Abstract
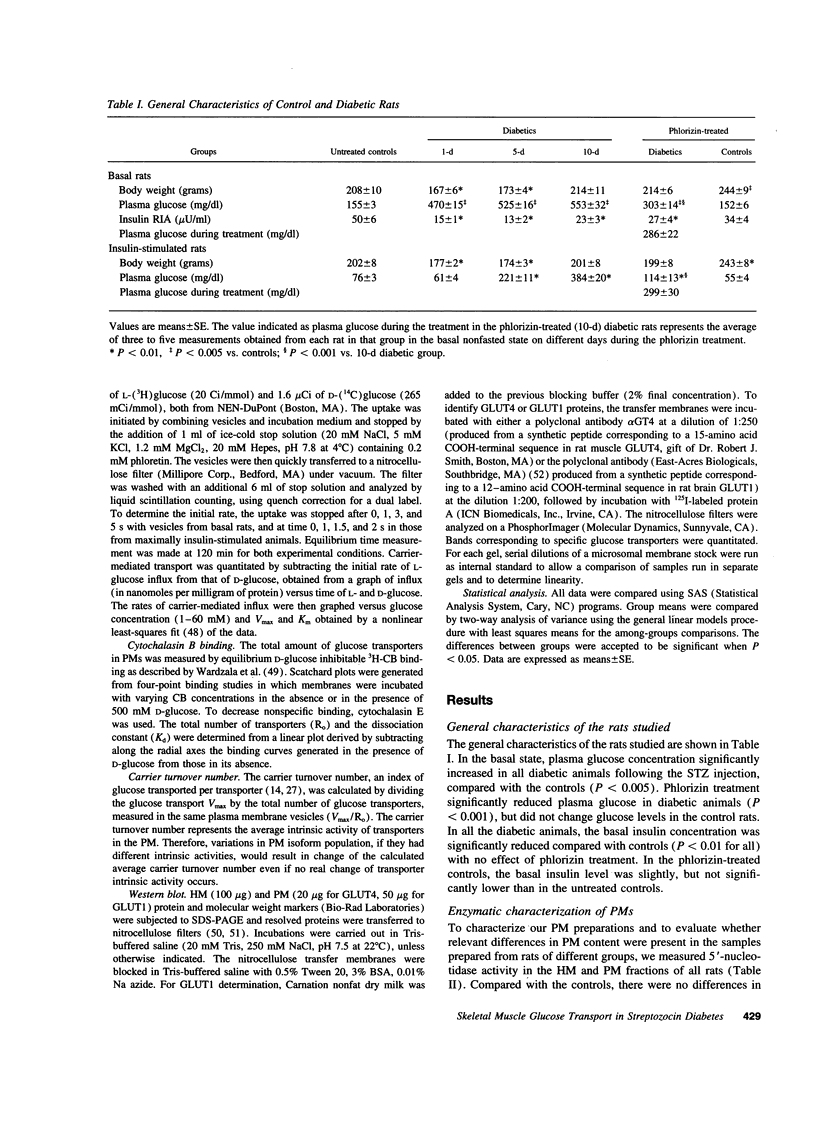
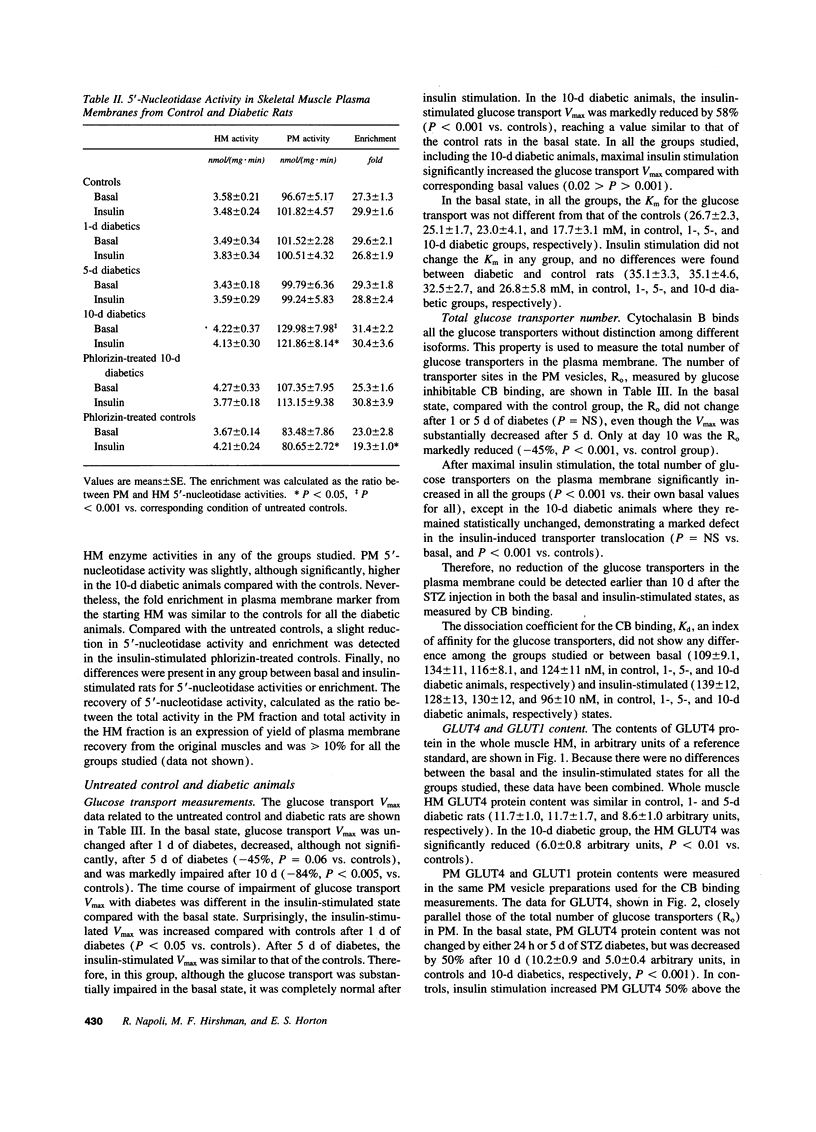
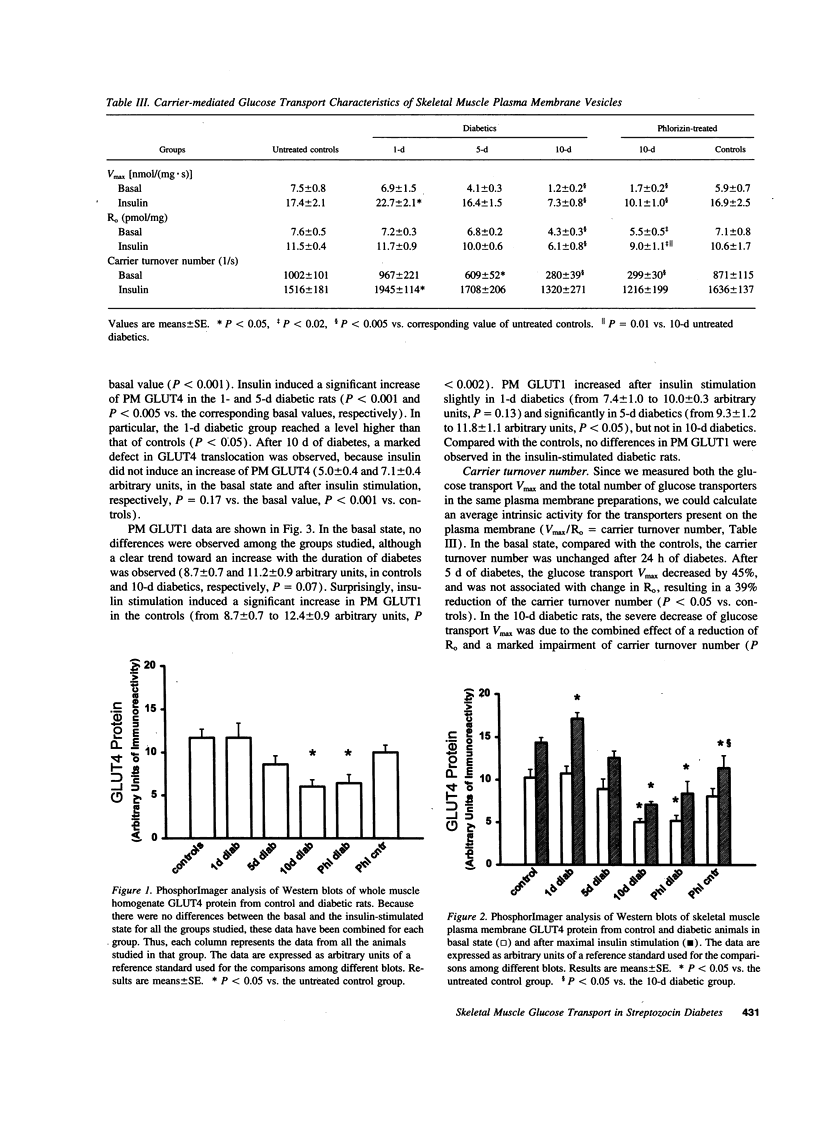
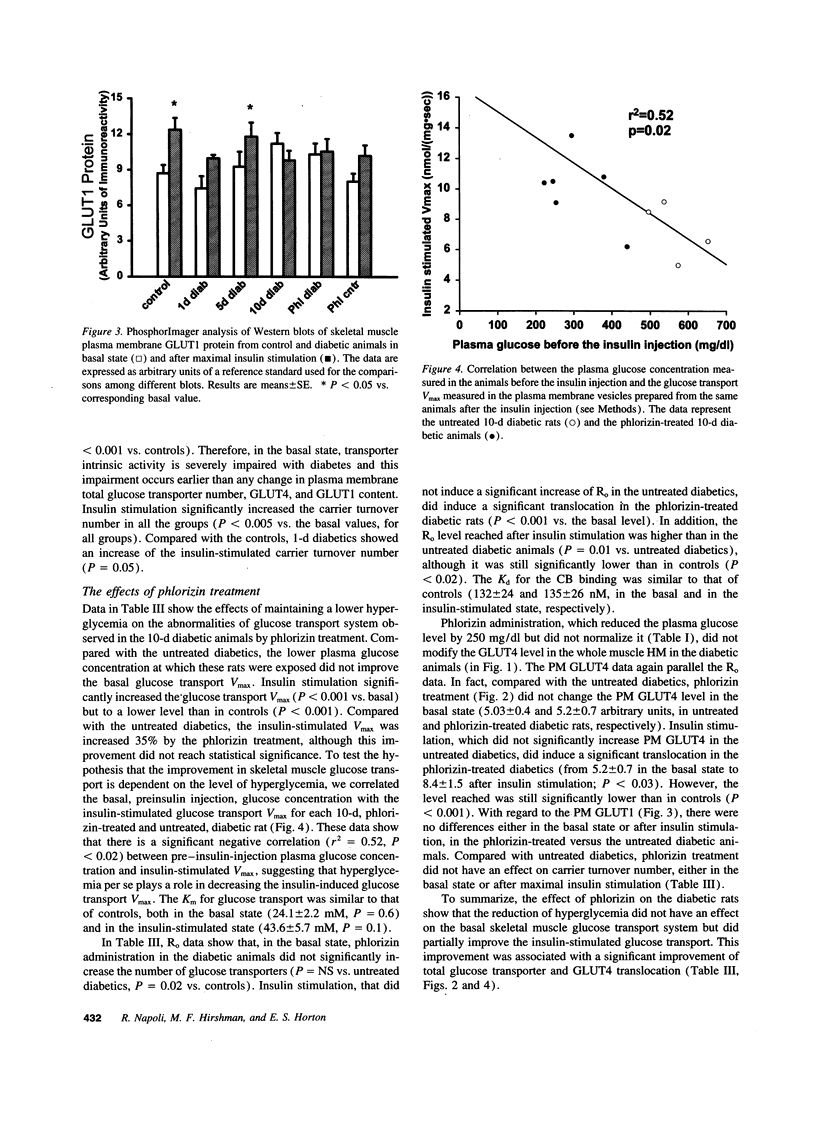
Skeletal muscle glucose transport is altered in diabetes in humans, as well as in rats. To investigate the mechanisms of this abnormality, we measured glucose transport Vmax, the total transporter number, their average intrinsic activity, GLUT4 and GLUT1 contents in skeletal muscle plasma membrane vesicles from basal or insulin-stimulated streptozocin diabetic rats with different duration of diabetes, treated or not with phlorizin. The glucose transport Vmax progressively decreased with the duration of diabetes. In the basal state, this decrease was primarily associated with the reduction of transporter intrinsic activity, which appeared earlier than any change in transporter number or GLUT4 and GLUT1 content. In the insulin-stimulated state, the decrease of transport was mainly associated with severe defects in transporter translocation. Phlorizin treatment partially increased the insulin-stimulated glucose transport by improving the transporter translocation defects. In conclusion, in streptozocin diabetes (a) reduction of intrinsic activity plays a major and early role in the impairment of basal glucose transport; (b) a defect in transporter translocation is the mechanism responsible for the decrease in insulin-stimulated glucose transport; and (c) hyperglycemia per se affects the insulin-stimulated glucose transport by altering the transporter translocation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. H., Lund S., Vestergaard H., Junker S., Kahn B. B., Pedersen O. Expression of the major insulin regulatable glucose transporter (GLUT4) in skeletal muscle of noninsulin-dependent diabetic patients and healthy subjects before and after insulin infusion. J Clin Endocrinol Metab. 1993 Jul;77(1):27–32. doi: 10.1210/jcem.77.1.8325952. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Barnard R. J., Youngren J. F., Kartel D. S., Martin D. A. Effects of streptozotocin-induced diabetes on glucose transport in skeletal muscle. Endocrinology. 1990 Apr;126(4):1921–1926. doi: 10.1210/endo-126-4-1921. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Laakso M., Brechtel G., Edelman S. V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest. 1991 Apr;87(4):1186–1194. doi: 10.1172/JCI115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. Isolation and characterization of cardiac sarcolemma. Biochim Biophys Acta. 1979 Jul 19;555(1):131–146. doi: 10.1016/0005-2736(79)90078-6. [DOI] [PubMed] [Google Scholar]
- Blok J., Gibbs E. M., Lienhard G. E., Slot J. W., Geuze H. J. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988 Jan;106(1):69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O., Portha B. Early appearance of in vivo insulin resistance in adult streptozotocin-injected rats. Diabete Metab. 1989 Nov-Dec;15(6):382–387. [PubMed] [Google Scholar]
- Bonadonna R. C., Del Prato S., Saccomani M. P., Bonora E., Gulli G., Ferrannini E., Bier D., Cobelli C., DeFronzo R. A. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest. 1993 Jul;92(1):486–494. doi: 10.1172/JCI116592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burcelin R., Printz R. L., Kande J., Assan R., Granner D. K., Girard J. Regulation of glucose transporter and hexokinase II expression in tissues of diabetic rats. Am J Physiol. 1993 Sep;265(3 Pt 1):E392–E401. doi: 10.1152/ajpendo.1993.265.3.E392. [DOI] [PubMed] [Google Scholar]
- Calderhead D. M., Kitagawa K., Lienhard G. E., Gould G. W. Translocation of the brain-type glucose transporter largely accounts for insulin stimulation of glucose transport in BC3H-1 myocytes. Biochem J. 1990 Aug 1;269(3):597–601. doi: 10.1042/bj2690597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo B., Napoli R., Di Marino L., Picardi A., Riccardi G., Sacca L. Quantitation of forearm glucose and free fatty acid (FFA) disposal in normal subjects and type II diabetic patients: evidence against an essential role for FFA in the pathogenesis of insulin resistance. J Clin Endocrinol Metab. 1988 Nov;67(5):893–898. doi: 10.1210/jcem-67-5-893. [DOI] [PubMed] [Google Scholar]
- Clancy B. M., Czech M. P. Hexose transport stimulation and membrane redistribution of glucose transporter isoforms in response to cholera toxin, dibutyryl cyclic AMP, and insulin in 3T3-L1 adipocytes. J Biol Chem. 1990 Jul 25;265(21):12434–12443. [PubMed] [Google Scholar]
- Clancy B. M., Harrison S. A., Buxton J. M., Czech M. P. Protein synthesis inhibitors activate glucose transport without increasing plasma membrane glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1991 Jun 5;266(16):10122–10130. [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Hendler R., Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982 Sep;31(9):795–801. doi: 10.2337/diab.31.9.795. [DOI] [PubMed] [Google Scholar]
- Deems R. O., Deacon R. W., Ramlal T., Volchuk A., Klip A., Young D. A. Insulin action on whole body glucose utilization and on muscle glucose transporter translocation in mice. Biochem Biophys Res Commun. 1994 Mar 15;199(2):662–670. doi: 10.1006/bbrc.1994.1279. [DOI] [PubMed] [Google Scholar]
- Dimitrakoudis D., Ramlal T., Rastogi S., Vranic M., Klip A. Glycaemia regulates the glucose transporter number in the plasma membrane of rat skeletal muscle. Biochem J. 1992 Jun 1;284(Pt 2):341–348. doi: 10.1042/bj2840341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Rastogi S., Bilan P. J., Cartee G. D., Vranic M., Holloszy J. O., Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990 Aug 15;265(23):13427–13430. [PubMed] [Google Scholar]
- Eboué-Bonis D., Clauser H. The combined action of insulin and phlorizin on transport and metabolism of sugars and nucleotide turnover in the isolated rat diaphragm. Biochimie. 1977;59(5-6):527–533. doi: 10.1016/s0300-9084(77)80058-8. [DOI] [PubMed] [Google Scholar]
- Fink R. I., Wallace P., Brechtel G., Olefsky J. M. Evidence that glucose transport is rate-limiting for in vivo glucose uptake. Metabolism. 1992 Aug;41(8):897–902. doi: 10.1016/0026-0495(92)90174-9. [DOI] [PubMed] [Google Scholar]
- Goodyear L. J., Hirshman M. F., Valyou P. M., Horton E. S. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes. 1992 Sep;41(9):1091–1099. doi: 10.2337/diab.41.9.1091. [DOI] [PubMed] [Google Scholar]
- Grimditch G. K., Barnard R. J., Kaplan S. A., Sternlicht E. Insulin binding and glucose transport in rat skeletal muscle sarcolemmal vesicles. Am J Physiol. 1985 Oct;249(4 Pt 1):E398–E408. doi: 10.1152/ajpendo.1985.249.4.E398. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M., Lavau M., Horne J. S., Wardzala L. J. Proposed mechanism for increased insulin-mediated glucose transport in adipose cells from young, obese Zucker rats. Large intracellular pool of glucose transporters. J Biol Chem. 1985 Feb 25;260(4):2197–2201. [PubMed] [Google Scholar]
- Handberg A., Vaag A., Damsbo P., Beck-Nielsen H., Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990 Oct;33(10):625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Clancy B. M., Czech M. P. Evidence that erythroid-type glucose transporter intrinsic activity is modulated by cadmium treatment of mouse 3T3-L1 cells. J Biol Chem. 1991 Oct 15;266(29):19438–19449. [PubMed] [Google Scholar]
- Harrison S. A., Buxton J. M., Czech M. P. Suppressed intrinsic catalytic activity of GLUT1 glucose transporters in insulin-sensitive 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7839–7843. doi: 10.1073/pnas.88.17.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Hirshman M. F., Goodyear L. J., Wardzala L. J., Horton E. D., Horton E. S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990 Jan 15;265(2):987–991. [PubMed] [Google Scholar]
- Holman G. D., Cushman S. W. Subcellular localization and trafficking of the GLUT4 glucose transporter isoform in insulin-responsive cells. Bioessays. 1994 Oct;16(10):753–759. doi: 10.1002/bies.950161010. [DOI] [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Mechanism for markedly hyperresponsive insulin-stimulated glucose transport activity in adipose cells from insulin-treated streptozotocin diabetic rats. Evidence for increased glucose transporter intrinsic activity. J Biol Chem. 1987 Apr 15;262(11):5118–5124. [PubMed] [Google Scholar]
- Kahn B. B. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992 May;89(5):1367–1374. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Rosen A. S., Bak J. F., Andersen P. H., Damsbo P., Lund S., Pedersen O. Expression of GLUT1 and GLUT4 glucose transporters in skeletal muscle of humans with insulin-dependent diabetes mellitus: regulatory effects of metabolic factors. J Clin Endocrinol Metab. 1992 May;74(5):1101–1109. doi: 10.1210/jcem.74.5.1569156. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Rossetti L., Lodish H. F., Charron M. J. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest. 1991 Jun;87(6):2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Simpson I. A., Cushman S. W. Divergent mechanisms for the insulin resistant and hyperresponsive glucose transport in adipose cells from fasted and refed rats. Alterations in both glucose transporter number and intrinsic activity. J Clin Invest. 1988 Aug;82(2):691–699. doi: 10.1172/JCI113649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Nyomba B. L., Bogardus C. No accumulation of glucose in human skeletal muscle during euglycemic hyperinsulinemia. Am J Physiol. 1988 Dec;255(6 Pt 1):E942–E945. doi: 10.1152/ajpendo.1988.255.6.E942. [DOI] [PubMed] [Google Scholar]
- King P. A., Horton E. D., Hirshman M. F., Horton E. S. Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J Clin Invest. 1992 Oct;90(4):1568–1575. doi: 10.1172/JCI116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A., Marette A., Dimitrakoudis D., Ramlal T., Giacca A., Shi Z. Q., Vranic M. Effect of diabetes on glucoregulation. From glucose transporters to glucose metabolism in vivo. Diabetes Care. 1992 Nov;15(11):1747–1766. doi: 10.2337/diacare.15.11.1747. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P. J., Cartee G. D., Gulve E. A., Holloszy J. O. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys Res Commun. 1990 Oct 30;172(2):728–736. doi: 10.1016/0006-291x(90)90735-6. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Kohn P. G., Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. VI. The effect of insulin, ouabain, and metabolic inhibitors on the transport of 3-O-methylglucose and glucose in rat soleus muscles. Biochim Biophys Acta. 1971 Feb 2;225(2):277–290. doi: 10.1016/0005-2736(71)90221-5. [DOI] [PubMed] [Google Scholar]
- Koopmans S. J., Maassen J. A., Radder J. K., Frölich M., Krans H. M. In vivo insulin responsiveness for glucose uptake and production at eu- and hyperglycemic levels in normal and diabetic rats. Biochim Biophys Acta. 1992 Jan 23;1115(3):230–238. doi: 10.1016/0304-4165(92)90059-4. [DOI] [PubMed] [Google Scholar]
- Kubo K., Foley J. E. Rate-limiting steps for insulin-mediated glucose uptake into perfused rat hindlimb. Am J Physiol. 1986 Jan;250(1 Pt 1):E100–E102. doi: 10.1152/ajpendo.1986.250.1.E100. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisato G., Cusin I., Tiengo A., Del Prato S., Jeanrenaud B. The contribution of hyperglycaemia and hypoinsulinaemia to the insulin resistance of streptozotocin-diabetic rats. Diabetologia. 1992 Apr;35(4):310–315. doi: 10.1007/BF00401197. [DOI] [PubMed] [Google Scholar]
- Lund S., Flyvbjerg A., Holman G. D., Larsen F. S., Pedersen O., Schmitz O. Comparative effects of IGF-I and insulin on the glucose transporter system in rat muscle. Am J Physiol. 1994 Sep;267(3 Pt 1):E461–E466. doi: 10.1152/ajpendo.1994.267.3.E461. [DOI] [PubMed] [Google Scholar]
- Lund S., Holman G. D., Schmitz O., Pedersen O. Glut 4 content in the plasma membrane of rat skeletal muscle: comparative studies of the subcellular fractionation method and the exofacial photolabelling technique using ATB-BMPA. FEBS Lett. 1993 Sep 20;330(3):312–318. doi: 10.1016/0014-5793(93)80895-2. [DOI] [PubMed] [Google Scholar]
- Lund S., Vestergaard H., Andersen P. H., Schmitz O., Gøtzsche L. B., Pedersen O. GLUT-4 content in plasma membrane of muscle from patients with non-insulin-dependent diabetes mellitus. Am J Physiol. 1993 Dec;265(6 Pt 1):E889–E897. doi: 10.1152/ajpendo.1993.265.6.E889. [DOI] [PubMed] [Google Scholar]
- Marette A., Richardson J. M., Ramlal T., Balon T. W., Vranic M., Pessin J. E., Klip A. Abundance, localization, and insulin-induced translocation of glucose transporters in red and white muscle. Am J Physiol. 1992 Aug;263(2 Pt 1):C443–C452. doi: 10.1152/ajpcell.1992.263.2.C443. [DOI] [PubMed] [Google Scholar]
- Mueckler M. The molecular biology of glucose transport: relevance to insulin resistance and non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1993 Apr-Jun;7(2):130–141. doi: 10.1016/1056-8727(93)90038-z. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Ramlal T., Rastogi S., Vranic M., Klip A. Decrease in glucose transporter number in skeletal muscle of mildly diabetic (streptozotocin-treated) rats. Endocrinology. 1989 Aug;125(2):890–897. doi: 10.1210/endo-125-2-890. [DOI] [PubMed] [Google Scholar]
- Richardson J. M., Balon T. W., Treadway J. L., Pessin J. E. Differential regulation of glucose transporter activity and expression in red and white skeletal muscle. J Biol Chem. 1991 Jul 5;266(19):12690–12694. [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., James D. E., Lienhard G. E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., James D. E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991 Apr;113(1):123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Moxley R., Geuze H. J., James D. E. No evidence for expression of the insulin-regulatable glucose transporter in endothelial cells. Nature. 1990 Jul 26;346(6282):369–371. doi: 10.1038/346369a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleminsky J., Burr I. M., Stauffacher W. Comparative study of early metabolic events resulting from the administration of the two diabetogenic agents alloxan and streptozotocin. Eur J Clin Invest. 1970 Aug;1(2):104–108. doi: 10.1111/j.1365-2362.1970.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Vogt B., Mühlbacher C., Carrascosa J., Obermaier-Kusser B., Seffer E., Mushack J., Pongratz D., Häring H. U. Subcellular distribution of GLUT 4 in the skeletal muscle of lean type 2 (non-insulin-dependent) diabetic patients in the basal state. Diabetologia. 1992 May;35(5):456–463. doi: 10.1007/BF02342444. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg-Henriksson H. Insulin treatment normalizes decreased glucose transport capacity in streptozotocin-diabetic rat muscle. Acta Physiol Scand. 1986 Dec;128(4):647–649. doi: 10.1111/j.1748-1716.1986.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Cushman S. W. Insulin stimulation of glucose transport activity in rat skeletal muscle: increase in cell surface GLUT4 as assessed by photolabelling. Biochem J. 1994 May 1;299(Pt 3):755–759. doi: 10.1042/bj2990755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Sahlin K., Ren J. M., Koivisto V. A. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990 Feb;39(2):157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Vuorinen-Markkola H., Koranyi L., Bourey R., Tordjman K., Mueckler M., Permutt A. M., Koivisto V. A. Defect in insulin action on expression of the muscle/adipose tissue glucose transporter gene in skeletal muscle of type 1 diabetic patients. J Clin Endocrinol Metab. 1992 Sep;75(3):795–799. doi: 10.1210/jcem.75.3.1517369. [DOI] [PubMed] [Google Scholar]
- Youn J. H., Kim J. K., Buchanan T. A. Time courses of changes in hepatic and skeletal muscle insulin action and GLUT4 protein in skeletal muscle after STZ injection. Diabetes. 1994 Apr;43(4):564–571. doi: 10.2337/diab.43.4.564. [DOI] [PubMed] [Google Scholar]
- Ziel F. H., Venkatesan N., Davidson M. B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988 Jul;37(7):885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]
- Zierath J. R., Galuska D., Nolte L. A., Thörne A., Kristensen J. S., Wallberg-Henriksson H. Effects of glycaemia on glucose transport in isolated skeletal muscle from patients with NIDDM: in vitro reversal of muscular insulin resistance. Diabetologia. 1994 Mar;37(3):270–277. doi: 10.1007/BF00398054. [DOI] [PubMed] [Google Scholar]


