Abstract
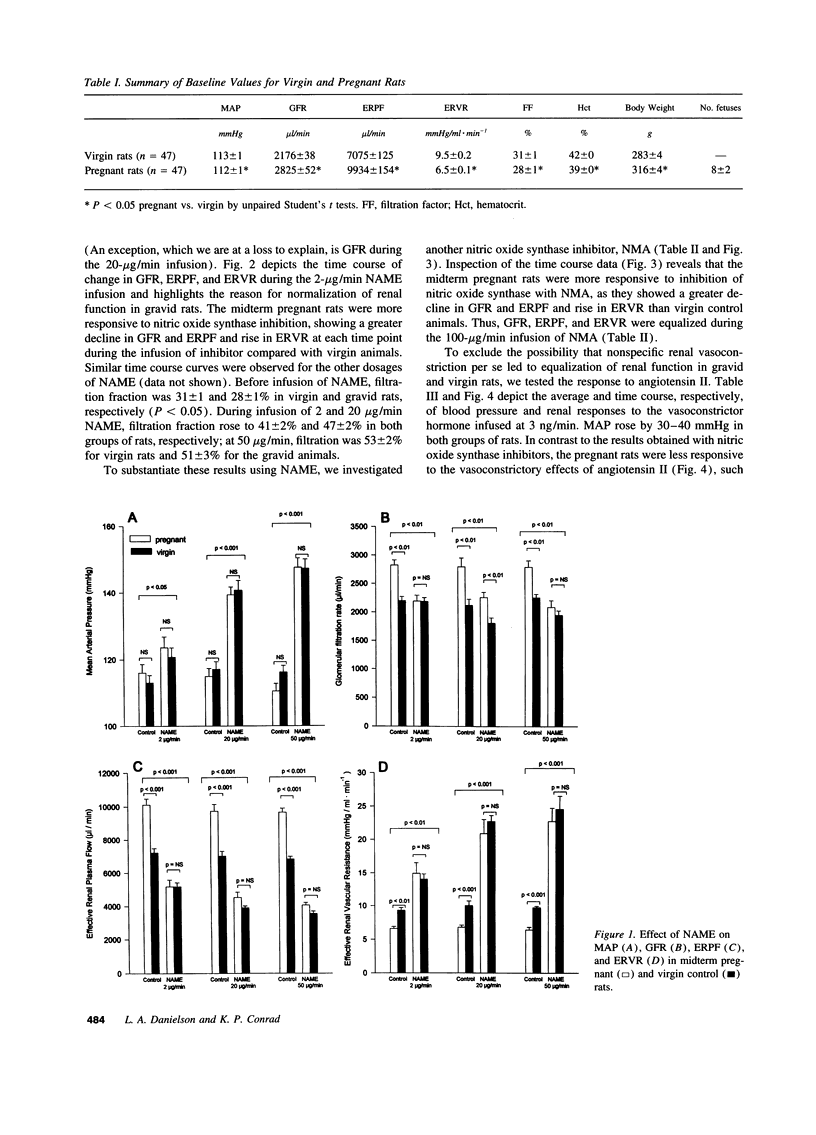
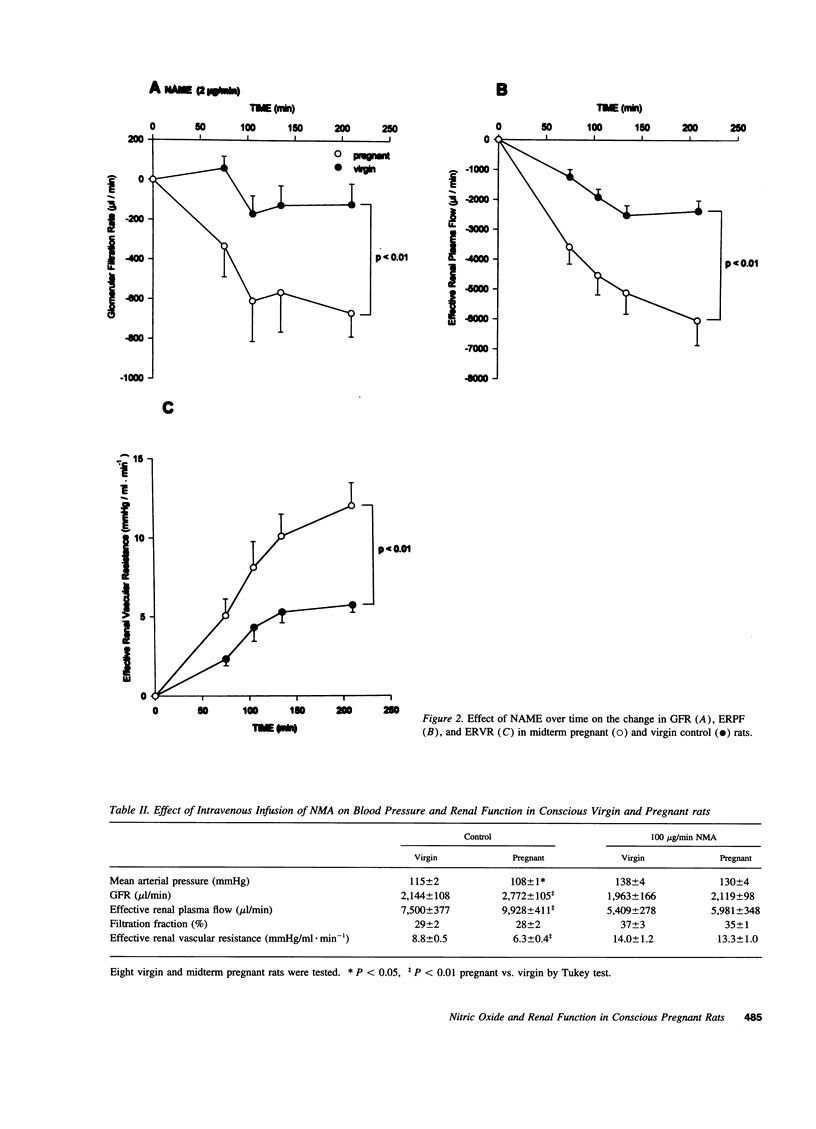
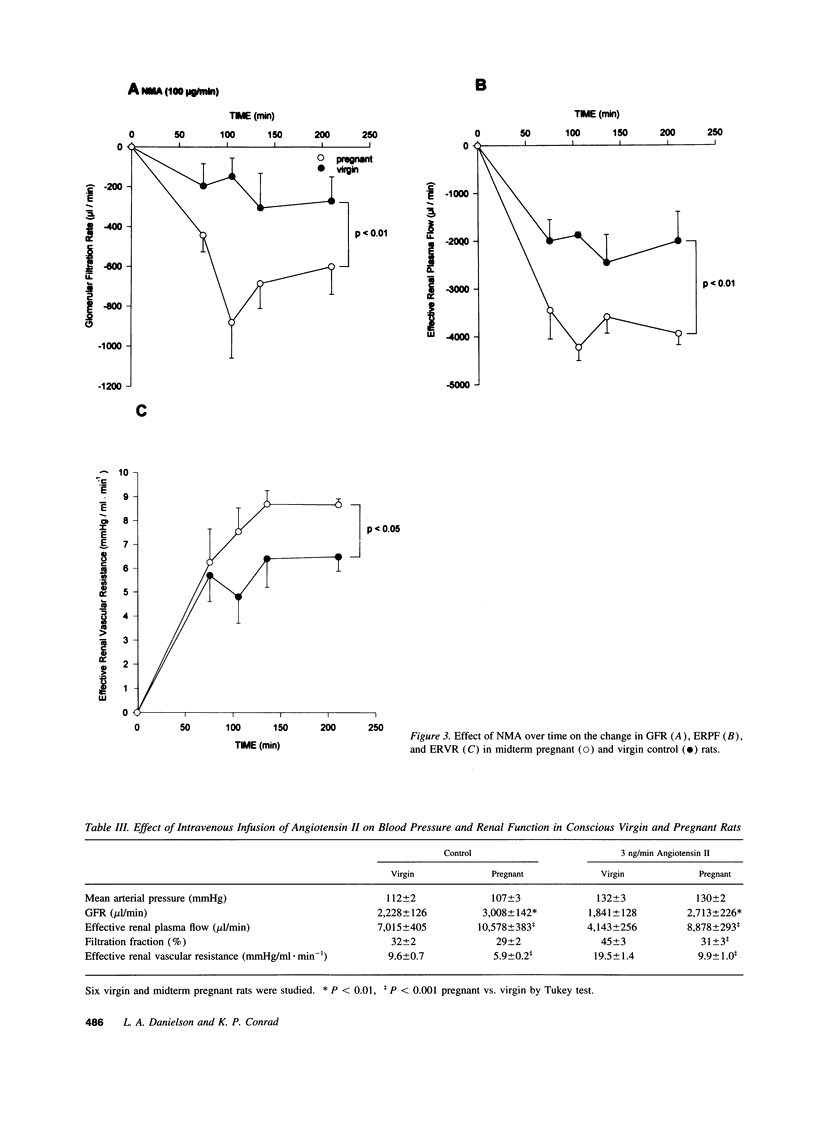
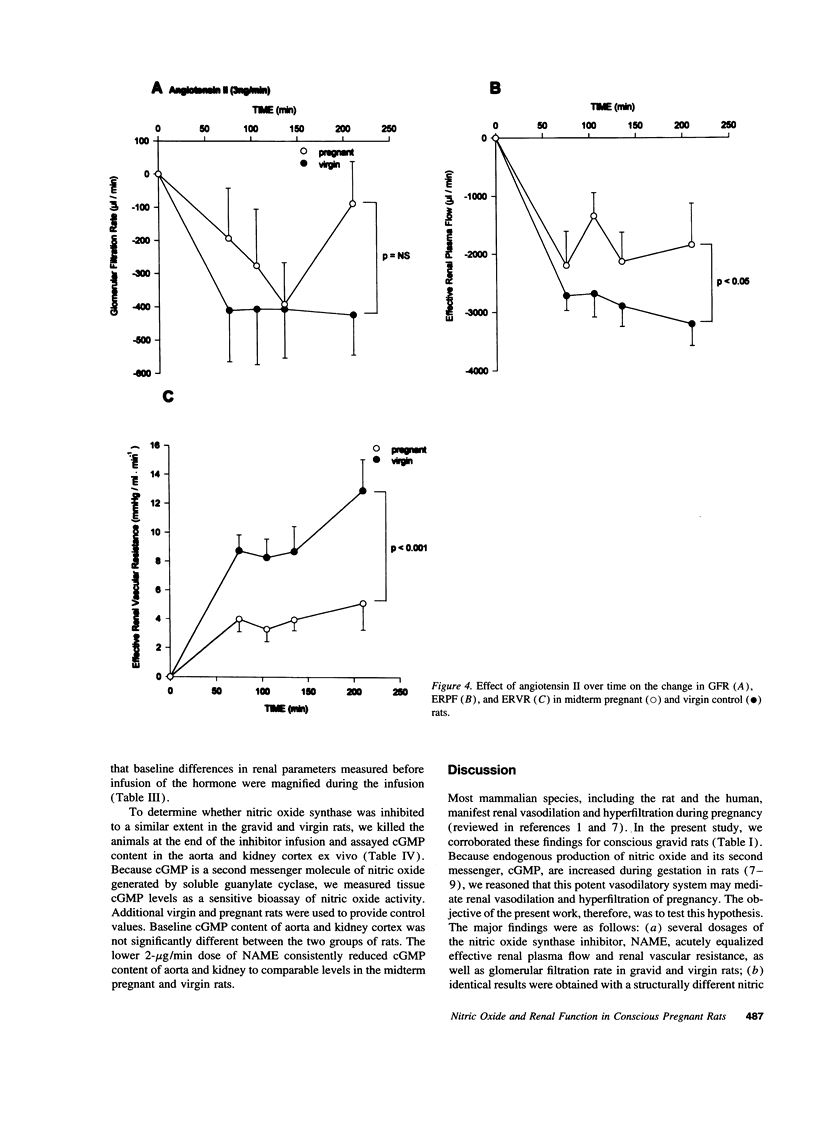
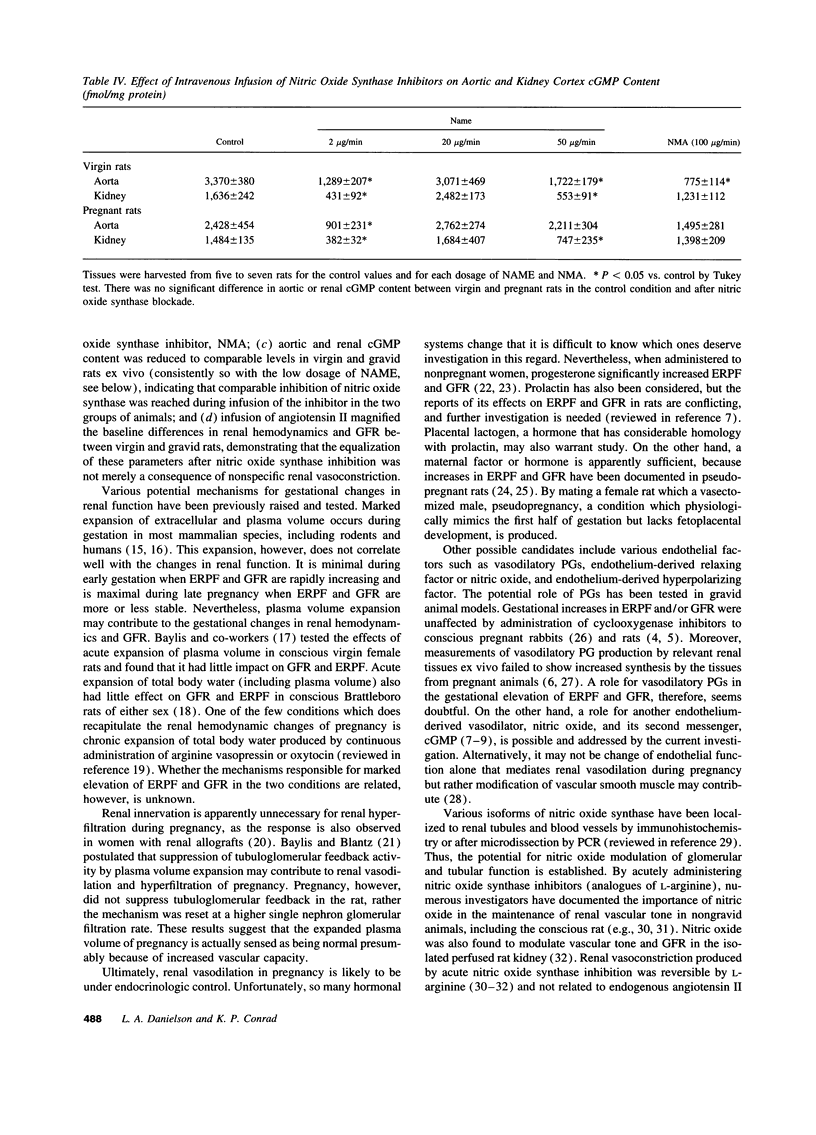
Because the kidneys are vasodilated and the endogenous production of nitric oxide is increased in gravid rats, we tested whether nitric oxide mediates the renal vasodilatory response to pregnancy. Chronically instrumented, conscious rats of gestational days 12-14 were studied concurrently with age-matched virgin control animals. GFR and effective renal plasma flow (ERPF) were determined by the renal clearances of inulin and para-aminohippurate before and during acute infusion of N omega-nitro-L-arginine methyl ester (NAME; 2, 20, and 50 micrograms/min) or NG-monomethyl-L-arginine (100 micrograms/min). Baseline GFR and ERPF were significantly increased, and effective renal vascular resistance was decreased by 30-40% in gravid rats compared with virgin controls. During infusion of all three dosages of NAME and NG-monomethyl-L-arginine, effective renal vascular resistance, GFR, and ERPF were equalized in the pregnant and virgin rats (the only exception being GFR during the 20 micrograms/min NAME infusion). When compared with virgin rats, the gravid animals were more responsive to nitric oxide synthase inhibition, showing a significantly greater decline in GFR and ERPF and rise in effective renal vascular resistance at each timepoint during the infusion of inhibitor. To exclude the possibility that nonspecific renal vasoconstriction per se led to equalization of renal function in the two groups of rats, we investigated angiotensin II. In contrast to the results observed with nitric oxide synthase inhibitors, pregnant rats were less responsive to the renal vasoconstrictory effects of angiotensin II, such that the baseline differences in renal parameters measured before infusion of the hormone were increased during the infusion. To determine whether nitric oxide synthase was inhibited to a similar extent in gravid and virgin rats, aortic and renal cortical cGMP content was assayed ex vivo at the end of inhibitor infusion. The lower 2-micrograms/min dose of NAME consistently reduced cGMP content of these tissues to comparable levels in the two groups of rats. In conclusion, we suggest that nitric oxide mediates reduced renal vascular resistance and hyperfiltration during pregnancy in conscious rats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnal J. F., Warin L., Michel J. B. Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J Clin Invest. 1992 Aug;90(2):647–652. doi: 10.1172/JCI115906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah A. N., Guimarães J. A., Gebara M., Sustovich D. R., Martinez T. R., Camano L. Progesterone increases glomerular filtration rate, urinary kallikrein excretion and uric acid clearance in normal women. Braz J Med Biol Res. 1988;21(1):71–74. [PubMed] [Google Scholar]
- Atherton J. C., Bu'lock D., Pirie S. C. The effect of pseudopregnancy on glomerular filtration rate and salt and water reabsorption in the rat. J Physiol. 1982 Mar;324:11–20. doi: 10.1113/jphysiol.1982.sp014097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J. C., Dark J. M., Garland H. O., Morgan M. R., Pidgeon J., Soni S. Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol. 1982 Sep;330:81–93. doi: 10.1113/jphysiol.1982.sp014330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann S., Mundel P. Nitric oxide in the kidney: synthesis, localization, and function. Am J Kidney Dis. 1994 Jul;24(1):112–129. doi: 10.1016/s0272-6386(12)80170-3. [DOI] [PubMed] [Google Scholar]
- Baylis C., Blantz R. C. Tubuloglomerular feedback activity in virgin and 12-day-pregnant rats. Am J Physiol. 1985 Jul;249(1 Pt 2):F169–F173. doi: 10.1152/ajprenal.1985.249.1.F169. [DOI] [PubMed] [Google Scholar]
- Baylis C., Engels K., Samsell L., Harton P. Renal effects of acute endothelial-derived relaxing factor blockade are not mediated by angiotensin II. Am J Physiol. 1993 Jan;264(1 Pt 2):F74–F78. doi: 10.1152/ajprenal.1993.264.1.F74. [DOI] [PubMed] [Google Scholar]
- Baylis C. Glomerular ultrafiltration in the pseudopregnant rat. Am J Physiol. 1982 Sep;243(3):F300–F305. doi: 10.1152/ajprenal.1982.243.3.F300. [DOI] [PubMed] [Google Scholar]
- Baylis C., Harton P., Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol. 1990 Dec;1(6):875–881. doi: 10.1681/ASN.V16875. [DOI] [PubMed] [Google Scholar]
- Baylis C. Renal effects of cyclooxygenase inhibition in the pregnant rat. Am J Physiol. 1987 Jul;253(1 Pt 2):F158–F163. doi: 10.1152/ajprenal.1987.253.1.F158. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Venuto R. C. Eicosanoid production in rabbit vascular tissues and placentas. Am J Physiol. 1990 Mar;258(3 Pt 1):E418–E422. doi: 10.1152/ajpendo.1990.258.3.E418. [DOI] [PubMed] [Google Scholar]
- CHESLEY L. C., WYNN R. M., SILVERMAN N. I. RENAL EFFECTS OF ANGIOTENSIN II INFUSIONS IN NORMOTENSIVE PREGNANT AND NONPREGNANT WOMEN. Circ Res. 1963 Sep;13:232–238. doi: 10.1161/01.res.13.3.232. [DOI] [PubMed] [Google Scholar]
- Chesley L. C., Tepper I. H. Effects of progesterone and estrogen on the sensitivity to angiotensin II. J Clin Endocrinol Metab. 1967 Apr;27(4):576–581. doi: 10.1210/jcem-27-4-576. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Barrera S. A., Friedman P. A., Schmidt V. M. Evidence for attenuation of myo-inositol uptake, phosphoinositide turnover and inositol phosphate production in aortic vasculature of rats during pregnancy. J Clin Invest. 1991 May;87(5):1700–1709. doi: 10.1172/JCI115187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K. P., Colpoys M. C. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J Clin Invest. 1986 Jan;77(1):236–245. doi: 10.1172/JCI112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K. P., Dunn M. J. Renal synthesis and urinary excretion of eicosanoids during pregnancy in rats. Am J Physiol. 1987 Dec;253(6 Pt 2):F1197–F1205. doi: 10.1152/ajprenal.1987.253.6.F1197. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Gellai M., North W. G., Valtin H. Influence of oxytocin on renal hemodynamics and electrolyte and water excretion. Am J Physiol. 1986 Aug;251(2 Pt 2):F290–F296. doi: 10.1152/ajprenal.1986.251.2.F290. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Gellai M., North W. G., Valtin H. Influence of oxytocin on renal hemodynamics and sodium excretion. Ann N Y Acad Sci. 1993 Jul 22;689:346–362. doi: 10.1111/j.1749-6632.1993.tb55559.x. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Joffe G. M., Kruszyna H., Kruszyna R., Rochelle L. G., Smith R. P., Chavez J. E., Mosher M. D. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993 Apr 1;7(6):566–571. [PubMed] [Google Scholar]
- Conrad K. P. Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models. Am J Kidney Dis. 1987 Apr;9(4):253–259. doi: 10.1016/s0272-6386(87)80118-x. [DOI] [PubMed] [Google Scholar]
- Conrad K. P. Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int. 1984 Jul;26(1):24–29. doi: 10.1038/ki.1984.129. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Vernier K. A. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol. 1989 Oct;257(4 Pt 2):R847–R853. doi: 10.1152/ajpregu.1989.257.4.R847. [DOI] [PubMed] [Google Scholar]
- Davison J. M. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int. 1985 Jan;27(1):74–79. doi: 10.1038/ki.1985.12. [DOI] [PubMed] [Google Scholar]
- Deng A., Baylis C. Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient. Am J Physiol. 1993 Feb;264(2 Pt 2):F212–F215. doi: 10.1152/ajprenal.1993.264.2.F212. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gellai M., Silverstein J. H., Hwang J. C., LaRochelle F. T., Jr, Valtin H. Influence of vasopressin on renal hemodynamics in conscious Brattleboro rats. Am J Physiol. 1984 Jun;246(6 Pt 2):F819–F827. doi: 10.1152/ajprenal.1984.246.6.F819. [DOI] [PubMed] [Google Scholar]
- Gilson G. J., Mosher M. D., Conrad K. P. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1992 Dec;263(6 Pt 2):H1911–H1918. doi: 10.1152/ajpheart.1992.263.6.H1911. [DOI] [PubMed] [Google Scholar]
- Imig J. D., Roman R. J. Nitric oxide modulates vascular tone in preglomerular arterioles. Hypertension. 1992 Jun;19(6 Pt 2):770–774. doi: 10.1161/01.hyp.19.6.770. [DOI] [PubMed] [Google Scholar]
- Lacolley P. J., Lewis S. J., Brody M. J. Role of sympathetic nerve activity in the generation of vascular nitric oxide in urethane-anesthetized rats. Hypertension. 1991 Jun;17(6 Pt 2):881–887. doi: 10.1161/01.hyp.17.6.881. [DOI] [PubMed] [Google Scholar]
- MacGillivray I., Rose G. A., Rowe B. Blood pressure survey in pregnancy. Clin Sci. 1969 Oct;37(2):395–407. [PubMed] [Google Scholar]
- Ohishi K., Carmines P. K., Inscho E. W., Navar L. G. EDRF-angiotensin II interactions in rat juxtamedullary afferent and efferent arterioles. Am J Physiol. 1992 Nov;263(5 Pt 2):F900–F906. doi: 10.1152/ajprenal.1992.263.5.F900. [DOI] [PubMed] [Google Scholar]
- Pucci M. L., Lin L., Nasjletti A. Pressor and renal vasoconstrictor effects of NG-nitro-L-arginine as affected by blockade of pressor mechanisms mediated by the sympathetic nervous system, angiotensin, prostanoids and vasopressin. J Pharmacol Exp Ther. 1992 Apr;261(1):240–245. [PubMed] [Google Scholar]
- Radermacher J., Klanke B., Schurek H. J., Stolte H. F., Frölich J. C. Importance of NO/EDRF for glomerular and tubular function: studies in the isolated perfused rat kidney. Kidney Int. 1992 Jun;41(6):1549–1559. doi: 10.1038/ki.1992.225. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Togashi H., Yoshioka M., Saito H., Yanagida M., Tamura M., Kobayashi T., Yasuda H., Gross S. S., Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992 Mar;70(3):607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuto R. C., Donker A. J. Prostaglandin E2, plasma renin activity, and renal function throughout rabbit pregnancy. J Lab Clin Med. 1982 Feb;99(2):239–246. [PubMed] [Google Scholar]
- Zatz R., de Nucci G. Effects of acute nitric oxide inhibition on rat glomerular microcirculation. Am J Physiol. 1991 Aug;261(2 Pt 2):F360–F363. doi: 10.1152/ajprenal.1991.261.2.F360. [DOI] [PubMed] [Google Scholar]