Abstract
Quiescent T cells express Tob, an APRO gene family member, which functions as a transcriptional regulator. Subtractive hybridization identified Twisted gastrulation (Tsg) as one of the genes suppressed by Tob. Tsg is a secreted protein that interacts with Drosophila decapentaplegic (Dpp) and its vertebrate orthologs BMP2/4 and regulates morphogenetic effects in embryos. Here, we report the expression and function of Tsg in human T cells. Tsg mRNA was almost undetectable in unstimulated T cells and was up-regulated after activation by TCR/CD3 and either CD28, IL-2, or PMA. Tsg protein had no effect on responses of primary T cells to TCR/CD3 stimulation but had a potent inhibitory effect on proliferation and cytokine production of primed alloreactive CD4+ cells. Surprisingly, Tsg did not affect phosphorylation of the BMP-specific Smad1 but induced phosphorylation of the TGF-β–specific Smad2 and mediated DNA binding on Smad3/4 consensus-binding sites, suggesting that it acted downstream of TGF-β. In vitro association assays revealed a direct interaction of Tsg and TGF-β proteins. Thus, Tsg functions as an agonist synergizing with TGF-β to inhibit T-cell activation. Modulation of Tsg signaling may represent a novel target for molecular intervention toward control of aberrant T-cell responses during ongoing graft-versus-host disease (GVHD) and autoimmune diseases.
Introduction
Morphogens are secreted signaling molecules produced at a localized source that specify different cell fates in a concentration-dependent manner. The generation of a concentration gradient of the morphogen by diffusion or movement from its source across the target cell field enable cells to respond according to their position within the field and patterning signals are thereby generated.1,2 In Drosophila melanogaster, Hedgehog (Hh), Wingless (Wg), and decapentaplegic (dpp) have been identified as morphogens during development.3,4 Their vertebrate orthologs, the Hh family, Wnt family, and bone morphogenetic proteins (BMPs) 2 and 4, likewise act as morphogens during vertebrate embryogenesis and organogenesis.1,4 Recent studies have revealed that these proteins not only determine patterning and cell fate during development but also function in cell-fate determination of self-renewing tissues in the adult, such as the hematopoietic system and the immune system.5,6 Specifically, evidence suggests a role of these morphogenetic proteins in the control of T-cell development in the thymus.7–10
BMPs belong to a family of secreted signaling molecules the founding member of which, TGF-β, is essential for immune homeostasis and maintenance of T-cell quiescence.11–13 BMPs 2 and 4 inhibit thymocyte differentiation7,9 and may mediate their effect at the same stage of thymocyte differentiation as TGF-β and Shh.8,14 Components of the BMP-signaling pathway are expressed in the thymus including BMP4,8 the BMP receptor components activin-like kinases ALK-3 (BMPR-Ia) and ALK-6 (BMPR-Ib), Smad proteins, the downstream mediators of BMP signaling,15 and the regulators of BMP signaling chordin,16 and Twisted gastrulation (Tsg).7
Tsg is an evolutionarily conserved, secreted protein that interacts with the Drosophila Dpp, the vertebrate Dpp orthologs BMP2/4, and also the extracellular Dpp/BMP inhibitors short gastrulation (sog) and chordin in Drosophila and vertebrate, respectively.17–21 Tsg can alter the proteolytic process of sog and chordin by extracellular metalloproteases.18,22 As a result, Tsg affects the binding of Dpp/BMP2/4 to their cellular receptors. Subsequently, the BMP downstream signaling events mediated by phosphorylation, nuclear translocation, DNA binding, and transcriptional activity of Smad proteins11 are regulated by Tsg positively17,22 or negatively.18–21 In the thymus, Tsg functions as a regulator of thymocyte differentiation. BMP2 and 4 inhibit thymocyte differentiation7,9 and this effect is antagonized by Tsg, which is produced by thymic epithelium and thymocytes.7
Here, we report that TSG is one of the genes regulated by Tob and acts as an inhibitor of activated mature CD4+ human T lymphocytes. TSG mRNA was expressed at very low levels in unstimulated T cells and was highly up-regulated after activation by TCR/CD3 and either CD28, IL-2, or PMA. Recombinant Tsg had a potent inhibitory effect on CD3-mediated proliferation and cytokine production of preactivated T cells, including IL-2, IL-4, IFN-γ, and IL-10. This effect was not altered by the presence of BMP2 or BMP4. In contrast, Tsg enhanced the inhibitory effect of TGF-β1 on preactivated T cells suggesting that Tsg regulates TGF-β and not BMP downstream signaling in mature CD4+ T cells. Consistent with this hypothesis, Tsg did not affect phosphorylation of the BMP-specific Smad1, but induced phosphorylation of the TGF-β–specific Smad2 and mediated DNA binding on Smad3/4 consensus sites. In vitro association assays using purified Tsg and TGF-β revealed a direct interaction of these proteins. Moreover, soluble TGF-β receptor II reversed the inhibitory effect of TGF-β and Tsg on preactivated T cells either in the presence or in the absence of TGF-β, providing functional evidence for the biologic significance of the Tsg/TGF-β interaction. Our results show that Tsg is a potent agonist of TGF-β downstream signaling in activated human CD4+ T cells and suggest that enhancement of TGF-β mediating signaling by Tsg may represent a novel target for molecular intervention for control of aberrant T-cell responses during ongoing graft-versus-host disease (GVHD) and autoimmune diseases.
Materials and methods
Transfections and suppression subtractive hybridization
Jurkat T cells were transiently transfected as described23 with full-length human Tob cDNA or with empty vector as control. Cells were collected at 12 and 24 hours after transfection, mRNA was isolated from each population with the RNAzol B RNA isolation kit (Tel-Test, Friendswood, Texas). cDNA was prepared by reverse transcription-polymerase chain reaction (RT-PCR), and subtractive suppressive hybridization24 was done with the use of the PCR-select cDNA subtraction kit (Clontech, Palo Alto, CA) according to the manufacturer's protocol, as previously described.23
Preparation and culture of T lymphocytes
CD4+ cells from peripheral blood lymphocytes were prepared from buffy coat leukophoresis residues obtained from the blood banks of the Dana-Farber Cancer Institute and the Brigham and Women's Hospital (Boston, MA). Mononuclear cells were isolated by Ficoll/Paque (Amersham-Pharmacia Biotech, Piscataway, NJ) gradient centrifugation. T cells were enriched by depletion of monocytes by plastic adherence and positive selection by E-rosetting using sheep red blood cells (BioWhittaker, Walkersville, MD). Subsequent preparation of CD4+ populations was done by negative selection using anti-CD8– or anti-CD4–specific monoclonal antibodies (mAbs), respectively, and anti-CD14, anti-CD11b, and anti-CD56 mAbs for removal of residual monocytes and NK cells, followed by incubation with goat anti–mouse IgG–coated magnetic beads (PerSceptive Biosystems, Framingham, MA). For RNA preparation, cells were cultured in 6-well plates at 5 × 106/mL for the indicated time intervals, in the presence of the indicated stimuli. Polyclonal preactivated CD4+ T-cell lines were previously described25 and were prepared by repetitive stimulation of CD4+ T cells from HLA-DR7− individuals with EBV-transformed B-cell lines from HLA-DR7 homozygous individuals alternating with NIH-3T3 cells stably transfected with HLA-DR7.
Proliferation and cytokine analysis
For proliferation assays, 5 × 104 cells/well were cultured in 96-well flat-bottom plates and 3H-thymidine incorporation was assessed for the last 16 hours of a 60-hour total period of culture. Culture supernatants were harvested at 24 hours and analyzed for levels of the indicated cytokines by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Agents and antibodies
TGF-β, BMP2, BMP4, mouse Tsg, and human TGF-β soluble receptor II were purchased from R&D Systems. Anti-CD3 and anti-CD28 mAbs were purchased from CLB (Flanders, NJ) and were used at a concentration of 100 ng/mL and 300 ng/mL, respectively.
Preparation of recombinant human Tsg and TGF-β
Full-length human Tsg cDNA was cloned from peripheral blood lymphocytes. Human Tsg was produced in COS cells transfected with full-length human Tsg cDNA tagged with a COOH-terminal V5 and His epitope and inserted into the pcDNA3.1/V5-His-TOPO vector. Supernatant was collected and concentrated, hTsg was purified by Nickel column (Pierce, Rockford, IL) and concentration was estimated by gel staining. Western blot analysis of the material with an anti-V5 mAb (Invitrogen, Grand Island, NY) and a His-specific antibody (Qiagen, Valencia, CA) showed a single band at approximately 25 kDa. As control of V5-tagged protein, B7-H3 cDNA was cloned in the pcDNA3.1/V5-His-TOPO vector. B7-H3-V5 was produced after transfection in COS cells as described for Tsg-V5. Supernatants from COS cells transfected with empty pcDNA3.1/V5-His-TOPO vector treated in the exact same way served as additional control. For preparation of tagged human TGF-β, IMAGE clone 4399762 (American Type Culture Collection, Manassas, VA; MGC-22008) was used. A bacterial culture was grown and 1 μL was vortexed vigorously in 10 μL water to extract DNA. Then, 1 μL of this preparation was subjected to PCR and the product was cloned into the pcDNA3.1/V5-His-TOPO vector. Supernatants were collected from transfected 293T cells cultured in serum-free media (Invitrogen; CD293 media 11913-019), and TGF-β–V5 protein was isolated as described for V5 tagged Tsg and B7-H3 proteins. Western blot analysis of TGF-β–V5 protein with an anti-V5 mAb (Invitrogen) and a His-specific antibody (Qiagen) showed a single band at approximately 35 kDa.
Northern blots
For Northern blot analysis 15 μg mRNA from human T cells was analyzed by electrophoresis on 1% agarose gel containing formamide and transferred on Hybond-XL blotting membrane (Amersham/Pharmacia Biotech). Human immune tissue blot and the human multiple tissue blot were purchased from Clontech. The Tsg probe consisted of full-length human Tsg cDNA and the Tob probe consisted of full-length human Tob cDNA. The G3PDH probe consisted of a 1-kb fragment of the cDNA spanning the coding region of human G3PDH. cDNA fragments were labeled by random priming with 32P-dATP using a labeling kit (Amersham Pharmacia Biotech). Blots were hybridized with labeled probes in hybridization buffer (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol. After hybridization blots were washed 4 times at room temperature in SSC (2 ×) and SDS (0.1%), followed by one more wash at 50°C and examined by autoradiography.
Immunoblotting
After the indicated conditions and time intervals of culture, T cells were isolated, cell lysates were prepared, and equal amounts of protein (50 μg/sample were analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred on nitrocellulose membranes, and immunoblotted with the indicated mAbs or antiserum, followed by immunodetection by incubation with horseradish peroxidase-conjugated anti–mouse IgG (1:5000) or anti–rabbit IgG (1:10 000; Promega, Madison, WI) and developed by chemiluminescence (NEN, Boston, MA). Preparation of whole-cell lysates, SDS-PAGE, stripping, and reprobing of the immunoblots were done as described.26 Erk1/2 and p-Erk1/2 mAbs were purchased from Santa Cruz Biotechnology (Palo Alto, CA); Smad1, p-Smad1, and p-Smad2 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY); and Smad2 was from Santa Cruz Biotechnology. All cultures from which cells were recovered for analysis of Smad phosphorylation were done in serum-free media to avoid potential Smad activation by TGF-β in serum. The DHL6 cell line was kindly provided by Dr Margaret Shipp (Dana-Farber Cancer Institute, Boston, MA) and was used as a control for Smad1 phosphorylation.27 In vitro association between hTsg-V5 and TGF-β, hTGF-β–V5 and Tsg, or B7-H3-V5 and TGF-β as control was conducted using roughly equimolar amounts of purified proteins as previously.19,28 Incubation of proteins was conducted overnight at 4°C, and subsequently immunoprecipitation was done with V5-specific antibody (Invitrogen) coupled on protein G. Complexes were analyzed by SDS-PAGE and immunoblot was conducted using TGF-β–specific mAb, with Tsg-specific mAb (R&D Systems), or with V5-specific mAb. Incubation of purified hTsg-V5 and B7-H3-V5 with only diluent used for reconstitution of lyophilized TGF-β was used as additional control.
Assessment of cell viability
Quantitative determination of viability cells was performed using an annexin V-based apoptosis detection kit and the manufacturer's protocol (R&D Systems, Minneapolis, MN). Briefly, after culture under various conditions, cells were harvested and suspended in the appropriate binding buffer, stained with FITC-conjugated annexin V and propidium iodide at room temperature for 15 minutes, and subsequently analyzed by flow cytometry. Cells positive for annexin V and annexin V/propidium iodide were considered early and late apoptotic cells, respectively. All cells negative for annexin V were considered viable cells.
Assessment of cell surface binding of TGF-β
To determine binding of hTGF-β on cell surface receptors, the Fluorokine detection kit (R&D Systems) was used. The cells were incubated with various concentrations of biotinylated TGF-β (provided in the Fluorokine detection kit) or with biotinylated TGF-β that had been preincubated with recombinant Tsg (R&D Systems). Detection of surface binding of TGF-β was determined by flow cytometry after incubation with avidin-fluorescein, according to the instructions of the manufacturer.
Nuclear extracts and electrophoretic mobility shift assay
After the indicated culture conditions, cells were recovered, nuclear extracts were prepared29 and electrophoretic mobility shift assays (EMSAs) were done according to a previously described protocol30 with 10 μg protein from each sample using 32P-labeled Smad consensus oligonucleotides (Santa Cruz Biotechnology). Samples were separated by 4% PAGE and gels were dried and exposed on film. All cultures from which cells were recovered for EMSA were done in serum-free media to avoid potential Smad activation by TGF-β in serum.
RT-PCR analysis
Purified cells were collected and mRNA was extracted by RNAzol-B (Tel-Test). The mRNA was reverse transcribed into cDNA by standard methods using Superscipt II (Gibco BRL, Grand Island, NY). PCR was performed for the detection of the indicated genes using the following primers: Smad1 forward: 5′-CGAATGCCTTAGTGACAG-3′; and Smad1 reverse 5′-GAGGTGAACCCATTTGAG-3′. BMPR-Ia forward: 5′-ACCATCGGAGGAGAACT-3′; and BMBR-Ia reverse: 5′-CTGCTGCGCTCATTTATC-3′. BMPR-Ib forward: 5′-AAGTTACGCCCCTCATTC-3′; and BMPR-Ib reverse: 5′-TGATGTCTTTTGCTCTGC-3′. BMPR-II forward: 5′-TTTCCCCACAGACATGCCTTCTTCCGTTTG-3′; and BMPR-II reverse: 5′-CATAGCCGTCTCTTGATTCTGCGAAGC-3′.
Results
Suppression of TSG gene expression by Tob
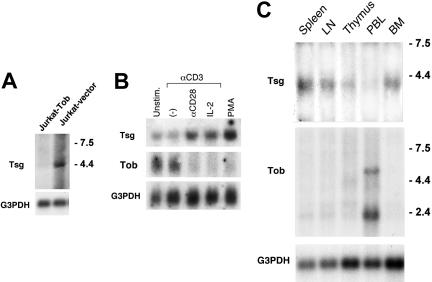
We have previously observed that TOB, a transcriptional regulator, is expressed in unstimulated T lymphocytes and functions as an inhibitor of cytokine transcription and cell cycle progression. When T cells are stimulated via TCR and CD28 costimulation, TOB is rapidly down-regulated. In contrast, forced expression of Tob in T cells prevents activation and mediates T-cell quiescence.23 To further understand the mechanisms via which Tob may regulate T-cell quiescence and activation, we sought to identify genes that are selectively induced or suppressed by Tob. Jurkat cells were transfected with Tob cDNA or empty vector and differential gene expression was determined by suppression subtraction hybridization. TSG was one of the genes that were selectively suppressed by Tob. The selective suppression of TSG in Tob-transfected cells was confirmed by Northern blot (Figure 1A).
Figure 1.
Northern blot analysis of TSG. (A) TSG mRNA is selectively suppressed in the presence of Tob. RNA extracted from Jurkat cells transfected with Tob cDNA or empty vector and expression of TSG was examined by Northern blot. (B) TSG mRNA is up-regulated in human CD4+ peripheral blood lymphocytes by mitogenic stimuli. Purified CD4+ T lymphocytes were cultured as indicated for 24 hours, RNA was extracted, and expression of TSG and TOB was examined by Northern blot. (C) TSG is expressed in human immune tissues. Northern blots of samples from human immune tissues were hybridized with human Tsg cDNA probe. In all experiments shown in panels A-C, after initial hybridization with Tsg probe, blots were striped and reprobed with human G3PDH cDNA probe. Relative molecular lengths, in kilobases, are indicated.
TSG mRNA is up-regulated after mitogenic activation of T cells
The expression and function of Tsg in mature T lymphocytes has not been examined. Therefore, to understand the significance of our finding, we sought to determine the regulation of Tsg expression and its physiologic role in primary human T lymphocytes. As shown in Figure 1B, TSG mRNA was expressed at very low levels in unstimulated CD4+ T cells and its expression was not affected by CD3-mediated signals alone. After 24 hours of culture with anti-CD3 mAb in the presence of anti-CD28 or IL-2, TSG mRNA was up-regulated (Figure 1B). In contrast to TSG, TOB was constitutively expressed at high levels in unstimulated primary human CD4+ T cells and was down-regulated to almost undetectable levels when T cells were activated via their T-cell receptor in the presence of CD28- or IL-2R–mediated signals (Figure 1B). After stimulation of T cells with phorbol ester, which directly activates protein kinase C and bypasses the requirements for costimulation,31 TSG was up-regulated, whereas TOB was down-regulated (Figure 1B).
Analysis of TSG expression in immune tissues by Northern blot showed that TSG transcript was detectable in spleen, lymph node, thymus, and bone marrow but a significantly lower level of expression was detected in peripheral blood cells. In contrast, the highest expression of TOB transcript among all immune tissues was detected in peripheral blood cells (Figure 1C). Taken together these results indicate that Tsg and Tob have a reciprocal pattern of expression.
Tsg does not affect TCR/CD3 responses of primary human T cells, but enhances the inhibitory effects of TGF-β on primed alloreactive T cells
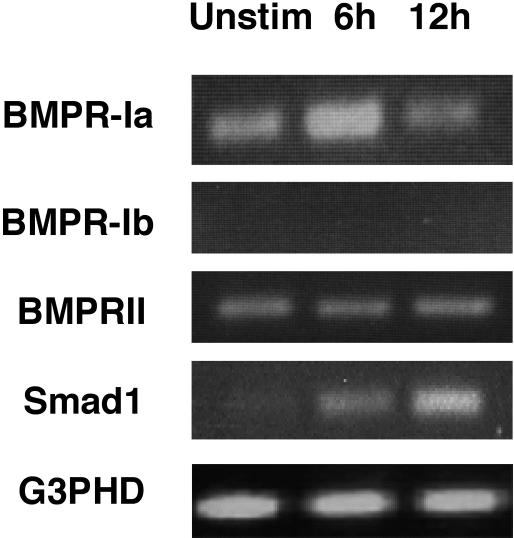
Based on the known effects of Tsg on thymocytes,7,9 we hypothesized that Tsg may regulate BMP2/4-mediated effects on mature T cells. Because no studies have reported effects of BMP on mature T lymphocytes, first we investigated whether expression of BMP receptors and the BMP-specific Smad1 could be detected in human CD4+ T cells. RT-PCR analysis demonstrated that BMPR-Ib was undetectable, but BMPR-Ia and BMPR-II were expressed in human CD4+ cells prior and after activation (Figure 2). Expression of the BMP-specific Smad1 was also detected in human CD4+ cells and was up-regulated after activation (Figure 2).
Figure 2.
Expression of BMP receptors and Smad1 in human CD4+ T lymphocytes. Human CD4+ T lymphocytes were either untreated or stimulated with anti-CD3 plus anti-CD28 mAbs for the indicated time intervals and RT-PCRs were performed using primers specific for the indicated genes. Primer pair for BMPR-Ib has been confirmed to generate the anticipated product in other cell types (data not shown).
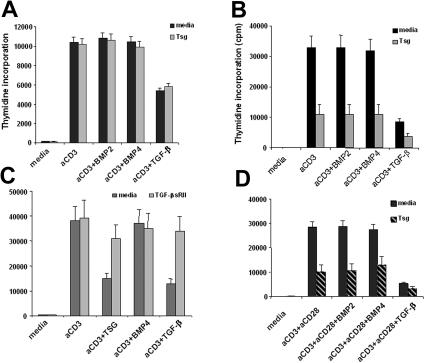
Because mature human CD4+ T cells express BMPR-II and BMPR-Ia that binds BMP2/4 with high affinity even in the absence of type II receptors5,15,32 and the BMP-specific Smad1, we hypothesized that mature CD4+ T cells might be able to respond to BMP-mediated signals. To study the role of Tsg in T-cell responses we generated recombinant human Tsg protein. We examined the effects of BMP on T-cell responses and the potential role of Tsg in regulating BMP-mediated responses. CD4+ T cells were cultured with anti-CD3 mAb in the presence of various concentrations (5-200 ng/mL) of either BMP2 or BMP4. As shown in Figure 3A, neither BMP2 nor BMP4 affected proliferation of CD4+ T cells. Moreover, addition of Tsg did not alter the responses of CD4+ T cells either in the presence or in the absence of BMP.
Figure 3.
Tsg selectively inhibits proliferation of primed alloreactive T cells and enhances TGF-β–mediated inhibition. Primary CD4+ T cells (A) or primed alloreactive CD4+ T cell lines (B) were cultured with anti-CD3 mAb in the presence of either BMP2, BMP4 (100 ng/mL), or TGF-β (2 ng/mL) in the absence or in the presence of Tsg (2 μg/mL). Proliferation was determined by 3H-thymidine incorporation for the last 16 hours of a 72-hour total period of culture. A wide range of BMP2/4 concentrations (5-200 ng/mL) and Tsg concentrations (1-10 μg/mL) showed a similar pattern of results (data not shown). Results are representative of 6 independent experiments using cells from different donors. Similar effects on proliferation and cytokine production by alloreactive primed CD4+ T cells line were observed when commercial recombinant Tsg (R&D Systems) was used (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). (C) sTGF-β RII reverses the inhibitory effect of Tsg on proliferation of primed alloreactive T lymphocytes. Primed alloreactive CD4+ T-cell lines were cultured with the indicated stimuli in the presence of Tsg alone or with TGF-β. Various concentrations (100-500 ng/mL) of TFG-β RII were added (results shown represent TFG-β RII concentrations 250 ng/mL). Proliferation was determined by 3H-thymidine incorporation for the last 16 hours of a 72-hour total period of culture. (D) Tsg inhibits proliferation of primary T lymphocytes that receive optimal activation by anti-CD3 plus anti-CD28 mAbs. Primary CD4+ T cells were cultured as in panels A-B but with anti-CD3 and anti-CD28 mAbs instead of anti-CD3 mAb alone. Proliferation was determined by 3H-thymidine incorporation for the last 16 hours of a 72-hour total period of culture. Error bars indicate variation of cpm values.
Because our RT-PCR analysis (Figure 2) showed that Smad1 expression was up-regulated after T-cell activation, we examined whether BMPs might have an effect on preactivated T lymphocytes. Polyclonal alloreactive CD4+ T cells lines were cultured with either BMP2 or BMP4 in the presence or in the absence of Tsg. Neither BMP2 nor BMP4 had any effect on proliferation of primed CD4+ T cells in response to CD3-mediated stimulation (Figure 3B). Strikingly, Tsg induced a significant inhibitory effect on CD3-mediated T-cell proliferation, but this effect was not affected by the presence of BMP2 or BMP4. In contrast, Tsg enhanced the inhibitory effect of TGF-β that was used as a positive control for inhibition of T-cell responses (Figure 3B). Similarly to proliferation, Tsg also inhibited cytokine production by alloreactive primed CD4+ T cell lines (Table 1). These results suggest that Tsg inhibits responses of primed CD4+ T cells and enhances TGF-β–mediated inhibitory signals in primed CD4+ T cells. The ability of Tsg to enhance TGF-β–mediated inhibitory effects was even more prominent when subinhibitory concentrations of TGF-β were used in the culture of alloreactive T-cell line stimulated with anti-CD3. Under these conditions, addition of increasing concentrations of Tsg induced a dose dependent inhibition of T-cell activation (Figure S2).
Table 1.
Tsg inhibits cytokine production
| Culture conditions | IL-2 | IFN-γ | IL-4 | IL-10 |
|---|---|---|---|---|
| Media | 20 (3) | 15 (2) | 1.5 (0.6) | 8 (2) |
| Anti-CD3 | 75 (8) | 345 (42) | 5 (1) | 119 (14) |
| Anti-CD3 + Tsg | 43 (5) | 175 (12) | 2.8 (0.8) | 32 (3) |
| Anti-CD3 + TGF-β | 40 (5) | 150 (18) | 2.6 (0.8) | 21 (2) |
| Anti-CD3 + Tsg + TGF-β | 25 (3) | 85 (11) | 1.5 (0.6) | 10 (2) |
CD4+ T cells were cultured under the indicated culture conditions, and cytokine concentrations were analyzed by ELISA in the supernatants of 24 hours of culture. Results show concentrations in picograms per milliliter and are representative of 3 independent experiments. The concentrations used were as follows: anti-CD3, 100 ng/mL; Tsg, 2 μg; TGF-β, 2 μg/mL. The lowest limits of ELISA detection were as follows: IL-2, 6 pg/mL; IFN-γ, 8 pg/mL; IL-4, 0.13 pg/mL; IL-10, 3.9 pg/mL.
Standard deviations of the values are shown in parentheses.
To further investigate the hypothesis that Tsg might mediate its effects in a TGF-β–dependent manner, we used TGF-β soluble receptor type II (sTGF-β RII), which inhibits the action of TGF-β by associating with TGF-β or by acting as dominant-negative receptor.33,34 Addition of sTGF-β RII reversed the inhibitory effects of TGF-β and Tsg in polyclonal alloreactive CD4+ T-cells lines (Figure 3C). Thus, Tsg mediates its effects in a TGF-β–dependent manner in activated mature CD4+ T cells.
Because Tsg inhibited responses of preactivated T-cell lines, we hypothesized that optimal activation of primary T lymphocytes might be required to render them capable of responding to Tsg-regulated signals. We cultured primary CD4+ T cells with anti-CD3 and anti-CD28 mAb either in media alone or with BMP2, BMP4, or TGF-β. As shown in Figure 3D, neither BMP2 nor BMP4 affected proliferation of CD4+ T cells in response to anti-CD3 plus anti-CD28 stimulation. However, Tsg induced a significant inhibitory effect on CD3 plus anti-CD28–mediated T-cell proliferation, but this effect was not altered by the presence of BMP2 or BMP4. In contrast, Tsg enhanced the inhibitory effect of TGF-β, similarly to its effect on primed, alloreactive T cells.
Tsg does not signal via the BMP-specific Smad 1 but via the TGF-β–specific Smad2/3 in T cells
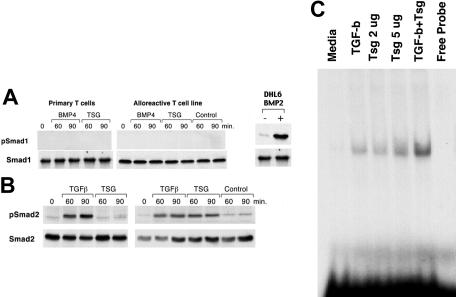
The observations that BMP2/4 did not alter T-cell proliferation and cytokine production in the presence or in the absence of Tsg suggest that either BMPs do not signal in mature CD4+ T cells or that BMPs regulate other functions independent of cellular proliferation and cytokine production. To dissect these possibilities we examined whether BMP2/4 could induce phosphorylation of the BMP-specific Smad1 in CD4+ T cells. In addition, an obvious question arising from the findings was whether Tsg might have an effect on phosphorylation of the BMP-specific Smad 1 or the TGF-β–specific Smad2 or both. Primary CD4+ T cells and primed alloreactive T cells were treated with either BMP2, BMP4, TGF-β, or Tsg and phosphorylation of Smad1 and Smad2 was examined by immunoblot with phospho-specific antibodies. BMP2 (data not shown), BMP4, and Tsg did not induce detectable phosphorylation of Smad1 in either primary or primed alloreactive T cells (Figure 4A). In contrast, TGF-β induced phosphorylation of Smad2 in both primary and primed alloreactive T cells (Figure 4B). Tsg also induced phosphorylation of Smad2 that was observed selectively in primed alloreactive T lymphocytes (Figure 4B).
Figure 4.
Tsg signals via the TGF-β–specific Smad2/3 in CD4+ T cells. (A) Primary CD4+ T cells or primed alloreactive CD4+ T-cell lines were cultured with BMP4 (100 ng/mL), Tsg (2 μg/mL), or control for 1 hour. Cell lysates were prepared and equal amounts (250 μg/sample) were used for immunoprecipitation with Smad1-specific antibody, followed by SDS-PAGE and Western blot with mAbs specific for p-Smad1 and Smad1. Results are representative of 4 independent experiments. DHL6 cell line was used as a positive control for Smad1 phosphorylation. (B) Primary CD4+ T cells or primed alloreactive CD4+ T-cell lines were cultured as in panel A and cell lysates (75 μg/sample) were analyzed by SDS-PAGE and Western blot with mAbs specific for p-Smad2 and Smad2. (C) Primed alloreactive CD4+ T-cell line was cultured for 1 hour with either media, TGF-β (2 ng/mL), Tsg (2 μg/mL), Tsg (5 μg/mL), or the combination of TGF-β (2 ng/mL) plus Tsg (2 μg/mL). Nuclear extracts were prepared and MSAs were done using Smad3/4 consensus oligonucleotides (Santa Cruz Biotechnology). Results are representative of 2 independent experiments.
Smads are transcription factors and have DNA-binding specificity. Smad2 and Smad3 are receptor Smads that become activated by TGF-β/activin, subsequently associate with Smad4, and translocate to the nucleus, where Smad3/4 bind to DNA to regulate gene transcription.35 Because Tsg induced phosphorylation of Smad2 and altered TGF-β–mediated responses, we examined whether the presence of Tsg potentially altered DNA-binding activity on Smad3/4 consensus-binding sites (Figure 4C). No DNA binding activity was observed by gel shift assays in primed alloreactive T cells prior to incubation with TGF-β family proteins. Incubation with TGF-β or with Tsg alone induced DNA-binding activity on Smad consensus sites. Combination of TGF-β and Tsg enhanced DNA-binding activity. These results indicate that Tsg enhances TGF-β–mediated signals in primed CD4+ T cells because it enhances TGF-β–mediated Smad activation.
Tsg interacts with TGF-β
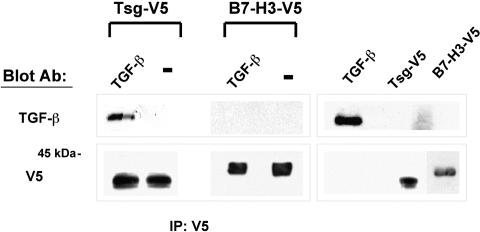
Previous studies in other systems in which Tsg has been shown to modify BMP-mediated signaling have revealed a direct interaction between Tsg and BMP proteins.17,19–21 Since our studies showed that Tsg regulated TGF-β–mediated signaling in T cells, we examined whether a direct association between Tsg and TGF-β could be detected. To address this issue purified hTsg-V5 was incubated with TGF-β and immunoprecipitation was done with V5-specific antibody. SDS-PAGE analysis followed by immunoblot with TGF-β–specific mAbs revealed a coprecipitation of Tsg and TGF-β indicating that these proteins had formed complexes. In contrast, such interaction was not detected between TGF-β and B7-H3-V5 that was used as control (Figure 5). Interaction between recombinant Tsg and TGF-β proteins was further confirmed by incubation of untagged, recombinant Tsg protein with TGF-β–V5 followed by immunoprecipitation with V5-specific antibody, SDS-PAGE analysis, and immunoblot with anti-Tsg mAb (Figure S3).
Figure 5.
Tsg interacts with TGF-β. Purified Tsg-V5 or B7-H3-V5 and TGF-β were incubated overnight at 4°C, and subsequently immunoprecipitation was done with V5-specific antibody coupled on protein G. Complexes (lanes 1-2) were analyzed by SDS-PAGE along with purified proteins (lanes 3-4) and immunoblot was conducted using TGF-β-specific mAb or V5-specific antibody. Incubation of purified Tsg-V5 or B7-H3-V5 with diluent alone was used as control (indicated as −).
Tsg does not affect activation of Erk1/2 MAP kinases
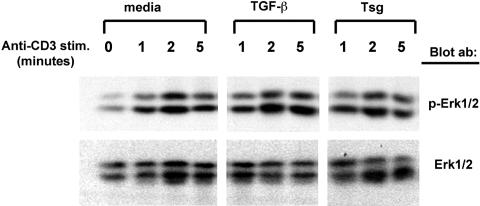
The MEK-Erk pathway is one of the pathways with a critical role in proliferation and cytokine production induced by TCR/CD3. Previous studies have shown cross-talk between Smads and MAP kinases.11 Specifically, Erk has been reported to mediate phosphorylation of Smad3 and to activate Smad3-mediated signaling.36 Consistently, activation of Erk has been reported to enhance TGF-β/Smad-mediated responses.37 In contrast, other studies have reported that TGF-β inhibits TCR-mediated Erk1/2 activation.38 Because our studies showed that Tsg inhibited T-cell responses in a TGF-β–dependent manner we examined whether Tsg might alter TCR/CD3-mediated activation of Erk1/2. Alloreactive CD4+ T-cell lines were stimulated by TCR/CD3 cross-linking with anti-CD3 antibody in the presence of media alone, TGF-β, or Tsg and activation of Erk1/2 was examined by immunoblot with p-Erk1/2–specific antibody. As shown in Figure 6, neither TGF-β nor Tsg had any effect on Erk1/2 activation. Thus, in our experimental system, the inhibitory effects of TGF-β and Tsg were not mediated by altered Erk1/2 activation and signaling.
Figure 6.
Tsg does not affect activation of Erk1/2. Primed alloreactive CD4+ T-cell lines were stimulated for the indicated time intervals by CD3 cross-linking after pretreatment either with media, TGF-β, or Tsg. Cell lysates were prepared and equal amounts (75 μg/sample) were analyzed by Western blot with mAbs specific for pErk1/2 and Erk1/2.
Apoptosis is not the major mechanism by which TGF-β and Tsg inhibit responses of human T cells
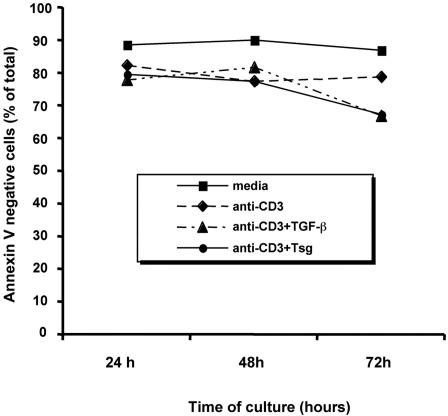
Previous studies showed that the inhibitory effects of TGF-β on proliferation of primed T cells are not due to induction of apoptosis.39 To investigate whether Tsg inhibited cell proliferation by reducing cell viability we used annexin V/propidium iodide staining. Alloreactive T cell lines were cultured with either media, anti-CD3, anti-CD3 plus TGF-β, or anti-CD3 plus Tsg and viability was assessed at 24, 48, and 72 hours of culture. As shown in Figure 7, culture with anti-CD3 induced a moderate but reproducible reduction of cell viability. Addition of either TGF-β or Tsg had no effect on cell viability at 24 and 48 hours, compared to cells cultured with anti-CD3 alone. We observed a slight reduction of cell viability (5-10%) by addition of either TGF-β or Tsg at 72 hours of culture. However, this degree of reduction was disproportional to the reduction of cellular proliferation (60-75%) observed under the same culture conditions (Figure 3). Thus, apoptosis was not the major mechanism by which either TGF-β or Tsg inhibited the proliferative capacity of T cells.
Figure 7.
Apoptosis is not the major mechanism by which TGF-β and Tsg inhibit responses of human T cells. Primed alloreactive CD4+ T-cell lines were cultured with media, anti-CD3 mAb in the presence of either media, TGF-β (2 ng/mL), or Tsg (2 μg/mL) for the indicated time intervals and were subsequently harvested and labeled with annexin V and propidium iodide, and viability was determined by fluorescence-activated cell sorting (FACS) analysis.
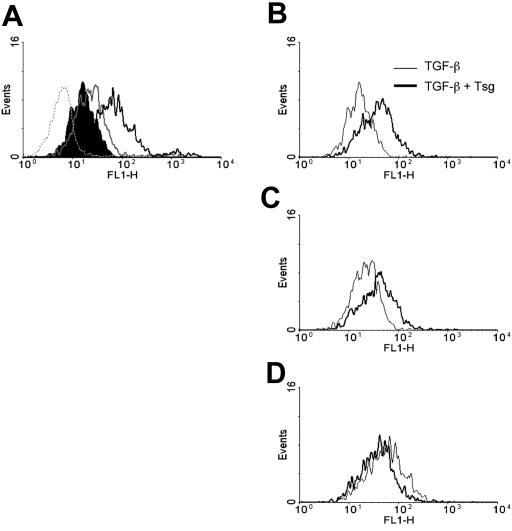
Tsg enhances binding of TGF-β on cell surface receptors
Our data showed that Tsg induced phosphorylation of Smad2 and enhanced TGF-β–mediated responses (Figure 4). These effects appeared to be mediated by altering signaling via the TGF-β receptor (Figure 3C). Therefore, we attempted to examine whether Tsg might alter binding of TGF-β to its receptors on the surface of activated T cells. To address this issue we used the Fluorokine detection system (R&D Systems) that allows detection of hTGF-β binding to its cell surface receptors. Alloreactive T cells were cultured either with media or anti-CD3 for 24 hours and cell surface binding was examined by incubation with increasing concentrations of biotinylated TGF-β. A dose-dependent binding of TGF-β was detected on activated cells (Figure 8A) but not on cells cultured with media alone (Figure 8A and data not shown). Next, cells were incubated with the same increasing concentrations of TGF-β that had been preincubated overnight with equimolar concentrations of Tsg (Figure 8B-D). Preincubation of TGF-β with Tsg enhanced cell surface binding of TGF-β as determined by a shift in mean fluorescence intensity (Figure 8B-C). This effect of Tsg in enhancing TGF-β binding on the cell was predominantly evident at low concentrations of TGF-β (Figure 8B-D). These results suggest that Tsg modulates binding of TGF-β on its specific receptors on activated T lymphocytes, thereby altering TGF-β–mediated signaling and functional outcome.
Figure 8.
Tsg enhances binding of TGF-β on cell surface receptors. (A) Primed alloreactive CD4+ T-cell line was cultured with media or anti-CD3 mAb for 24 hours and subsequently cells were incubated with increasing concentrations of biotinylated TGF-β (1.25 ng/sample, shaded histogram; 2.5 ng/sample, gray line histogram; 12.5 ng/sample black line histogram). A sample of cells from the media-only culture incubated with 12.5 ng biotinylated TGF-β (dotted line histogram) is included as negative control. (The data of this sample were comparable to those obtained when these cells were incubated with 1.25 ng/sample and 2.5 ng/sample of TGF-β [data not shown]). Following incubation with avidin-fluorescein, samples were analyzed by flow cytometry. (B-D) Alloreactive CD4+ T cells cultured with anti-CD3 for 24 hours were incubated with increasing concentrations of TGF-β (B, 1.25 ng/sample; C, 2.50 ng/sample; D, 12.5 ng/sample) that had been preincubated overnight with equimolar concentrations of Tsg. Samples were subsequently incubated with avidin-fluorescein and were analyzed by flow cytometry. Results are representative of 2 independent experiments.
Discussion
The key and unexpected observation of our present study is that mature CD4+ T cells express Tsg, a secreted morphogenetic protein. TSG mRNA is induced by optimal stimulation by TCR/CD3 and CD28 costimulation or by TCR/CD3 and IL-2 receptor stimulation. Tsg functions as an inhibitor of T-cell activation. Interestingly, human T cells respond to the effects of Tsg only when previously activated, whereas Tsg does not alter responses of primary T cells at first encounter of antigen.
Because Tsg has been recently shown to interact with extracellular components of the BMP2/4 signaling pathway in various cell types, including developing thymocytes,17–21 we investigated the effects of BMP on mature CD4+ T cells. Interestingly, despite the fact that mature CD4+ T cells expressed the BMP receptors BMPR-Ia and BMPR-II, as well as the BMP-specific Smad1, we did not observe an effect of BMP2/4 on proliferation or cytokine production of primary or preactivated T cells. Similarly, we did not observe phosphorylation of Smad1 by BMP. These observations suggest that BMP2/4 may not mediate signals in mature T cells. Consistent with our findings that BMPs did not induce detectable phosphorylation of Smad1, studies in thymocytes failed to determine that BMPs induced Smad1 phosphorylation.7 However, neither in thymocytes7 nor in our mature CD4+ T cells, did BMP2/4 use the canonical TGF-β–signaling pathway as indicated by phosphorylation of Smad2 by TGF-β but not by BMP2/4 in both cell types. Although these findings suggest that BMPs do not signal in mature T cells, we emphasize that BMPs may have a function in these cells, but their effect is not reflected by altered T-cell expansion or cytokine production. Alternatively, BMPs may be able to mediate effects on mature T cells under different conditions.
Because Tsg is a secreted, diffusible protein17,19,21 it is possible that Tsg, produced by T cells, may regulate the function of other cell types in the proximal or distal microenvironment. Such cell types may include monocytes, B lymphocytes, and lymphoid progenitors that can respond to BMP.41–43 Subsequently, the effects of Tsg may alter the activation, maturation, and function of these cells and affect the outcome of APC/T-cell interactions, either for enhancement or for attenuation of the immune responses.
Strikingly, we observed that Tsg selectively inhibited proliferation and cytokine production of preactivated CD4+ T cells and augmented the inhibitory effect of TGF-β. Consistently, Tsg enhanced DNA-binding activity on Smad3/4 consensus sites induced by TGF-β. Interestingly, in preactivated T cells, Tsg appeared to mediate signals that resulted in inhibition of proliferation, phosphorylation of Smad2, and DNA binding on Smad consensus sites, even in the absence of TGF-β. Because T cells produce TGF-β during activation,44 it is possible that in preactivated T cells endogenously produced TGF-β provided a subsaturating signal to the TGF-β receptor that was enhanced by Tsg. Our present studies showed that Tsg can associate with TGF-β and mediates its effects in a TGF-β–dependent manner in mature T lymphocytes. It was previously considered that Tsg-mediated effects require direct binding on chordin, by which Tsg regulates BMP signaling.17,19,20 However, recent studies have shown that Tsg can function independently of Tsg-chordin-BMP interactions.45 Taken together with those studies, our present observations suggest that Tsg may regulate not only signals mediated by BMP but also by other members of the TGF-β superfamily.
Our present data showed that TSG is induced after mitogenic activation of T cells and functions as a negative regulator of T-cell proliferation and cytokine production. This expression pattern is reciprocal to the expression of TOB that also functions as a negative regulator of T-cell proliferation and cytokine production.23 Interestingly, both Tob and Tsg enhance Smad DNA binding and appear to inhibit T-cell activation by regulating Smad activity. Although it is still unclear how TGF-β– and Smad-mediated signals induce their functional effects, it is well established that they inhibit T-cell proliferation and IL-2 production.12,13,46–48 Increasing evidence indicates that their inhibitory effect on cell proliferation is mediated by transcriptional induction of cell cycle inhibitors including p15 and p21cip1 thereby inducing blockade of cell cycle progression.40 There is also accumulating evidence that the inhibitory effect of TGF-β– and Smad-mediated signals on IL-2 production may occur at the level of transcription. An intriguing hypothesis is that Smads may directly inhibit transcription of the IL-2 gene by binding on Smad-binding sites that lie within the IL-2 promoter.23,48 Dependent on the context of promoters, activated Smads interact with different DNA-binding cofactors recruiting transcriptional coactivators or corepressors to distinct target gene promoters. By recruiting transcriptional coactivators (eg, p300/CBP, P/CAF) Smads can induce gene transcription, whereas by recruiting corepressors and histone deacetylases (eg, mSin3/HDAC1, Runx2/HDAC4) repress transcription.49 By this mechanism, TGF-β–activated Smads may positively regulate transcription of cdk inhibitors and negatively regulate transcription of IL-2 gene.
Extensive ongoing studies are investigating the molecular mechanisms via which TGF-β superfamily members mediate their signaling and function. The most prominent functional effect of TGF-β on lymphoid cells is to suppress their proliferative capacity without affecting their viability.40 An intriguing finding of our studies was that Tsg enhanced binding of TGF-β on activated T cells. Our present studies cannot determine at this point whether this effect is mediated by conformational changes of TGF-β induced by its interaction with Tsg, that may result in affinity or avidity changes of TGF-β for its receptor. In addition, this may not be the only mechanism via which Tsg affects TGF-β signaling. However, our observations suggest that local production of Tsg during T-cell activation may enhance TGF-β–mediated signaling and functional outcome. Interestingly, the effect of Tsg in augmenting TGF-β–mediated inhibition on activated T cells was mostly evident at low concentrations of TGF-β, suggesting that this mechanism may function as a safeguard pathway to ensure prevention or suppression of aberrant T-cell activation induced by a low potency/affinity antigen. Further studies will be required to determine the precise molecular mechanisms by which Tsg modulates TGF-β signaling thereby regulating its downstream functions.
The expression pattern of Tsg is similar to that observed in other inducible negative regulators of T-cell immune responses, such as CTLA4 or PD-1,50,51 which are not expressed in unstimulated cells but are induced after stimulation. Costimulation via CD28, a pathway with a dominant role in T-cell proliferation, cytokine production, and cell cycle progression mediated rapid up-regulation of TSG mRNA. Importantly, resting T cells did not respond to Tsg but acquired the ability to respond to Tsg only when previously activated. Thus, Tsg appears to be expressed and to mediate its negative regulatory function only after T-cell activation. To date, there is no ideal approach for selective immunosuppression, and unwanted side effects of compromising global immunity are inevitable. Because Tsg selectively inhibits preactivated T cells, T cells that are activated during GVHD or autoimmunity may be more sensitive to the inhibitory effects of Tsg than previously unstimulated T lymphocytes that encounter antigen for the first time. Thus, Tsg may represent a novel target for molecular intervention toward control of aberrant T-cell responses during ongoing GVHD and autoimmune diseases. Future studies will be required to address the in vivo effect of Tsg in the immune responses of the intact host, which involve multiple cell types and signaling pathways.
Supplementary Material
Acknowledgment
This work was supported by National Institutes of Health grants CA104596 and AI 43552 (V.A.B.) and AI 56299 (G.J.F.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.T. conducted subtraction hybridization, Northern blots, cloning of human Tsg, generation and purification of recombinant Tsg protein, proliferation assays, EMSA, and Western blots and generated the first draft of the manuscript; L.L. conducted ELISA, PCR, Western blots, in vitro association assays between B7-H3 and TGF-β, annexin V/propidium iodide experiments, assessment of TGF-β binding on cell surface, and contributed to the preparation of the manuscript; E.M.L. conducted Northern blots, proliferation assays, Western blots, and in vitro association between purified Tsg and TGF-β, and contributed to the preparation of the manuscript; A.B. prepared purified cell populations for all experiments, conducted SDS-PAGE analysis, Western blot, EMSA, and 3H-thymidine incorporation assays, and maintained cell lines; G.J.F. contributed to the design and cloning of human Tsg and TGF-β, prepared B7-H3-V5 protein, and contributed to the preparation of the manuscript; V.A.B. contributed to the cloning of human Tsg, did the cloning and expression of TGF-β, supervised D.T., E.M.L., L.L., and A.B., and had the responsibility for the entire project and the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
D.T. and L.L. contributed equally to the completion of this work.
Correspondence: Vassiliki A. Boussiotis, Massachusetts General Hospital, Bldg CNY 149-5102, 13th St, Boston, MA 02129; e-mail: vboussiotis@partners.org.
References
- 1.Christian JL. BMP Wnt and Hedgehog signals: how far can they go? Curr Opin Cell Biol. 2000;12:244–249. doi: 10.1016/s0955-0674(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 2.Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 3.Podos SD, Ferguson EL. Morphogen gradients: new insights from DPP. Trends Genet. 1999;15:396–402. doi: 10.1016/s0168-9525(99)01854-5. [DOI] [PubMed] [Google Scholar]
- 4.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Bonnet D, Wu D, et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 7.Graf D, Nethisinghe S, Palmer DB, Fisher AG, Merkenschlager M. The developmentally regulated expression of Twisted gastrulation reveals a role for bone morphogenetic proteins in the control of T cell development. J Exp Med. 2002;196:163–171. doi: 10.1084/jem.20020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outram SV, Varas A, Pepicelli CV, Crompton T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13:187–197. doi: 10.1016/s1074-7613(00)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Hager-Theodorides AL, Outram SV, Shah DK, et al. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J Immunol. 2002;169:5496–5504. doi: 10.4049/jimmunol.169.10.5496. [DOI] [PubMed] [Google Scholar]
- 10.Takagi T, Harada J, Ishii S. Murine Schnurri-2 is required for positive selection of thymocytes. Nat Immunol. 2001;2:1048–1053. doi: 10.1038/ni728. [DOI] [PubMed] [Google Scholar]
- 11.Itoh S, Itoh F, Goumans M-J, ten Dijke P. Signaling of transforming growth factor-b family members through Smad proteins. Eur J Biochem. 2000;267:6954–6957. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorelik L, Flavell RA. Abrogation of TGFb signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-b. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahama Y, Letterio JJ, Suzuki H, Farr AG, Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J Exp Med. 1994;179:1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewulf N, Verschueren K, Lonnoy O, et al. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- 16.Scott IC, Steiglitz BM, Clark TG, Pappano WN, Greenspan DS. Spatiotemporal expression patterns of mammalian chordin during postgastrulation embryogenesis and in postnatal brain. Dev Dyn. 2000;217:449–456. doi: 10.1002/(SICI)1097-0177(200004)217:4<449::AID-DVDY12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu K, Srinivasan S, Shimmi O, et al. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]
- 19.Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KWY, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signaling. Nature. 2001;410:475–478. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- 20.Chang C, Holtzman DA, Chau S, et al. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- 21.Ross JJ, Shimmi O, Vilmos P, et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–443. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 22.Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzachanis D, Freeman GJ, Hirano N, et al. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 24.Diatchenko L, Lau Y-FC, Campbell AP, et al. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci U S A. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boussiotis VA, Freeman GJ, Gray G, Gribben J, Nadler LM. B7 but not ICAM-1 costimulation prevents the induction of human alloantigen specific tolerance. J Exp Med. 1993;178:1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boussiotis VA, Freeman GF, Taylor PA, et al. p27kip1 functions as an anergy factor inhibiting IL-2 transcription and clonal expansion of alloreactive human and murine helper T lymphocytes. Nat Med. 2000;6:290–297. doi: 10.1038/73144. [DOI] [PubMed] [Google Scholar]
- 27.Munoz O, Fend F, de Beaumont R, Husson H, Astier A, Freedman AS. TGFbeta-mediated activation of Smad1 in B-cell non-Hodgkin's lymphoma and effect on cell proliferation. Leukemia. 2004;18:2015–2025. doi: 10.1038/sj.leu.2403485. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, McAlinden A, Sandell LJ. Type IIA procollagen in development of the human intervertebral disc: regulated expression of the NH(2)-propeptide by enzymic processing reveals a unique developmental pathway. Dev Dyn. 2001;220:350–362. doi: 10.1002/dvdy.1115. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts,” prepared from a small number of cells. Nucleic Acid Rec. 1989;17:6419–6420. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dent CL, Latchman DS. The DNA mobility shift assay. In: Rickwood D, Hames BD, editors. Transcription Factors: A Practical Approach. New York, NY: Oxford University Press; 1994. pp. 1–26. [Google Scholar]
- 31.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 32.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 33.Isaka Y, Akagi Y, Ando Y, et al. Gene therapy by transforming growth factor-beta receptor-IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1999;55:465–475. doi: 10.1046/j.1523-1755.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Okada H, Takemura G, Kosai K, et al. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–2437. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 35.Jonk LJC, Itoh S, Heldin C-H, ten Dijke P, Kruijer W. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-b, activin, and bone morphogenetic protein-inducible enhancer. J Biol Chem. 1998;273:21145–21152. doi: 10.1074/jbc.273.33.21145. [DOI] [PubMed] [Google Scholar]
- 36.Biggs JR, Kraft AS. The role of the Smad3 protein in phorbol ester-induced promoter expression. J Biol Chem. 1999;274:36987–36994. doi: 10.1074/jbc.274.52.36987. [DOI] [PubMed] [Google Scholar]
- 37.Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a Smad-dependent pathway. J Biol Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- 38.Chen CH, Seguin-Devaux C, Burke NA, et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003;197:1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiemessen MM, Kunzmann S, Schmidt-Weber CB, et al. Transforming growth factor-beta inhibits human antigen-specific CD4+ T cell proliferation without modulating the cytokine response. Int Immunol. 2003;15:1495–1504. doi: 10.1093/intimm/dxg147. [DOI] [PubMed] [Google Scholar]
- 40.Letterio JJ. TGF-beta signaling in T cells: roles in lymphoid and epithelial neoplasia. Oncogene. 2005;24:5701–5712. doi: 10.1038/sj.onc.1208922. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci U S A. 1992;89:11740–11744. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kersten C, Dosen G, Myklebust JH, et al. BMP-6 inhibits human bone marrow B lymphopoiesis-Upregulation of Id1 and Id3. Exp Hematol. 2006;34:72–81. doi: 10.1016/j.exphem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Kersten C, Sivertsen EA, Hystad ME, Forfang L, Smeland EB, Myklebust JH. BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1. BMC Immunol. 2005 doi: 10.1186/1471-2172-6-9. Prepublished ahead of print as DOI: 10.1186/1471-2172-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kehrl JH, Wakefield LM, Roberts AB, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- 46.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakao A, Miike S, Hatano M, et al. Blockade of transforming growth factor b/smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192:151–158. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- 49.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 50.Tivol EA, Borriello F, Schweitzer NA, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 51.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.