Abstract
The Rag2-γC double-knockout (DKO) mouse lacks T, B, and natural killer (NK) cells, and allows development of a functional human immune system with human CD34+ hematopoietic stem/progenitor cells (DKO-hu HSCs). Normal human T, B, and dendritic cells are present in peripheral blood, thymus, spleen, and lymph nodes. We report that both CCR5 and CXCR4 are expressed on human immature and mature T cells. DKO-hu HSC mice allow efficient HIV-1 infection with plasma high viremia. High levels of productive infection occur in the thymus, spleen, and lymph nodes. Human CD4+ T cells are gradually depleted by HIV-1 in a dose-dependent manner. In addition, HIV-1 infection persists in infected DKO-hu HSC mice for at least 19 weeks, with infectious HIV-1 in lymphoid tissues. Thus, the DKO-hu HSC mouse can serve as a relevant in vivo model to investigate mechanisms of HIV-1 infection and immunopathogenesis as well as to develop anti–HIV-1 therapeutics.
Introduction
The hallmarks of AIDS are high levels of HIV-1 infection, depletion of CD4+ T cells, and loss of immunity. The mechanism of HIV-1 immunopathogenesis is not clear because human patients are the only hosts that support high levels of HIV-1 replication and develop AIDS. Extensive studies have been performed in primate models with either low levels of HIV-1 replication (HIV-1 in chimps) or a different virus (simian immunodeficiency virus [SIV] or simian/human immunodeficiency virus [SHIV] in monkeys).1–3 HIV-1 fails to infect murine cells due to blocks at multiple steps of the HIV life cycle. The mouse with a reconstituted human immune system would be an ideal small animal model for studying HIV-1 infection and pathogenesis. A number of human-mouse chimeric models have been developed, but with only limited success. The severe combined immunodeficient (SCID)–hu Thy/Liv mouse has an intact human thymus organ, thus allowing investigation of HIV-1 pathogenesis in a human lymphoid organ.4–6 However, very low levels of human T cells are detected in the peripheral lymphoid organs or blood. The hu–peripheral blood lymphocyte (PBL)–SCID mouse supports transient and selective engraftment of xeno-reactive human T cells.7,8 Human CD34+ cells transplanted into SCID or nonobese diabetic (NOD)/SCID mice lead to generation of mainly human myeloid and B cells in the mouse bone marrow, but inefficient generation of human T cells.9,10 When CD34+ human hematopoietic stem cells (HSCs)/hematopoietic stem progenitor cells (HSPCs) are injected directly into the liver of newborn Rag2-γC double-knockout (DKO) mice, which lack T, B, and natural killer (NK) cells, the newborn liver environment of DKO mice supports efficient engraftment and development of a functional human immune system in central and peripheral lymphoid organs.11,12
To demonstrate that the DKO-hu HSC mouse can support efficient HIV-1 infection with relevant immunopathogenesis, we show that CCR5 and CXCR4, the HIV-1 coreceptors, are both expressed on human T cells in central and peripheral lymphoid organs. DKO-hu HSC mice allow high plasma viremia with high levels of HIV-1 infection in lymphoid organs. Both CD4+CD45RO+ and CD4+CD45RO− T cells are productively infected in lymphoid organs. Human CD4+ T cells are gradually depleted by HIV-1 in a dose-dependent manner. In addition, HIV-1 infection persists in infected DKO-hu HSC mice for at least 19 weeks, with recoverable infectious HIV-1 in lymphoid tissues.
Materials and methods
Construction of DKO-hu HSC mice with human CD34+/− cells from cord blood or fetal liver
Human CD34+ cells were isolated from cord blood (CB) or fetal liver (FL) tissues. FL CD34+ (1 × 106) or CB CD34+ (1 × 105) cells were injected intrahepatically into each newborn DKO mouse previously irradiated at 400 rad.11 Human leukocytes (CD45+) were analyzed for CD3, CD4, CD8, CD45RO, CD19, CCR5(2D7), and CXCR4(12G5) by fluorescence-activated cell sorting (FACS).13
HIV-1 infection and pathogenesis in DKO-hu HSC mice
At least 15 weeks after CD34 HSPC transfer, DKO-hu HSC mice with stable human leukocyte reconstitution were intravenously infected with X4/R5 dual tropic, R5-tropic, or X4-tropic HIV-1 (NL4-R3A, JRCSF, or NL4-3, respectively). Mock supernatant or heat-inactivated HIV-1 stocks were used as negative controls. Plasma viral load was monitored with the Roche Amplicor Monitor v.1.5 assay (Roche Diagnostics, Indianapolis, IN). Blood CD4+ T cells were measured by GUAVA Easycytes (GUAVA, Hayward, CA) and by FACS. Central and peripheral lymphoid organs were harvested to investigate HIV replication and pathogenesis in the thymus, spleen, and lymph nodes.13–16 To detect infectious HIV-1 in lymphoid organs, splenocytes or thymocytes were cocultured with PHA-activated peripheral blood mononuclear cells (PBMCs) for 14 days.13,17
Results and discussion
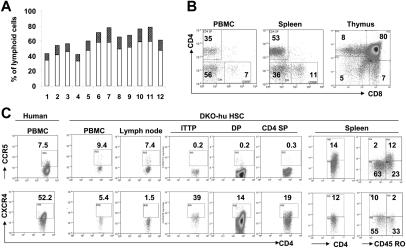
We transplanted CD34+ HSPCs derived from human cord blood or fetal liver into newborn DKO mice intrahepatically.11 Between 8 and 33 weeks after transplantation, we analyzed human leukocytes in the peripheral blood and lymphoid organs of these DKO-hu HSC mice and found that more than 90% of the DKO mice that underwent transplantation had stable engraftment with human CD45+ cells (Figure 1A). CD3+CD4+ cells (10%-20% of total PBLs) were detected in mice that underwent transplantation, at 14 weeks after transplantation (Figure 1A). Long-term development of normal T-cell subsets (33 weeks after transplantation) is also observed in mice that underwent transplantation (Figure 1B), suggesting maintenance of human HSPCs in DKO-hu HSC mice as previously reported.18,19 The HIV-1 entry coreceptors CCR5 and CXCR4 are both expressed on human CD4+ T cells (Figure 1C). While the levels of CCR5 expressed on human CD4 T cells derived from DKO-hu HSC mice are comparable to those from primary human tissues or cells, a reduced expression of CXCR4 is detected on human CD4 T cells from DKO-hu HSC mice.20,21 As in human primary T cells, CD45RO+ T cells predominantly express CCR5, whereas CD45RO− T cells efficiently express CXCR4 (Figure 1C).
Figure 1.
Human T cells express both CCR5 and CXCR4 in DKO-hu HSC mice. Human CD34+ cells were injected intrahepatically in newborn DKO mice. (A) A representative cohort of 12 DKO-hu HSC mice reconstituted with FL CD34+ cells is analyzed by FACS. Individual open bars represent percentage of human CD45+ cells in total peripheral blood lymphoid cells (14 weeks after transplantation). The dark bars indicate the percentage of human CD3+CD4+ cells. (B) Long-term T-cell reconstitution in DKO-hu HSC mice. At 33 weeks after transplantation, the CD4+/CD8+ pattern on human CD45+ cells in the PBMCs, spleen, and thymus are analyzed. (C) Expression of HIV-1 coreceptors on human CD4+ T cells. Representative FACS analysis of CCR5 (top) or CXCR4 (bottom) on human CD45+CD3+CD4+ cells in PBMCs and lymphoid organs of DKO-hu HSC mice. ITTP indicates intrathymic T progenitor (CD4+CD8−CD3+); DP, double positive (CD4+CD8+ thymocytes); CD4SP, CD4+ single positive (CD4+CD8− thymocytes).
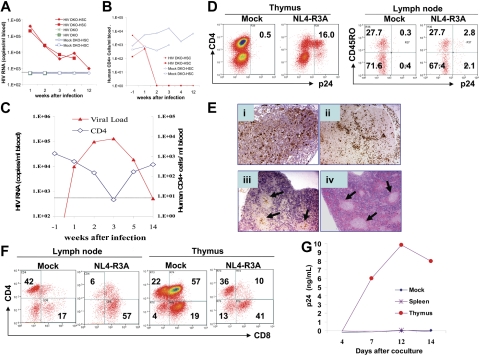
To test HIV-1 infection of DKO-hu HSC mice in vivo, we first used the NL4-R3A recombinant virus with the R5/X4 dual tropic R3A Env. R3A was transmitted in the patient (an intravenous drug user) with rapid disease progression, and infected both macrophages and T cells.22 When inoculated with a high dose (20 000 IU/mouse) of the NL4-R3A virus, DKO-hu HSC mice showed very high plasma viral loads (200 000-400 000 copies/mL) at 1 week after infection and 10 000 copies/mL at 2 to 4 weeks after infection. Interestingly, HIV-1 plasma viremia was still detectable (947 copies/mL) with infectious HIV-1 at 12 weeks after infection (Figure 2A and data not shown). The mock-infected DKO-hu HSC mice or HIV-infected control DKO mice with no human cells showed no detectable HIV-1 genome (Figure 2A and Table 1). In the infected DKO-hu HSC mice, CD4+ T-cell numbers in the blood were significantly depleted at 2, 3, 4, and 12 weeks after infection (Figure 2B).
Figure 2.
HIV-1 replication and pathogenesis in DKO-hu HSC mice. Intravenous infection of HIV-R3A at high (A-B) and low (C-G) doses was performed in DKO-hu HSC mice. (A) NL4-R3A stock (5 ng p24 or 20 000 IU/mouse) was used to infect DKO-hu HSC mice (HIV DKO-HSC, solid diamond and square) and DKO mice (HIV DKO, crosses). Two DKO-hu HSC mice were also mock infected as controls (mock DKO-HSC, open diamond and square). Plasma samples were collected at 1, 2, 3, 4, and 12 weeks after infection and HIV genome copy numbers were determined. The sensitivity of detection is 500 copies/mL due to necessary sample dilution. (B) Human CD4+ T-cell number in the blood of DKO-hu HSC mice was determined at 15 weeks after HSPC transfer (1 week prior to HIV infection, −1 week after infection), and determined again at 1, 2, 3, 4, and 12 weeks after infection. (C) DKO-hu HSC mice were infected with low-dose NL4-R3A (1 ng p24 or 4000 IU/mouse). Plasma HIV genome (triangle, left Y axis) and CD4 cell counts (diamond, right Y axis) in mouse no. 3 (Table 1) are shown. (D) FACS analysis of CD45+ thymocytes by costaining p24 with CD4 or of CD45+CD4+ lymph node cells with CD45RO from mice infected with NL4-R3A (1 week after infection). (E) Immunohistochemistry was performed with anti-p24 antibody on paraffin sections of lymphoid tissues. Lymph node (i), thymus (ii), spleen (iii), and the hematoxylin and eosin (H&E) staining of adjacent section of the spleen (iv). Arrows in subpanels iii and iv indicate the CD45+ follicles in the spleen. Slides were observed under a Nikon Microphot FXA microscope equipped with a PlanApo 4×/0.20 numerical aperture (NA) i-ii) or a PlanApo 2×/0.08 NA (iii-iv) objective lens (Nikon, Garden City, NY). Images were captured using a QImaging Micropublisher 3.3 CCD digital camera and QCapture software version 3.0 (QImaging, Surrey, BC, Canada). (F) Depletion of CD4+ T cells in lymphoid organs. At 1 week after infection, human CD4 and CD8 in the thymus and lymph nodes of mock- or NL4-R3A–infected mice were analyzed by FACS. The percentage of each population is indicated. (G) Thymocytes (4500 CD45+ cells) or splenocytes (17 000 CD45+ cells) from mouse no. 4 (Table 1) harvested at 19 weeks after infection were cocultured with PHA-activated PBMCs to detect infectious HIV-1 (p24/mL). Similar results were observed with cells from mouse no. 3 at 14 weeks after infection.
Table 1.
Summary of HIV-1 infection in the DKO-HSC mice
| Mouse no. | CD34+ cells | Time after transplantation, wk | HIV (ng/mouse)* | HIV RNA per mL blood (CD4/CD8 ratio)† |
||||
|---|---|---|---|---|---|---|---|---|
| −1 wk after infection | 1 wk after infection | 2 wk after infection | 3 wk after infection | 4 wk after infection‡ | ||||
| 1 | CB | 16 | NL4-R3A (5) | ND (6.8) | 4.0 × 105 (0.6) | 2.4 × 104 (0.3) | 3.7 × 103§ | 8.5 × 103§ |
| 2 | CB | 16 | NL4-R3A (5) | ND (1.0) | 2.0 × 103 (0.5) | 2.6 × 104 (0.0) | 6.9 × 103§ | 4.2 × 103§ |
| 3 | CB | 15 | NL4-R3A (1) | ND (3.0) | 9.7 × 103 (2.9) | 9.9 × 104 (1.7) | 1.3 × 105 (0.2) | 1.9 × 104 (3.7) |
| 4 | FL | 19 | NL4-R3A (1) | ND (1.6) | 3.2 × 103 (0.5) | 2.5 × 103 (0.8) | 2.1 × 103 (0.1) | ND§ |
| 5 | FL | 19 | NL4-R3A (1) | ND (1.7) | 4.0 × 103 (1.1) | — | — | — |
| 6 | FL | 41 | NL4-R3A (1) | ND (13.5) | 1.0 × 105 (1.8) | — | — | — |
| 7 | FL | 22 | JR-CSF (1) | ND (4.0) | 1.8 × 104 (5.1) | 2.1 × 105 (2.5) | — | — |
| 8 | FL | 22 | JR-CSF (1) | ND (3.6) | 2.4 × 105 (3.7) | 4.0 × 105 (3.4) | — | — |
| 9 | CB | 17 | NL4-3 (1) | ND (2.7) | 7.8 × 104 (0.9) | 7.6 × 103 (0.8) | — | 8.2 × 104 (0.3) |
| 10 | CB | 17 | NL4-3 (1) | ND (0.9) | ND (1.5) | ND (3.0) | — | — |
| 11 | — | — | NL4-R3A (1) | ND§ | ND§ | ND§ | ND§ | ND§ |
| 12 | — | — | NL4-R3A (1) | ND§ | ND§ | ND§ | ND§ | ND§ |
| 13 | CB | 16 | Mock | ND (1.3) | ND (2.4) | ND (1.7) | ND (3.2) | ND (3.6) |
| 14 | CB | 16 | Mock | ND (1.1) | ND (2.2) | ND (1.7) | — | ND (2.0) |
Time after transplantation refers to initiation of HIV-1 infection.
ND indicates nondetectable, below the detection level (< 500 copies/mL); —, not done.
The NL4-R3A and NL4 were titered on Hela-CD4-LTR-β-gal cell line. For NL4-R3A, 1 ng p24 equals 4000 infection units. For NL4-3, 1 ng p24 equals 500 infection units. JRCSF was titered on U373-MAGI-CCR5E cells, 1 ng p24 equals 20 infection units. (Both cell lines obtained from NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID.)
Percentage of CD4+ cells divided by percentage of CD8+ in the blood.
Mouse no. 3 sample is from 5 weeks after infection.
No human CD45+ cells detected in the blood.
When infected with a lower dose of NL4-R3A (4000 IU or 1 ng/mouse), HIV-1 replication peaked at 2 to 3 weeks after infection, correlated with depletion of CD4+ T cells (Figure 2C). Viremia decreased to low or undetectable levels in the infected DKO-hu HSC mouse, correlated with an increase of CD4+ T cells (Figure 2C). At 19 weeks after infection with no detectable HIV-1 viremia (< 500 copies/mL), infectious HIV-1 was recovered from thymocytes, but not from splenocytes or PBMCs in the mouse (Figure 2G). In the lymphoid organs at 1 week after infection, high levels of productive infection were detected in the thymus, spleen, and LN (Figure 2D–E). Interestingly, both CD45RO+ and CD45RO− T cells are infected (Figure 2D) as in HIV-infected lymphoid organs.23 The CD4+ T cells are also preferentially depleted in the thymus and LN (Figure 2F and Table 1). Efficient HIV-1 infection is also detected with R5-tropic JRCSF and X4-tropic NL4-3 in DKO-hu HSC mice (Table 1).
The DKO-hu HSC mouse will serve as a relevant HIV-1 infection and pathogenesis model. First, the mouse supports efficient and stable development of human T, B, and myeloid cells in central and peripheral lymphoid organs.11,12 Second, HIV-1 infection leads to high and persistent HIV-1 viremia in PB and in lymphoid organs (this report and a recent report24). Third, human CD4+ T cells are depleted by HIV-1 in a dose- and virus-dependent fashion. However, the human antibody or T-cell response is weak in the DKO-hu HSC mouse (L.Z. and L.S., unpublished results and Baenziger et al24). In a recent report, NOD/SCID/IL2Rγ-null mice humanized by cord blood–derived CD34+ cells have been shown to support HIV-1 infection with both CCR5- and CXCR4-tropic HIV-1 isolates for more than 40 days.25 Anti–HIV-1 antibodies are detected only in animals with high levels of viral infection. The DKO-hu HSC mouse, and its improved derivatives, will facilitate development of novel therapeutic strategies for controlling HIV-1 diseases.
Acknowledgments
We thank Dr M. Kondo for providing the mutant mice, H. Su and S. Barbour for technical support, University of North Carolina (UNC)–Center for AIDS Research (CFAR) virology core and the UNC FACS and Division of Laboratory Animal Medicine (DLAM) core facilities for assistance.
This work was supported in part by grants from the National Institutes of Health (CA99 939, AI041 356, and AI/GM 48 407), a 2006 developmental grant from the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR; P30 AI50 410), a Developmental Award from the Southeast Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB), and the American Foundation for AIDS Research (amFAR 106 693) (L.Z.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.Z. and G.I.K. participated in designing and performing the research; L.S. designed the experiments, analyzed data, and wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
L.Z. and G.I.K. contributed equally to this study.
Correspondence: Lishan Su, The Lineberger Comprehensive Cancer Center, CB#7295, UNC-CH, Chapel Hill, NC 27599-7295; e-mail: lsu@med.unc.edu.
References
- 1.Desrosiers RC. Simian immunodeficiency viruses. Annu Rev Microbiol. 1988;42:607–625. doi: 10.1146/annurev.mi.42.100188.003135. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Lord CI, Haseltine W, Letvin NL, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 3.Levy JA. The value of primate models for studying human immunodeficiency virus pathogenesis. J Med Primatol. 1996;25:163–174. doi: 10.1111/j.1600-0684.1996.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 4.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 5.McCune J, Kaneshima H, Krowka J, et al. The SCID-hu mouse: a small animal model for HIV infection and pathogenesis. Annu Rev Immunol. 1991;9:399–429. doi: 10.1146/annurev.iy.09.040191.002151. [DOI] [PubMed] [Google Scholar]
- 6.Su L. HIV-1 pathogenesis and therapeutic intervention in the SCID-hu Thy/Liv mouse: a model for primary HIV-1 infection in the human thymus. Rev Med Virol. 1997;7:157–166. doi: 10.1002/(sici)1099-1654(199709)7:3<157::aid-rmv197>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 8.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817–1827. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick JE. Human stem cell assays in immune-deficient mice. Curr Opin Hematol. 1996;3:405–409. doi: 10.1097/00062752-199603060-00002. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 11.Traggiai E, Chicha L, Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 12.Gimeno R, Weijer K, Voordouw A, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–3893. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 13.Su L, Kaneshima H, Bonyhadi M, et al. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 14.Miller ED, Duus KM, Townsend M, et al. Human immunodeficiency virus type 1 IIIB selected for replication in vivo exhibits increased envelope glycoproteins in virions without alteration in coreceptor usage: separation of in vivo replication from macrophage tropism. J Virol. 2001;75:8498–8506. doi: 10.1128/JVI.75.18.8498-8506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duus KM, Miller ED, Smith JA, Kovalev GI, Su L. Separation of human immunodeficiency virus type 1 replication from nef-mediated pathogenesis in the human thymus. J Virol. 2001;75:3916–3924. doi: 10.1128/JVI.75.8.3916-3924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meissner EG, Coffield VM, Su L. Thymic pathogenicity of an HIV-1 envelope is associated with increased CXCR4 affinity and V5-gp41 dependent-cytopathicity, but not V1/V2-associated CD4 affinity and viral entry. Virology. 2005;336:84–97. doi: 10.1016/j.virol.2005.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meissner EG, Duus KM, Loomis R, D'Agostin R, Su L. HIV-1 replication and pathogenesis in the human thymus. Curr HIV Res. 2003;1:275–285. doi: 10.2174/1570162033485258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chicha L, Tussiwand R, Traggiai E, et al. Human adaptive immune system Rag2−/−{gamma}c−/− mice. Ann N Y Acad Sci. 2005;1044:236–243. doi: 10.1196/annals.1349.029. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski MA, Justement SJ, Catanzaro A, et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–3201. [PubMed] [Google Scholar]
- 22.Meissner EG, Duus KM, Gao F, Yu XF, Su L. Characterization of a thymus-tropic HIV-1 isolate from a rapid progressor: role of the envelope. Virology. 2004;328:74–88. doi: 10.1016/j.virol.2004.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckstein DA, Penn ML, Korin YD, et al. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity. 2001;15:671–682. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- 24.Baenziger S, Tussiwand R, Schlaepfer E, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−{gamma}c−/− mice. Proc Natl Acad Sci U S A. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe S, Terashima K, Ohta S, et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]