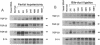Abstract
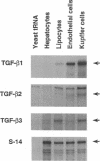
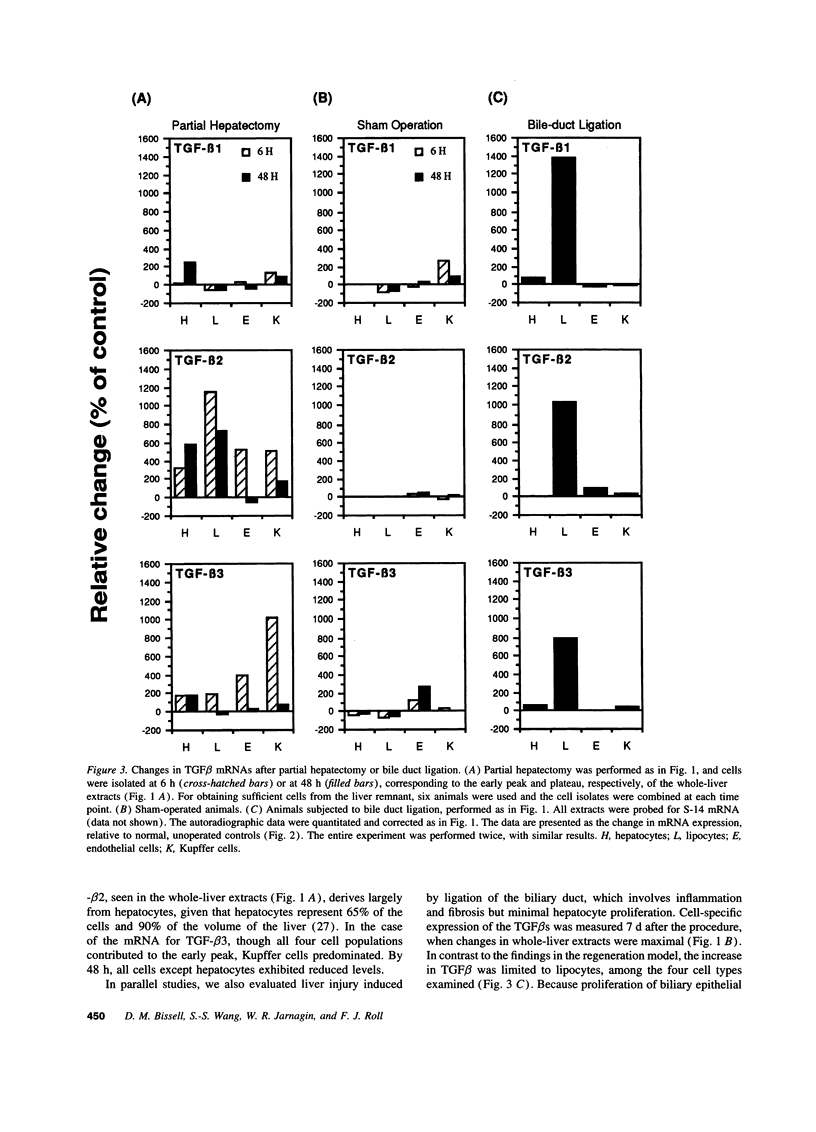
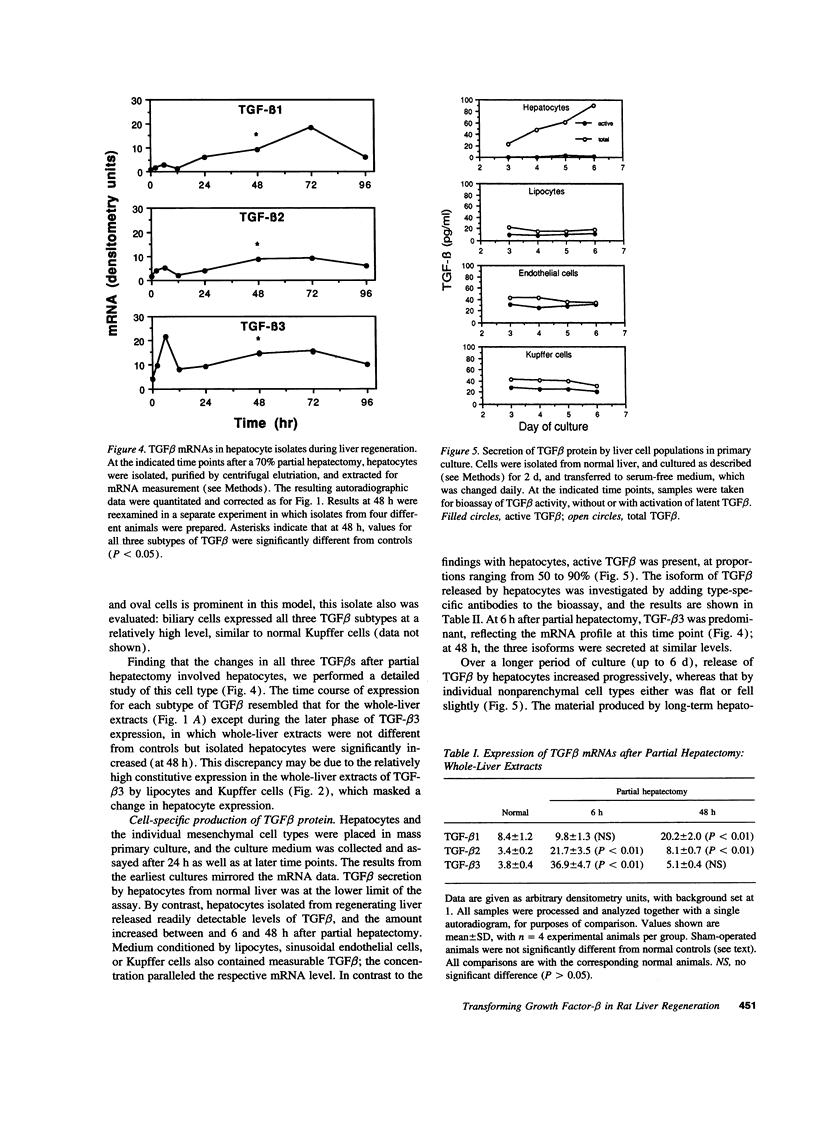
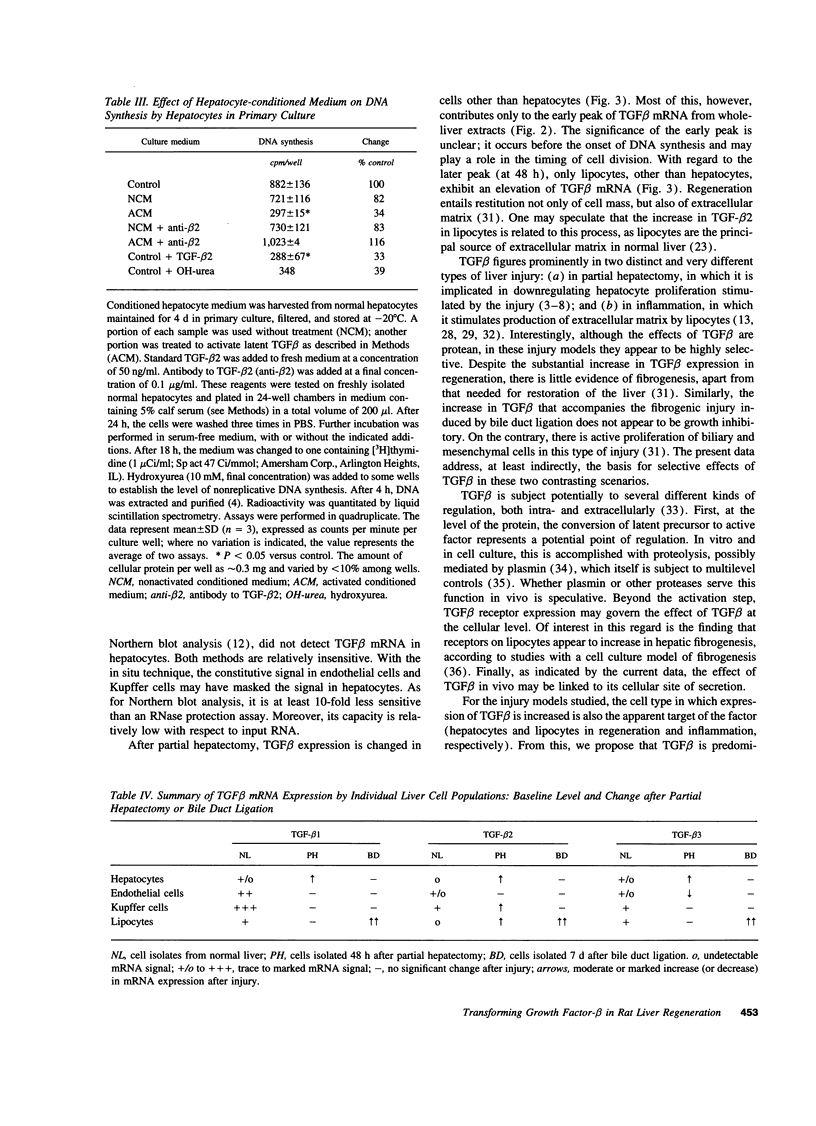
Expression of the group of cytokines known as transforming growth factor-beta (TGF-beta 1, -beta 2 and -beta 3) is increased during liver regeneration induced by a 70% partial hepatectomy. The origin of these changes was examined in purified isolates of hepatocytes, sinusoidal endothelial cells, Kupffer cells (liver macrophages), and lipocytes (Ito or stellate cells) from normal and regenerating liver. In normal liver, TGF-beta 1 and -beta 2 levels were relatively high in sinusoidal endothelial cells and Kupffer cells. After partial hepatectomy, an early peak of TGF-beta 2 and -beta 3 was present in all four cell types, followed by a sustained increase in mRNA for TGF-beta 1, -beta 2, and -beta 3 primarily in the hepatocyte population. The specificity of these changes was established by examining a mechanistically different injury model, fibrosis induced by ligation of the biliary duct. In this model, TGF beta mRNA was increased only in lipocytes and the increase was progressive over a 7-d period of observation. Secretion of TGF beta protein was examined in cell isolates placed in short-term primary culture and generally reflected the corresponding mRNA level. The TGF beta released by hepatocytes was entirely in the latent form, whereas the individual nonparenchymal cell isolates released 50-90% active TGF beta. Hepatocyte-conditioned culture medium, after treatment to activate latent TGF beta, inhibited hepatocellular DNA synthesis as did the authentic factor. The data indicate that after injury TGF beta increases selectively in the cells that are the target of the factor, i.e., in hepatocytes after partial hepatectomy and in lipocytes in inflammation and fibrosis. We conclude that the effects of TGF beta in liver regeneration and fibrogenesis are predominantly, if not exclusively, autocrine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowski R. S., Cowan M. J., McDonald J. A., Crystal R. G. Degradation of newly synthesized collagen. J Biol Chem. 1978 Jun 25;253(12):4356–4363. [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Caron J. M., Babiss L. E., Friedman J. M. Transcriptional regulation of the albumin gene in cultured rat hepatocytes. Role of basement-membrane matrix. Mol Biol Med. 1990 Apr;7(2):187–197. [PubMed] [Google Scholar]
- Bissell D. M., Guzelian P. S. Phenotypic stability of adult rat hepatocytes in primary monolayer culture. Ann N Y Acad Sci. 1980;349:85–98. doi: 10.1111/j.1749-6632.1980.tb29518.x. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Liver sinusoidal cells. Identification of a subpopulation for erythrocyte catabolism. J Cell Biol. 1972 Jul;54(1):107–119. doi: 10.1083/jcb.54.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr B. I., Hayashi I., Branum E. L., Moses H. L. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986 May;46(5):2330–2334. [PubMed] [Google Scholar]
- Carr B. I., Huang T. H., Itakura K., Noël M., Marceau N. TGF beta gene transcription in normal and neoplastic liver growth. J Cell Biochem. 1989 Apr;39(4):477–487. doi: 10.1002/jcb.240390413. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Kost L. J., Lyons R. M., Moses H. L., LaRusso N. F. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987 Sep;80(3):750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Dickson M. C., Slager H. G., Duffie E., Mummery C. L., Akhurst R. J. RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development. 1993 Feb;117(2):625–639. doi: 10.1242/dev.117.2.625. [DOI] [PubMed] [Google Scholar]
- Fausto N., Mead J. E., Gruppuso P. A., Braun L. TGF-beta in liver development, regeneration, and carcinogenesis. Ann N Y Acad Sci. 1990;593:231–242. doi: 10.1111/j.1749-6632.1990.tb16115.x. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Abe M., Mignatti P., Rifkin D. B. Basic fibroblast growth factor-induced activation of latent transforming growth factor beta in endothelial cells: regulation of plasminogen activator activity. J Cell Biol. 1992 Aug;118(4):901–909. doi: 10.1083/jcb.118.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987 Feb 15;161(1):207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Yamasaki G., Wong L. Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem. 1994 Apr 8;269(14):10551–10558. [PubMed] [Google Scholar]
- Glick A. B., McCune B. K., Abdulkarem N., Flanders K. C., Lumadue J. A., Smith J. M., Sporn M. B. Complex regulation of TGF beta expression by retinoic acid in the vitamin A-deficient rat. Development. 1991 Apr;111(4):1081–1086. doi: 10.1242/dev.111.4.1081. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Mead J. E., Danielpour D., Wu J., Roberts A. B., Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991 Jul;2(7):535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin W. R., Rockey D. C., Koteliansky V. E., Wang S. S., Bissell D. M. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994 Dec;127(6 Pt 2):2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Maher J. J., Bissell D. M., Friedman S. L., Roll F. J. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Pham N. T., Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989 Apr;9(2):71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- McMahon J. B., Richards W. L., del Campo A. A., Song M. K., Thorgeirsson S. S. Differential effects of transforming growth factor-beta on proliferation of normal and malignant rat liver epithelial cells in culture. Cancer Res. 1986 Sep;46(9):4665–4671. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Stein H., Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991 Dec;139(6):1221–1229. [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K., Pimstone N. R., Burns M. Dual mechanism of inhibition of rat liver uroporphyrinogen decarboxylase activity by ferrous iron: its potential role in the genesis of porphyria cutanea tarda. Gastroenterology. 1984 Dec;87(6):1248–1254. [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Hirai R., Yamaoka K., Kaji K., Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H., Evarts R. P., Hsia C. C., Thorgeirsson S. S. Transforming growth factor-beta 1 and type I procollagen transcripts during regeneration and early fibrosis of rat liver. Lab Invest. 1990 Aug;63(2):171–180. [PubMed] [Google Scholar]
- Nakatsukasa H., Nagy P., Evarts R. P., Hsia C. C., Marsden E., Thorgeirsson S. S. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990 Jun;85(6):1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar V. M., Vukicevic S., Reddi A. H. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991 Feb;143(2):303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Hogan B. L., Miller D. A., Moses H. L. Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev Biol. 1990 Oct;141(2):456–460. doi: 10.1016/0012-1606(90)90401-4. [DOI] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder H., Armbrust T., Meyer zum Büschenfelde K. H., Ramadori G. Contribution of sinusoidal endothelial liver cells to liver fibrosis: expression of transforming growth factor-beta 1 receptors and modulation of plasmin-generating enzymes by transforming growth factor-beta 1. Hepatology. 1993 Oct;18(4):937–944. doi: 10.1002/hep.1840180427. [DOI] [PubMed] [Google Scholar]
- Robinson S. D., Silberstein G. B., Roberts A. B., Flanders K. C., Daniel C. W. Regulated expression and growth inhibitory effects of transforming growth factor-beta isoforms in mouse mammary gland development. Development. 1991 Nov;113(3):867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Schwall R. H., Robbins K., Jardieu P., Chang L., Lai C., Terrell T. G. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993 Aug;18(2):347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. TGF-beta: problems and prospects. Cell Regul. 1990 Nov;1(12):875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain A. J., Hill D. J., Milner R. D. Divergent action of transforming growth factor beta on DNA synthesis in human foetal liver cells. Cell Biol Int Rep. 1986 Nov;10(11):855–860. doi: 10.1016/0309-1651(86)90102-5. [DOI] [PubMed] [Google Scholar]
- Strain A. J. Transforming growth factor-beta: the elusive hepatic chalone? Hepatology. 1992 Jul;16(1):269–270. doi: 10.1002/hep.1840160139. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Bonney R. J., Becker J. E., Potter V. R. Pyruvate kinase, hexokinase, and aldolase isozymes in rat liver cells in culture. In Vitro. 1972 Sep-Oct;8(2):107–114. doi: 10.1007/BF02615969. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg G. K., Semple E., Quinn B. A., Hayes M. A. Inhibition of proliferation of normal, preneoplastic, and neoplastic rat hepatocytes by transforming growth factor-beta. Cancer Res. 1987 Dec 15;47(24 Pt 1):6595–6599. [PubMed] [Google Scholar]
- Yamamoto H., Murawaki Y., Kawasaki H. Hepatic collagen synthesis and degradation during liver regeneration after partial hepatectomy. Hepatology. 1995 Jan;21(1):155–161. [PubMed] [Google Scholar]
- Yasuda H., Mine T., Shibata H., Eto Y., Hasegawa Y., Takeuchi T., Asano S., Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993 Sep;92(3):1491–1496. doi: 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]