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3440–3444. doi: 10.1073/pnas.71.9.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Bill J., Kubo R. T., Marrack P., Kappler J. W. Analysis of the interaction site for the self superantigen Mls-1a on T cell receptor V beta. J Exp Med. 1991 May 1;173(5):1183–1192. doi: 10.1084/jem.173.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosnek E., Lanzavecchia A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J Exp Med. 1991 Feb 1;173(2):487–489. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffet E., Pirès R., Pillot J., Bouvet J. P. Activation of the classical pathway of complement by non-immune complexes of immunoglobulins with human protein FV (FV fragment-binding protein). Scand J Immunol. 1994 Sep;40(3):359–362. doi: 10.1111/j.1365-3083.1994.tb03474.x. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Sasano M., Burton D. R., Silverman G. J. Molecular selection of human antibodies with an unconventional bacterial B cell antigen. J Immunol. 1993 Nov 15;151(10):5822–5839. [PubMed] [Google Scholar]
- Sasso E. H., Silverman G. J., Mannik M. Human IgA and IgG F(ab')2 that bind to staphylococcal protein A belong to the VHIII subgroup. J Immunol. 1991 Sep 15;147(6):1877–1883. [PubMed] [Google Scholar]
- Sasso E. H., Silverman G. J., Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989 Apr 15;142(8):2778–2783. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Walter M. A., Hofker M. H., Ebens A., Willems van Dijk K., Liao L. C., Cox D. W., Milner E. C., Perlmutter R. M. Physical linkage of a human immunoglobulin heavy chain variable region gene segment to diversity and joining region elements. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8196–8200. doi: 10.1073/pnas.85.21.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte M. E., van Es J. H., Silberstein L. E., Logtenberg T. VH4.21-encoded natural autoantibodies with anti-i specificity mirror those associated with cold hemagglutinin disease. J Immunol. 1993 Dec 1;151(11):6569–6576. [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Heavy-chain directed B-cell maturation: continuous clonal selection beginning at the pre-B cell stage. Immunol Today. 1994 Jan;15(1):27–32. doi: 10.1016/0167-5699(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Silberstein L. E., Jefferies L. C., Goldman J., Friedman D., Moore J. S., Nowell P. C., Roelcke D., Pruzanski W., Roudier J., Silverman G. J. Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood. 1991 Nov 1;78(9):2372–2386. [PubMed] [Google Scholar]
- Silverman G. J. Human antibody responses to bacterial antigens: studies of a model conventional antigen and a proposed model B cell superantigen. Int Rev Immunol. 1992;9(1):57–78. doi: 10.3109/08830189209061783. [DOI] [PubMed] [Google Scholar]
- Silverman G. J., Sasano M., Wormsley S. B. Age-associated changes in binding of human B lymphocytes to a VH3-restricted unconventional bacterial antigen. J Immunol. 1993 Nov 15;151(10):5840–5855. [PubMed] [Google Scholar]
- Silverman G. J., Schrohenloher R. E., Accavitti M. A., Koopman W. J., Carson D. A. Structural characterization of the second major cross-reactive idiotype group of human rheumatoid factors. Association with the VH4 gene family. Arthritis Rheum. 1990 Sep;33(9):1347–1360. doi: 10.1002/art.1780330907. [DOI] [PubMed] [Google Scholar]
- Stevenson F. K., Smith G. J., North J., Hamblin T. J., Glennie M. J. Identification of normal B-cell counterparts of neoplastic cells which secrete cold agglutinins of anti-I and anti-i specificity. Br J Haematol. 1989 May;72(1):9–15. doi: 10.1111/j.1365-2141.1989.tb07643.x. [DOI] [PubMed] [Google Scholar]
- Stewart A. K., Huang C., Stollar B. D., Schwartz R. S. High-frequency representation of a single VH gene in the expressed human B cell repertoire. J Exp Med. 1993 Feb 1;177(2):409–418. doi: 10.1084/jem.177.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Es J. H., Raaphorst F. M., van Tol M. J., Meyling F. H., Logtenberg T. Expression pattern of the most JH-proximal human VH gene segment (VH6) in the B cell and antibody repertoire suggests a role of VH6-encoded IgM antibodies in early ontogeny. J Immunol. 1993 Jan 1;150(1):161–168. [PubMed] [Google Scholar]
- Varade W. S., Insel R. A. Isolation of germinal centerlike events from human spleen RNA. Somatic hypermutation of a clonally related VH6DJH rearrangement expressed with IgM, IgG, and IgA. J Clin Invest. 1993 Apr;91(4):1838–1842. doi: 10.1172/JCI116397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varade W. S., Marin E., Kittelberger A. M., Insel R. A. Use of the most JH-proximal human Ig H chain V region gene, VH6, in the expressed immune repertoire. J Immunol. 1993 Jun 1;150(11):4985–4995. [PubMed] [Google Scholar]
- Wolf J. L., Bye W. A. The membranous epithelial (M) cell and the mucosal immune system. Annu Rev Med. 1984;35:95–112. doi: 10.1146/annurev.me.35.020184.000523. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Johnson G., Kabat E. A. Length distribution of CDRH3 in antibodies. Proteins. 1993 May;16(1):1–7. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Reichard B. A., Shane S., Caton A. J., Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991 Feb 1;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Zelenetz A. D., Chen T. T., Levy R. Clonal expansion in follicular lymphoma occurs subsequent to antigenic selection. J Exp Med. 1992 Oct 1;176(4):1137–1148. doi: 10.1084/jem.176.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es J. H., Meyling F. H., Logtenberg T. High frequency of somatically mutated IgM molecules in the human adult blood B cell repertoire. Eur J Immunol. 1992 Oct;22(10):2761–2764. doi: 10.1002/eji.1830221046. [DOI] [PubMed] [Google Scholar]



