Abstract
Pulmonary hypertension is a highly prevalent complication of sickle cell disease and is a strong risk factor for early mortality. However, the pathophysiologic mechanisms leading to pulmonary vasculopathy remain unclear. Transgenic mice provide opportunities for mechanistic studies of vascular pathophysiology in an animal model. By microcardiac catheterization, all mice expressing exclusively human sickle hemoglobin had pulmonary hypertension, profound pulmonary and systemic endothelial dysfunction, and vascular instability characterized by diminished responses to authentic nitric oxide (NO), NO donors, and endothelium-dependent vasodilators and enhanced responses to vasoconstrictors. However, endothelium-independent vasodilation in sickle mice was normal. Mechanisms of vasculopathy in sickle mice involve global dysregulation of the NO axis: impaired constitutive nitric oxide synthase activity (NOS) with loss of endothelial NOS (eNOS) dimerization, increased NO scavenging by plasma hemoglobin and superoxide, increased arginase activity, and depleted intravascular nitrite reserves. Light microscopy and computed tomography revealed no plexogenic arterial remodeling or thrombi/emboli. Transplanting sickle marrow into wild-type mice conferred the same phenotype, and similar pathobiology was observed in a nonsickle mouse model of acute alloimmune hemolysis. Although the time course is shorter than typical pulmonary hypertension in human sickle cell disease, these results demonstrate that hemolytic anemia is sufficient to produce endothelial dysfunction and global dysregulation of NO.
Introduction
Pulmonary hypertension is a highly prevalent complication of sickle cell disease that is associated with early mortality.1–4 The putative mechanisms responsible for pulmonary hypertension are the focus of intense current research and remain incompletely defined.5 One mechanism proposed is that hemolytic anemia and decompartmentalization of erythrocyte hemoglobin and arginase into plasma leads to nitric oxide (NO) scavenging and arginine degradation, limiting the bioavailability of NO.3,6–10 This process would ultimately lead to acute changes in pulmonary vascular endothelial and vasomotor function and chronic pathologic intimal and smooth muscle hyperplasia. Alternatively, chronic lung disease caused by recurrent pulmonary infarction, pneumonia, acute chest syndrome, and thromboembolism could lead to chronic hypoxemia, pulmonary fibrosis, thrombotic vascular obliteration, and secondary pulmonary hypertension.11–15 Pulmonary hypertension could also arise from chronic hypoxia or chronic nocturnal hypoxia.16–18 Additional factors contributing to pulmonary hypertension include right-heart failure secondary to a chronic high cardiac output as compensation for chronic anemia and left ventricular diastolic dysfunction secondary to cardiac tissue microinfarction and/or iron overload.19–22 In short, is exposure to hemoglobin S (HbS) erythrocytes sufficient to cause pulmonary hypertension, or are additional pathophysiologic challenges necessary?
Transgenic sickle mouse models now exist that allow for the physiologic, biochemical, and pathologic interrogation of the pulmonary vasculature during different stages of development, allowing us to test the mechanisms responsible for the development of pulmonary hypertension. One such model, also known as the Berkeley mouse, expresses exclusively human HbS generated by knockout of mouse α- and β-globins and insertion of a single transgene that expresses human α- and βS-globin.23–25 This animal model develops severe extravascular and intravascular hemolytic anemia, sickle deformation of erythrocytes, microvascular occlusion following inflammatory or hypoxic stress, vasomotor dysregulation,9,26 multiorgan infarction,25,27 and evidence of chronic inflammation and enhanced oxidative stress.28–31 These sickle mice develop exaggerated pulmonary inflammatory responses to acute hypoxia30 and lipopolysaccharide inflammation.32 However, their baseline cardiac and pulmonary vascular functions have not been examined in detail.
We therefore sought to determine whether pulmonary hypertension develops in this mouse model of severe sickle cell disease and to evaluate the various putative mechanisms associated with the development of pulmonary hypertension, including lung infarction and fibrosis, thromboembolism, and reduced NO bioavailability and endothelial/vasomotor dysfunction. Closed-chest right- and left-heart microcardiac catheterization in live animals detected pulmonary hypertension in all sickle mice studied but not in hemizygous colony controls or wild-type controls. The pulmonary hypertension occurred spontaneously, unlike the need for experimental triggers in other animal models,33–36 and without evidence of thrombosis or major vascular remodeling. The pulmonary hypertension was mediated by a functional impairment in endothelial-dependent vasodilation, NO resistance, and increased vasoconstrictor tone. Additional mice generated by bone marrow transplantation had similar functional vascular abnormalities when receiving transplants with sickle marrow but normal vascular responsiveness when receiving transplants with normal mouse marrow. As additional evidence for a central role of hemolytic anemia in driving this process, acute hemolytic transfusion reaction in mice without HbS was associated with a similar pulmonary vascular endothelial dysfunction. Two mechanisms were identified that could contribute to low bioavailability of NO in the lung vasculature of sickle mice: loss of functional dimerization of endothelial nitric oxide synthase (eNOS) associated with reduced enzymatic activity and hemolysis-related NO scavenging by free hemoglobin in plasma and increased arginase activity. These studies provide important novel insights into the pathogenesis of pulmonary hypertension in human diseases characterized by chronic hemolytic anemia.3,5,8,37–40
Materials and methods
Mouse model of human sickle cell disease
Sickle mice with knockout of all mouse hemoglobin genes and expressing exclusively human sickle hemoglobin were developed at Lawrence Berkeley National Laboratory.23 A breeding colony at the National Institutes of Health (NIH) generated animals for this study by mating sickle male mice to hemizygous females (approximately 15 generations). Because C57BL/6 is one of the background strains for the transgenic sickle mice,23 C57BL/6 was chosen as wild-type control. Additional control animals were hemizygous littermates, which have anemia and increased oxidative stress but no sickle deformation.23,41 All were males ages 3 to 5 months old, except a series of retired breeder males (13 to 15 months old) to determine the effects of aging. Transgenic mice expressing human blood group antigen Duffy b (Fyb) were used as blood donors for B6CBA-F1 mice, which lack human Fyb, as a model for red blood cell alloimmunization.42 Mice were pathogen free and received routine NIH rodent chow and water. Studies were approved by the animal care and use committees of the National Institute of Diabetes and Digestive and Kidney Diseases and of the Johns Hopkins Medical Institute. A breeding colony of transgenic sickle cell mice is maintained at the National Cancer Institute at Frederick (NCI-Frederick), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals.43
Cardiac catheterization with vasoactive challenges
Intact-chest catheterization of mice via the jugular vein was previously described,44 with isoflurane anesthesia and orotracheal intubation for mechanical ventilation plus continuous temperature and electrocardiographic monitoring and intravenous fluids. Baseline hemodynamics were measured using standard methods for cardiac output and pulmonary and systemic vascular resistance.45 Under conditions of controlled blood flow and constant left atrial pressure, the thromboxane A2 analog U-46619 was infused intravenously to increase basal vascular tone to a uniform pulmonary arterial pressure of 30 mm Hg as a reference point prior to each vasodilator challenge, as previously described.46 Mice then had measurements taken of pulmonary vascular responsiveness to several pharmacologic agents (1) to assess NO resistance and the NO axis (authentic inhaled NO, infusion of the NO donor sodium nitroprusside, and infusion of the phosphodiesterase-5 inhibitor sildenafil); (2) to examine other endothelium-dependent vasodilators (infusion of bradykinin and adrenomedullin); (3) to examine endothelium-independent vasodilation (calcitonin gene-related peptide [CGRP]); and (4) to examine pulmonary vasoconstriction (infusion of norepinephrine or angiotensin II or 10 of minutes inhalation of 10% oxygen [balanced with nitrogen]) to determine pulmonary hypoxic vasoconstriction response.
Generating chimeric mice by bone marrow transplantation
C57BL/6 mice received transplants with whole bone marrow (5 × 106 cells per recipient) after myeloablative irradiation (900 cGy) and 4 daily intraperitoneal injections of recombinant human erythropoietin ([rHuEPO] 300 U/kg; Amgen, Thousand Oaks, CA) to promote erythropoiesis. Twelve mice received marrow from sickle mice, and 5 mice received marrow from C57BL/6 donors. Ninety percent survived to erythroid engraftment (HbS exceeding 90% of the total hemoglobin) at 10 weeks after transplantation and proceeded to cardiac catheterization at 14 to 15 weeks after transplantation (age 5 months).
Generating alloimmune hemolysis in mice
After being bled 200 μL, B6CBA-F1 mice received 2 weekly immune-sensitizing blood transfusions from transgenic mice expressing the human Duffy b antigen (Fyb), as previously described.42 A subsequent transfusion of 300 μL transgenic Fyb blood into these alloimmunized mice triggered immune destruction of 70% of the transfused erythrocytes over the next 3 days,42 and during this period of acute hemolysis these mice went to microcardiac catheterization.
Chest computed tomography scans in vivo
High-resolution micro–computed tomography (micro-CT) (Imtek, Knoxville, TN) obtained images in vivo with respiratory gating (Biopac Systems, Goleta, CA) in mice anesthetized but maintaining spontaneous respiration, as previously described.47
Lung homogenate assays
After right-heart perfusion with saline to wash away blood, mouse lung and pulmonary arteries were frozen in liquid nitrogen and pulverized. Resulting tissue powder was combined 1:4 (wt/vol) with ice-cold buffer (20 mM HEPES [pH 7.4] and 0.25 M sucrose) and homogenized on ice in the presence of protease inhibitors (PMSF, leupeptin, aprotinin). The homogenate was centrifuged at 3000g for 20 minutes, and the supernatant was removed for arginase activity assay,48–50 nitric oxide synthase activity by radiolabeled l-arginine to l-citrulline conversion,51,52 and nitrotyrosine assay (Oxis International, Foster City, CA).
NOS dimer-monomer ratio by Western blot
Sodium dodecyl sulfate (SDS)–resistant eNOS dimers and monomers were assayed using low-temperature SDS–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions, as previously described.53 The eNOS was immunoprecipitated as previously described,54 and the resulting samples were added to Tris glycine 6% gels (Invitrogen, Carlsbad, CA) without 2-mercaptoethanol. To provide fully denatured controls, samples were boiled for 15 minutes prior to loading. Electrophoresis was performed in an ice bath at 4°C, and the gel was stained (SimplyBlue; Invitrogen) and destained with water.
Statistical analysis
Data are reported as mean ± SEM and were analyzed using paired t tests unless otherwise specified. A P value of less than .05 was used as the criterion for statistical significance.
Results
Sickle cell mice develop spontaneous pulmonary hypertension and right-heart failure
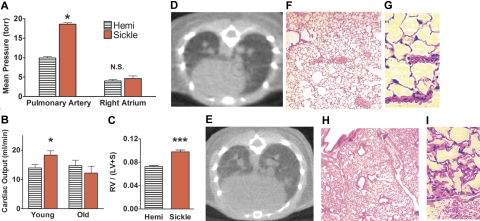
Pulmonary hypertension was detected in all sickle mice, with pulmonary arterial pressures nearly double those of age-matched hemizygous controls (P < .05; Figure 1A). Likewise, pulmonary vascular resistance of sickle mice was twice that of hemizygous controls (P < .05; Table 1). Right atrial pressures of young adult sickle mice and hemizygotes were not significantly different (Figure 1A). Although known to have abnormal hematology and kidneys,41,57 hemizygotes did not differ from wild-type mice in baseline hemodynamics (data not shown). Despite the observed increases in pulmonary artery pressures, the systemic blood pressure did not differ between sickle, hemizygote, and wild-type mice. Elevated cardiac output was expected as a physiologic compensation for severe anemia, and this was a consistent finding in the younger sickle cell mice (Figure 1B). Old sickle mice (aged 13 to 15 months) were similarly anemic but did not have elevated cardiac output, indicating progressive heart failure and loss of the critical physiologic compensation for severe anemia (P < .05 for cardiac output in young versus old sickle mice; Table 1). Accordingly, old sickle mice developed increased right atrial pressure consistent with progressive right-heart failure (P < .05). To determine the mechanisms of elevated pulmonary vascular resistance in sickle mice independent of effects of heart failure, the rest of this study focused on the younger adult mice. Right ventricular hypertrophy in younger sickle mice was evident by a Fulton ratio of right ventricular weight to left ventricle including septum (Figure 1C), not previously assessed in these mice. Overall cardiomegaly was clearly documented on micro-CT of hemizygous control versus sickle mice (Figures 1D–E), consistent with previous histologic reports.23,25
Figure 1.
Pulmonary artery pressures were elevated in sickle mice and associated with decreasing cardiac output and right-heart failure with advancing age. (A) Elevated pulmonary artery pressures in younger sickle mice (3 to 5 months old, n = 7) were associated with normal right atrial pressures. (B) Elevated cardiac output in younger sickle mice contrasted with low cardiac output in older sickle mice (13 to 15 months old, n = 4). (C) The Fulton ratio of ventricular weights (right ventricle–left ventricle including septum) indicated disproportionately heavier right ventricles in sickle mice than in hemizygous controls (n = 11 and 16, respectively). Hearts were excised and sectioned using the method of Fulton55,56 from younger mice not used for other cardiac studies. (D) Thoracic micro-CT of the hemizygous control in vivo demonstrated normal heart size, lung vasculature, and lung aeration. (E) CT in vivo of the sickle mouse at the same axial level showed an enlarged heart. Lungs were very similar to normal, except for dilated pulmonary vessels and mildly increased attenuation with a speckled pattern consistent with perfusion heterogeneity (n = 11). (F) Hemizygous control mouse lung histology at low magnification had normal lung parenchyma and vascularity and (G) at high magnification demonstrated normal thin alveolar capillaries. (H) Sickle mouse lung histology at low magnification confirmed the radiologic findings of dilated pulmonary vessels and patchy vascular congestion. However, pulmonary arterial walls were not significantly thickened, and no plexiform lesions were observed. (I) Histology at high magnification showed engorged alveolar capillaries. No sickle mouse of any age had intra-alveolar edema, fat and/or bone marrow emboli, thromboemboli, pulmonary infarcts, or significant interstitial fibrosis. *P < .05 versus age-matched hemizygous control. ***P < .001 versus age-matched hemizygous control. CT scans (panels D-E) acquired by MicroCAT II (Imtek, Knoxville, TN) were processed by image analysis software (Amira 3.0, TGS Inc, San Diego, CA) and formatted using Adobe Photoshop (Adobe Systems, San Jose, CA). Photomicrographs of lung sections stained with hematoxylin and eosin (panels F-I) were visualized using an Olympus IX70 microscope equipped with UPlanFl 10×/0.30 numerical aperture (NA) and LCPlanFl 40×/0.60 NA PH2 objective lenses (Olympus, Melville, NY); images were acquired by Spot Flex digital camera (Spot Diagnostic Instruments, Sterling Heights, MI) with Spot 3.02 application software, and were formatted using Adobe Photoshop (Adobe Systems, San Jose, CA) and ImageJ software (National Institutes of Health, Bethesda, MD).
Table 1.
Hemodynamics of sickle mice show pulmonary hypertension and age-related heart failure; n = 4 to 7 per group
| Mice 3 to 5 mo of age |
Mice 13 to 15 mo of age |
|||
|---|---|---|---|---|
| Hemizygous | Sickle | Hemizygous | Sickle | |
| Pulmonary arterial pressure, mm Hg | ||||
| Systolic | 12.7 ± 0.8 | 23.0 ± 0.6 | 13.9 ± 0.7 | 22.2 ± 0.5 |
| Diastolic | 6.8 ± 0.8 | 15.3 ± 0.7 | 8.1 ± 0.4 | 13.8 ± 0.7 |
| Pulmonary vascular resistance, mm Hg/mL/min | 0.37 ± .05 | 0.80 ± .07* | 0.36 ± .06 | 0.75 ± .04* |
| Mean right atrial pressure, mm Hg | 4.1 ± 0.3 | 4.3 ± 0.4 | 4.0 ± 0.9 | 6.5 ± 0.8*† |
| Cardiac output, mL/min | 13.7 ± 1.9 | 17.1 ± 2.3* | 14.7 ± 1.2 | 12.2 ± 2.1*† |
| Total peripheral resistance, mm Hg/mL/min | 5.8 ± 0.5 | 4.1 ± 0.6 | 5.7 ± 0.8 | 7.2 ± 0.6* |
| Systemic systolic pressure, mm Hg | 103 ± 5 | 99 ± 9 | 109 ± 7 | 119 ± 12 |
| Systemic diastolic pressure, mm Hg | 76 ± 6 | 71 ± 8 | 81 ± 6 | 87 ± 8 |
| Mean systemic arterial pressure, mm Hg | 83 ± 6 | 74 ± 8 | 88 ± 8 | 94 ± 9 |
| Heart rate, bpm | 600 ± 29 | 639 ± 30 | 605 ± 24 | 611 ± 28 |
Numbers are mean ± SEM; P < .05 by t test. Statistically significant results by t test are denoted as
P < .05 for sickle versus age-matched hemizygotes and
P < .05 for young versus old mice.
Histologic and CT scan studies suggest a functional rather than structural etiology of pulmonary hypertension in sickle mice
Due to early observations that sickle cell pulmonary hypertension correlated with progressive fibrosis and restrictive chronic lung disease,14 histologic scoring by light microscopy was performed on sickle mice and hemizygous controls by 2 pathologists not aware of age or genotype. Thickness of the medial layer was evaluated in large and small arteries.
Hemizygous controls had normal lung histology (Figure 1F–G). The lungs of sickle mice (Figure 1H–I) did not differ significantly in any histologic feature, except for the elevated intravascular leukocytes (Table 2; P < .01), which is consistent with the baseline leukocytosis previously described in the sickle mice.23,27 Importantly, we detected no in situ or embolic thrombosis. Pulmonary arteries did not have significantly thicker walls in the sickle mice compared with hemizygous controls (Table 2), and wall thicknesses of young and old sickle mice did not significantly differ (data not shown). Interstitial fibrosis was not seen in sections stained with hematoxylin and eosin, and collagen stain with Masson trichrome revealed only mild and scattered fibrosis (data not shown). The characteristic lesions seen in advanced pulmonary arterial hypertension of humans, such as vascular obliteration or plexogenic vascular lesions, were not observed in the sickle mice. These histologic findings were consistent with chest CT in vivo of the sickle mice as previously described47 and with reports that the mixed restrictive and obstructive lung function abnormalities seen commonly in patients with sickle cell disease have no correlation with pulmonary hypertension.4 Vascular congestion of large and small pulmonary vessels (Figure 1) may suggest that abnormal hemorheology and adherent sickle erythrocytes contributed in part to the pulmonary hypertension. However, the impaired vascular responsiveness described in human sickle cell disease6,58 and in the cremasteric microcirculation of sickle mice9,59 made it logical to extend the hemodynamic studies to examine pulmonary vascular responsiveness in these sickle mice.
Table 2.
Lung histologic features of pulmonary hypertension
| Feature | Sickle mice | Hemizygous mice |
|---|---|---|
| Number of mice | 8 | 9 |
| Histology score | ||
| Thrombi, emboli | 0 | 0 |
| Plexiform vascular lesion | 0 | 0 |
| Vascular congestion | 3.9 ± 0.8 | 3.0 ± 0.8 |
| Capillary dilation | 3.6 ± 1.0 | 2.4 ± 0.7 |
| Large vessel dilation | 3.2 ± 1.0 | 2.7 ± 0.9 |
| Intravascular leukocytes | 3.1 ± 0.6* | 1.3 ± 0.2 |
| Intra-alveolar leukocytes | 1.0 ± 0.5 | 0.2 ± 0.2 |
| Arterial wall thickness, μm | ||
| Large pulmonary arteries | 14.3 ± 1.2 | 12.6 ± 0.8 |
| Small pulmonary arteries | 2.8 ± 0.3 | 2.4 ± 0.3 |
Histology scores range from 0 to 6; numbers are mean ± SEM.
P < .01 by t test.
Pulmonary hypertension was associated with a global and specific impairment in the pulmonary vascular responsiveness to NO in vivo
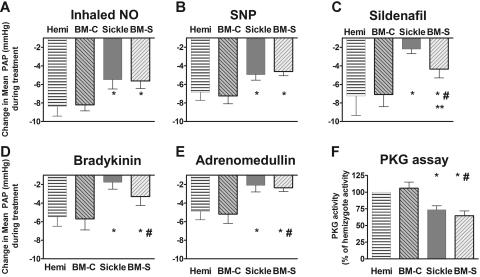
Each group of young adult mice received a series of challenges with vasoactive agents to determine responsiveness to authentic inhaled NO, the responsiveness to NO donors, and responsiveness to other endothelium-dependent vasodilators sharing the cyclic guanosine monophosphate (cGMP)–dependent signaling pathway (Figure 2). In these studies, the mice first received the thromboxane A2 analog U46619 to set a high pulmonary vascular tone in order to provide a constant baseline for vasodilation comparisons. Administrations of inhaled NO gas and the NO donor, sodium nitroprusside, were associated with blunted vasodilator responses (Figures 2A–B). Vasodilation following exposure to sildenafil, a phosphodiesterase-5 inhibitor that increases cGMP half-life and augments NO-dependent vasodilation, was also blunted (Figure 2C), suggesting a reduction in basal NO bioavailability. Consistent with this observation and with the dominant contribution of NO to endothelium-dependent vasodilation, vasodilators dependent on endothelial function (bradykinin and adrenomedullin) also elicited blunted vasodilation in the sickle mouse (Figure 2D–E), indicating endothelial dysfunction. Furthermore, lung tissue cGMF–dependent protein kinase (PKG) activity was impaired in the sickle mice under basal conditions (Figure 2F). This observation and the reduced responsiveness to sildenafil (Figure 2C) suggest a reduction in steady-state activation of the NO-soluble guanylate cyclase–cGMP–PKG pathway. In each of these tests, the hemizygous mice were similar to wild-type mice (data not shown).
Figure 2.
Sickle mice and mice receiving transplants with marrow from sickle mice (BM-S) exhibit blunted pulmonary vasodilatory responses to NO and endothelium-dependent vasodilators, showing mean ± SEM for 5 or 6 mice per group. Responses to each vasodilator challenge are shown by the magnitude of drop in mean pulmonary artery pressure (PAP). (A) Sickle mice demonstrated less decrease in pulmonary arterial pressure in response to inhaled NO (data shown for 4 ppm). A similarly blunted response was observed at a lower NO dose (0.4 ppm, P < .05, data not shown). There was no systemic response to inhaled NO (not shown), consistent with the very brief half-life of NO in blood. (B) The NO donor sodium nitroprusside (10 μg/kg intravenous bolus) was similar to inhaled NO in the blunted pulmonary vasodilator response in sickle mice and BM-S mice compared with controls. Similar blunted responsiveness was observed at lower doses (3 μg/kg intravenous bolus, P < .05, data not shown). (C) The phosphodiesterase-5 inhibitor sildenafil (30 μg/kg/min), which acts through the cGMP pathway like NO, had blunted pulmonary vasodilatory responses in sickle mice. BM-S mice preserved more vasodilatory responsiveness to sildenafil than did sickle mice but were still significantly less responsive than controls (P < .05). (D) Bradykinin (3 μg/kg intravenous bolus) response was also blunted in the sickle mice. Similar results were observed at lower doses (0.3 and 1μg/kg, P < .05, data not shown). (E) Pulmonary vascular response to another endothelium-dependent vasodilator, adrenomedullin (0.3 μg/kg intravenous bolus), was likewise blunted in sickle mice and in BM-S mice. Similar results were observed at lower doses (0.1 μg/kg intravenous bolus, P < .05, data not shown). (F) Lung homogenate assays demonstrated significantly lower activity of cGMP-dependent protein kinase in mice with circulating sickle erythrocytes compared with wild-type and hemizygous controls (4 to 7 mice per group) using a colorimetric assay (CycLex, Nagano, Japan)52,60 according to followed manufacturer's specifications. Statistically significant differences, P < .05 by t test, are indicated as follows: *, versus hemizygous controls, #, versus recipients of marrow from wild-type mice (BM-C); **, sickle versus BM-S mice.
To rule out a major pathophysiologic contribution from the background strains of these transgenic sickle mice, wild-type C57BL6 mice were converted to the sickle hematologic phenotype using bone marrow transplantation of sickle mouse marrow (BM-S mice). Comparisons of these BM-S mice and their controls (BM-C mice) are discussed in “Transplantation of sickle bone marrow.”
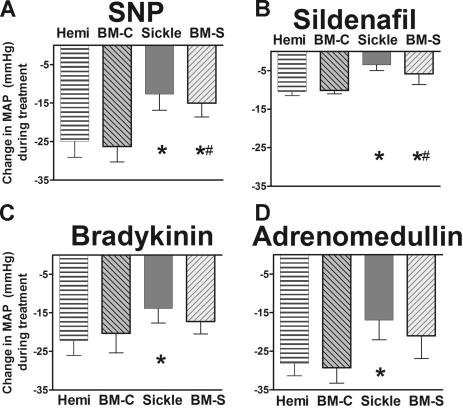
A similar state of endothelial dysfunction was observed in the systemic vasodilation, characterized by resistance to authentic NO, NO donors, and endothelium-dependent vasodilation (Figure 3A–D). These parallel findings in the systemic as well as the pulmonary circulation indicate that the endothelial dysfunction in sickle mice is global and not a specific deficit in only the lungs.
Figure 3.
Systemic responsiveness to endothelium-dependent vasodilators was blunted in sickle mice and BM-S mice, showing mean ± SEM for 5 or 6 mice per group. (A) NO donor sodium nitroprusside (10 μg/kg intravenous bolus) had blunted systemic vasodilator response in sickle mice and BM-S mice compared with controls. Similar blunted responsiveness was observed at a lower dose (3 μg/kg intravenous bolus, P < .05, data not shown). (B) Sildenafil (30 μg/kg/min) had significantly blunted systemic vasodilatory responses in sickle mice and in BM-S mice compared with controls. (C) Bradykinin had blunted mean arterial pressure response in the sickle mice at 3 μg/kg intravenous bolus. Similar results were observed at lower doses for BM-S (0.3 and 1 μg/kg, P < .05 and <.05, respectively; data not shown). Mixed response was observed at lower doses for sickle mice (0.3 and 1 μg/kg, P = NS and P < .05, respectively; data not shown). (D) Response to another endothelium-dependent vasodilator, adrenomedullin (1 μg/kg intravenous bolus) was likewise blunted in sickle mice but not in BM-S mice. Neither type of mouse was significantly different from controls at a lower dose of adrenomedullin (0.3 μg/kg, P = NS, data not shown). Statistically significant results noted as follows: *P < .05 versus hemizygotes; #P < .05 compared with mice receiving transplants with wild-type marrow (BM-C).
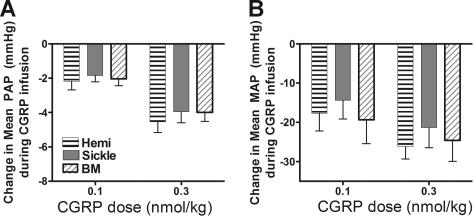
In contrast, sickle mice had normal responsiveness to CGRP, which vasodilates through cyclic adenosine monophosphate pathways rather than endothelial cyclic guanosine monophosphate or NO61 (Figure 4). Preservation of this response indicated that the sickle mice were still capable of vasodilation in both pulmonary and systemic vasculature but had a specific resistance to NO-mediated pathways of vasodilation.
Figure 4.
Preserved vasodilator responses to CGRP. CGRP was infused as an intravenous bolus (0.1 or 0.3 nmol/kg) to probe whether endothelium-independent vascular regulation remained normal. The responses of sickle mice and BM-S mice were no different from hemizygous controls. Pulmonary responsiveness (A) was mirrored by systemic responsiveness (B) in 5 to 6 mice per group. Numbers represent mean ± SEM.
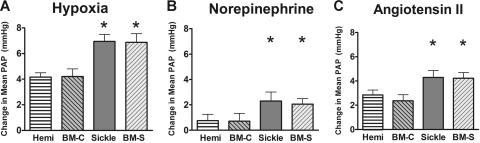
Augmented vasoconstrictor responses were demonstrated in the sickle mice after challenge with acute hypoxia, norepinephrine, and angiotensin II (Figure 5A–C, respectively). The increased change in pulmonary arterial pressure in sickle mice is consistent with a dominant vasoconstrictor phenotype likely produced by the decrease in NO-dependent vasomotor tone.
Figure 5.
Enhanced responses to pulmonary vasoconstrictors. The vasoconstrictor challenges triggered enhanced pulmonary arterial pressure in the sickle mice and BM-S mice. (A) Hypoxic pulmonary vasoconstrictor response. Acute exposure to 10% oxygen increased pulmonary artery pressure in both sickle and mice receiving transplants with marrow from sickle mice compared with hemizygous controls. (B) Norepinephrine (0.3 μg/kg/min) was associated with increased pulmonary artery pressure in both sickle mice and BM-S mice compared with controls. This difference was also observed at other doses of norepinephrine (0.1 and 1.0 μg/kg/min, P < .05 for both, data not shown). (C) Angiotensin II (0.3 μg/kg/min) was also associated with increased pulmonary artery pressure in sickle mice and BM-S mice compared with controls. Similar results were observed at a lower dose (0.1 μg/kg intravenous bolus, P < .05, data not shown). Statistically significant differences from hemizygous controls are noted by *P < .05. Numbers represent mean ± SEM for n = 4-7 per group.
Transplantation of sickle cell bone marrow into strain controls produces pulmonary hypertension in recipients
To more rigorously demonstrate that the pulmonary hypertension was associated with the presence of HbS-containing erythrocytes rather than nonhematologic genetic factors in the background of the sickle mice, we performed bone marrow transplantation from sickle mice and wild-type mice into wild-type recipients prepared with myeloablative irradiation. Transplantation also provides additional control animals to compare with the vascular responsiveness of hemizygous mice, which differ from wild-type mice in many respects, including a high hemoglobin oxygen affinity.41,57 Mice engrafted by 2½ months after transplantation, with peripheral blood counts identical to the sickle mouse donors and demonstrating more than 90% HbS by hemoglobin electrophoresis. Cardiac catheterization was performed in all mice that survived transplantation, at a similar age as the mice that did not receive transplants. Control mice that received transplants with bone marrow from wild-type donors did not have hemolytic anemia or erythrocytes with HbS and had normal pulmonary and systemic blood pressures and normal NO-dependent vascular responsiveness. In contrast, recipients of marrow from sickle mice had similar hemodynamic findings as the transgenic sickle cell mice with lifelong exposure to sickle erythrocytes. Pulmonary vasodilation (Figure 2A–E) and systemic vasodilation (Figure 3) in response to inhaled NO, sodium nitroprusside, sildenafil, bradykinin, and adrenomedullin were blunted for mice receiving transplants with marrow from sickle mice compared with mice receiving transplants with marrow from wild-type mice and lifelong hemizygous mice. Mice receiving transplants all preserved normal responsiveness to CGRP (Figure 4), again linking exposure to HbS erythrocytes with specific resistance to NO pathways of vasodilation, not global resistance to vasodilation. Enhanced responsiveness to vasoconstrictor challenges (acute hypoxia, norepinephrine, and angiotensin II) in mice receiving transplants with sickle marrow was also similar to the lifelong sickle mice (Figure 5).
Evidence for eNOS monomerization and functional uncoupling in the sickle mouse pulmonary vasculature
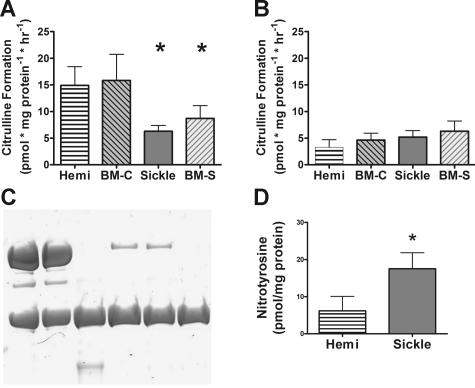
We next explored a number of potential mechanisms of endothelial dysfunction at the level of NO production from eNOS and inducible NO synthase (iNOS). Lung tissue from sickle mice had significantly lower constitutive NO synthase activity (eNOS) assayed using the calcium-dependent ornithine to citrulline activity assay (Figure 6A). Calcium-independent ornithine to citrulline activity (iNOS) was similar in all of the mice studied (Figure 6B). Western blotting under nondenaturing conditions demonstrated that the sickle mice had decreased active eNOS dimer versus inactive monomer (ratio, 1:10) compared with hemizygous and control mice (ratio, approximately 3:2) (Figure 6C). Such an observation is consistent with functional uncoupling of eNOS by monomerization. Further scavenging reactions of NO with superoxide derived from uncoupled eNOS, xanthine oxidase,28 or NADPH oxidase62 were supported by increases in the measured levels of nitrated tyrosine residues in lung homogenate (Figure 6D). Such tyrosine nitration can arise from NO reactions with superoxide to form peroxynitrate or by nitrite-myeloperoxidase reactions.
Figure 6.
Mechanisms of abnormal NO synthase activity. Lung homogenate analysis is shown for 4 to 7 mice per group. (A) Lung NO synthase activity in the presence of calcium (constitutive NOS activity in the lung, the vast majority of which is eNOS) was decreased in sickle mice. (B) Calcium-independent citrulline formation (iNOS activity) did not differ between groups. (C) Western blot under nondenaturing conditions demonstrated 280 kDa eNOS dimer (active form) and 140 kDa eNOS monomer, which is inactive.53,54 Hemizygous mice had slightly more eNOS dimer than monomer, but sickle mice had almost completely lost dimerized eNOS. Positive controls show eNOS dissociated completely to monomeric form by boiling. Images were acquired by scanning the gel (Microtek [Carson, CA] i700 flatbed scanner with a transparency adapter) and saved without processing as a “tiff” image and then converted to “jpg” using Adobe Photoshop CS. (D) Lung nitrotyrosine, evidence of NO scavenging by reaction with superoxide or nitrite myeloperoxidase, was elevated in sickle mice and nitrotyrosine when measured by colorimetric assay (Oxis International) following the manufacturer's specifications. Statistically significant results indicated as follows: *P <.05 versus hemizygote; #P < .05 versus mice receiving transplants with wild-type marrow (BM-C). Numbers represent mean ± SEM.
Increased NO consumption in sickle mice
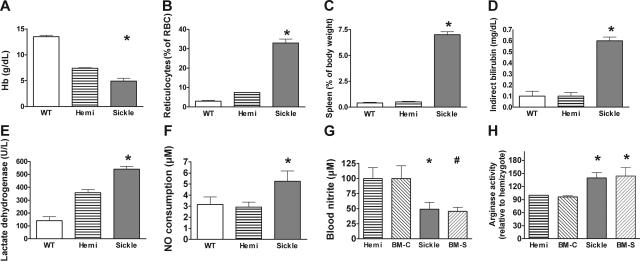
In humans with sickle cell disease, intravascular hemolysis releases erythrocyte hemoglobin and arginase into plasma. Plasma hemoglobin is maintained in a ferrous oxidation state (ferrous oxyhemoglobin), which reacts with NO in a nearly diffusion-limited dioxygenation reaction to form nitrate. This mechanism of NO consumption severely reduces NO bioavailability in patients with sickle cell disease.6 Plasma arginase further degrades arginine, the substrate for NO synthesis.8 Consistent with these mechanisms contributing to the observed resistance state to NO (Figure 2) and reduced L-arginine to L-citrulline conversion rates (Figure 6A), the sickle cell mice exhibited severe hemolytic anemia, including severe anemia, reticulocytosis, splenomegaly (a site of erythropoiesis in mice), elevated bilirubin, lactate dehydrogenase, and cellfree hemoglobin in plasma23,27 (Figure 7A–E). Plasma was assayed for its ability to instantaneously scavenge NO,6 and the sickle cell mouse exhibited a higher NO consumption activity by plasma under baseline conditions compared with controls (P < .01 by Kruskal-Wallis test comparing mice of all 3 hemoglobin types [Figure 7F]). Plasma NO consumption correlated with plasma oxyhemoglobin concentration determined by spectroscopy (r2 = 0.90, data not shown), just as in human sickle cell disease.6 Also consistent with reduced intravascular NO bioavailability, the whole blood nitrite (NO2−) concentration, a biomarker of eNOS-derived NO production63,64 and considered an intravascular endocrine reservoir of NO,65,66 was significantly decreased in sickle cell mice (Figure 7G). Consistent with increased arginase release into plasma,8 the arginase activity in lung homogenate was significantly elevated in the sickle mice and the mice receiving transplants with sickle bone marrow (Figure 7H).
Figure 7.
Laboratory indicators of hemolytic anemia. Mean ± SEM are shown for 6 to 10 animals of each genotype. (A) Hemoglobin levels of sickle mice were lower than hemizygotes, but both were below wild-type, consistent with previous reports.23,27 The hemizygous mouse is anemic but does not have erythrocyte sickling. (B) Elevated reticulocyte fraction indicated markedly increased erythropoiesis in sickle mice. (C) Spleen as a fraction of body weight was elevated in sickle mice. The spleen is a normal site of erythropoiesis in adult mice and greatly increases in conditions of increased erythropoiesis. (D) Indirect bilirubin was elevated in sickle mouse plasma. (E) Lactate dehydrogenase in plasma was elevated in sickle mice as a nonspecific indicator of hemolysis. (F) Plasma NO consumption in blood processed under low shear to avoid artifactual hemolysis was measured using the gas-phase chemiluminescent NO analyzer (Sievers, Boulder, CO) as previously described.6 Nitric oxide consumption measurement was elevated in sickle mice (n = 21) compared with hemizygotes (n = 11) or wild-type controls (n = 6) by Kruskal-Wallis test. Absorbance spectrophotometry in the Soret band determined that plasma hemoglobin concentration correlated with NO consumption in mice (r2 = 0.96, n = 49, data not shown). The wide variability in NO consumption in the sickle mice was similar to the variability of plasma NO consumption in humans with sickle cell disease.6 (G) Whole blood nitrite levels were obtained by mixing fresh blood immediately with nitrite preservation solution63 and then assaying by chemiluminescence (Sievers, Boulder, CO) as an indirect measure of NO synthase activity. Whole blood nitrite levels were lower in sickle mice than hemizygous and wild-type controls by the Mann-Whitney test (n = 4 to 7 per group). Nitrite in the BM-S mice (n = 9) was lower than the nitrite in the controls receiving transplants with wild-type marrow (BM-C, n = 4). (H) Lung homogenate assays demonstrated significantly higher arginase activity in sickle mice and in BM-S mice (n = 4 to 7 mice per group). Statistically significant results are indicated as follows: *P < .05 versus hemizygous controls; #P < .05 versus BM-C controls.
Similar endothelial dysfunction and loss of NO bioavailability in another hemolytic condition without sickle hemoglobin
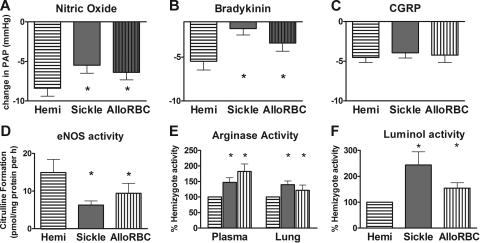
To determine whether the phenotype of the endothelial dysfunction and impaired NO axis characteristic of the sickle mice is mechanistically linked to hemolysis per se or to the numerous features of sickle cell disease beyond hemolysis (such as sickle hemoglobin polymerization, vasoocclusion, abnormal endothelial adhesion, and leukocytosis), we developed and evaluated mice with alloimmune hemolytic anemia but no sickle hemoglobin.42 The severity of acute hemolysis in these alloimmune mice is indicated by plasma lactate dehydrogenase elevation to 1700 ± 200 U/L (n = 5) compared with 540 ± 57 U/L and 360 ± 73 U/L (Figure 7E) for sickle mice (n = 7) and hemizygous controls (n = 10), respectively, and increased plasma hemoglobin42 (data not shown). Vasoresponsiveness in these alloimmune hemolytic mice showed blunted vasodilation to NO and endothelium-dependent vasodilators compared with hemizygous controls, similar to sickle mice (Figure 8A–B). Vasodilation to the endothelium-independent CGRP was preserved, just as in sickle mice (Figure 8C). Likewise, blunted response to other endothelium-dependent vasodilators and enhanced response to vasoconstrictors in the alloimmune hemolytic mice were similar to the sickle mice (data not shown). The NO axis was impaired, with decreased eNOS activity and increased arginase activity in both plasma and in lung (Figure 8D–E). Luminol activity, a measure of reactive oxygen species, was elevated (Figure 8F).
Figure 8.
Pulmonary vascular responsiveness and lung homogenate activity of alloimmune hemolytic mice were similar to sickle mice and not to hemizygous sickle controls (mean ± SEM for n = 4 to 6 per group). A mouse model of acute hemolytic transfusion reaction was studied during alloimmune hemolysis by microcardiac catheterization. Because mice studied on the third day of hemolysis did not appear to have greater abnormality in the parameters measured than mice studied on the first or second days of hemolysis, data were pooled together. (A) Vasodilation to inhaled nitric oxide at 4 ppm was significantly blunted in alloimmune hemolytic mice compared with hemizygous controls (P < .05). (B) Vasodilation to bradykinin infusion was significantly blunted at 3 μg/kg in alloimmune hemolytic mice (P < .05). (C) The endothelium-independent agent CGRP (0.3 nmol/kg intravenous bolus) produced the same vasodilation response in alloimmune hemolytic mice and sickle mice as in hemizygous controls (P = NS). (D) eNOS assay of lung homogenate was significantly lower in alloimmune hemolytic mice than in hemizygous controls (P < .05), similar to sickle mice. (E) Arginase assay of plasma and lung homogenate was significantly elevated in both alloimmune hemolytic mice and sickle mice compared with hemizygous controls (P < .05). (F) Luminol assay of lung homogenate indicated significantly elevated reactive oxygen species in alloimmune hemolytic mice and sickle mice compared with hemizygous controls (P < .05). Statistically significant results are indicated by an asterisk (P < .05 versus hemizygous controls).
Discussion
In the current study we find that mice expressing exclusively human sickle hemoglobin and mice with acute alloimmune hemolytic anemia develop pulmonary hypertension associated with pulmonary vascular endothelial dysfunction. The global reduction in NO responsiveness and bioavailability is associated with an augmented vasoconstrictor tone. Histologic and radiologic studies reveal no evidence of thromboembolism and no significant vascular remodeling, suggesting that in this mouse model a severe impairment in NO-dependent endothelial function may be sufficient to cause pulmonary hypertension. This is the first study to demonstrate that pulmonary hypertension caused by pulmonary vascular endothelial dysfunction can be triggered by hemolytic anemia with secondary NO and arginine catabolism and eNOS uncoupling.
Cardiac catheterization of these mice provides the first hemodynamic demonstration of pulmonary hypertension in sickle mice. All sickle mice had pulmonary hypertension and increased pulmonary vascular resistance comparable to pulmonary pressures reported in humans with sickle cell disease. Chimeric mice derived by bone marrow transplantation from sickle mice (BM-S) developed similar findings of pulmonary hypertension and blunted responsiveness to NO-dependent and endothelium-dependent vasodilators. Sickle mice had elevated cardiac outputs as young adults, an expected compensation for the severe anemia, while older mice developed heart failure characterized by reductions in cardiac output and increases in right atrial pressure. Alloimmune hemolytic mice had similar findings, although sometimes not as severe as the sickle mice or chimeric BM-S mice. Hemizygous mice were similar at cardiac catheterization to wild-type mice (data not shown), suggesting that the oxidative stress in hemizygotes62 is not sufficient to cause vascular pathology. Taken together, the studies of age dependence and the studies of chimeric mice imply that the vascular dysfunction phenotype develops after exposure to HbS erythrocytes or severe hemolysis. Vascular dysfunction in these mice was detected after weeks or months of exposure to hemolysis, in contrast to the typical diagnosis of pulmonary hypertension in human sickle cell disease in the third or fourth decade of life. Acute hemolysis produces vasoconstriction of pulmonary vessels in canine models.67 Future studies might explore how vasculopathy depends on chronic exposure to intense hemolysis and whether there are species differences in susceptibility to vascular dysfunction that might affect how these results can be extrapolated to patients with sickle cell disease.
Lung histologic studies were notable for the absence of thrombi, emboli, interstitial fibrosis, or inflammatory infiltrates to suggest pneumonia, thromboemboli, or pulmonary infarction as an etiology for pulmonary hypertension. Pulmonary vascular remodeling was mild, and there was no evidence of obliterative hyperplasia or plexogenic lesions. Relatively normal lung histology was also observed in another subcolony of sickle mice.25 These data imply that thromboembolism, chest syndrome, or pulmonary fibrosis are not necessary for pulmonary hypertension in the sickle mouse. The similar patterns observed in mice with alloimmune transfusion reaction reinforce that pulmonary vascular dysfunction can simply occur with acute hemolysis without time to develop chronic lung disease. Taken together, these animal models support the hypothesis that pulmonary hypertension in patients with sickle cell disease, thalassemia, and other chronic hereditary or acquired hemolytic anemias3,39,68–71 develops as a consequence of hemolytic anemia, oxidative stress, and endothelial dysfunction rather than acute chest syndrome or pulmonary fibrosis.
Consistent with a number of recent studies evaluating the systemic resistance vessels in mouse models and human sickle cell disease,6,9,58,59 the sickle mice in the current study exhibited blunted pulmonary and systemic vascular responses to NO. Recognizing that NO pathways can have complex abnormalities in other conditions of pulmonary hypertension,72 the NO axis in sickle mice was assessed by multiple methods. Diminished vasodilation was observed with exposure to authentic NO gas and NO donors as well as endothelium-dependent vasodilators (bradykinin and adrenomedullin). Blunted vasodilation to sildenafil, a phosphodiesterase-5 inhibitor that increases cGMP half-life and augments NO-dependent vasodilation, was also consistent with a reduction in basal NO bioavailability. However, sickle mice retained their responsiveness to the endothelium-independent vasodilator CGRP, indicating that the mice do possess a vasoregulatory reserve. Lastly, the vasoconstriction responses to hypoxia, norepinephrine, and angiotensin II were augmented in sickle mice, consistent with a dysregulation of the normal balance of vasodilator/vasoconstrictor tone.
Markedly diminished NOS activity was demonstrated in the lungs. The low NOS activity can be attributed to loss of the active homodimeric form of eNOS, with predominance of inactive monomeric eNOS in the sickle mice. The dual findings of low constitutive (calcium-dependent) NO synthase activity and loss of eNOS dimerization can help explain previous demonstrations of increased eNOS protein expression in mouse models of sickle cell disease,9,73 which measured eNOS using denaturing conditions that cannot distinguish between eNOS dimer and eNOS monomer. The mice with acute alloimmune hemolysis and no sickle hemoglobinopathy also exhibited decreased eNOS activity and increased lung arginase compared with hemizygous controls, although not as severe as sickle mice (Figure 8), suggesting that this process occurs secondary to intravascular hemolysis.
In addition to NOS dysfunction characterized by reduced L-arginine to L-citrulline turnover, the blunted vasodilation to NO and NO donors suggests a state of resistance to NO in sickle cell disease. This has been observed in the human forearm during infusions of sodium nitroprusside6 and nitroglycerin74 and in transgenic mouse models during infusions of NONOate NO donors59,75 and sodium nitroprusside.75 This state of resistance to NO may not be unique to sickle cell disease, and pulmonary hypertension has been described in cross-sectional surveys of other hemolytic conditions such as thalassemia intermedia.38,39 In contrast, patients with coronary artery disease and its risk factors have endothelial dysfunction characterized by blunted responses to endothelial-dependent vasodilators, such as acetylcholine, but preserved responses to exogenous NO donors, such as nitroprusside. This NO resistance in sickle cell disease has been associated with increased levels of free hemoglobin in plasma (also observed in the current study) and xanthine oxidase–derived superoxide, both high-affinity and irreversible NO scavengers.6,28 In addition to releasing hemoglobin into plasma, hemolysis releases red cell arginase, which further contributes to substrate depletion and reductions in NO formation from NOS.8 The similar findings in alloimmune hemolytic mice and a human report71 are consistent with a major role for hemolysis in the vasculopathy of sickle mice.
The pulmonary hypertension in these mice did not appear to have progressed to the plexiform and obliterative vascular lesions characteristic of advanced pulmonary hypertension in humans with idiopathic pulmonary hypertension or sickle cell–associated pulmonary hypertension.76 In this respect, the sickle mice are similar to hph-1 mice with uncoupled eNOS, which develop mild pulmonary hypertension without vascular lesions.77 Perhaps due to species differences, most animal models of pulmonary hypertension do not develop plexiform vascular lesions, with the exception of hypoxic rats treated with inhibitors of vascular endothelial growth factor34 and monocrotaline-challenged rodents with inflammatory pulmonary vascular lesions.35,36 Whether plexiform vascular lesions develop in humans only in the advanced stages of pulmonary hypertension is controversial,78,79 and pulmonary hypertension is not always associated with vascular remodeling.80 Current understanding of the pathogenesis of early human pulmonary arterial hypertension is that pulmonary arteries and arterioles have abnormally high vasoconstrictor tone, and the initial vascular defect is functional rather than structural, just as the cardiac catheterization studies demonstrate in these sickle mice.
These data in animal models are consistent with epidemiologic and human physiologic evidence for hemolysis as a major contributor to pulmonary hypertension in sickle cell disease and other hemolytic anemias.3,7,38,39,71 In this study, alloimmune hemolytic mice were chosen for comparison in order to generalize beyond hemoglobinopathies, but future studies might examine thalassemic mice for mechanistic insights on pulmonary hypertension in human thalassemia intermedia.38,39 The finding that abnormalities were more severe in sickle mice compared with alloimmune hemolytic mice does open additional mechanistic questions about the pathophysiologic effects of sickling and vasoocclusion, abnormal adherence of blood cells to endothelium, abnormal blood rheology, greater oxidative stress, and other abnormal characteristics of sickle cell disease. Because the duration of hemolysis differs in the 3 mouse model systems studied—lifelong hemolysis in sickle mice, up to 3 months of hemolysis in chimeric BM-S mice, and a few days in alloimmune hemolytic mice—it is possible that blunted vascular responsiveness and impaired NO axis require more prolonged or intense exposure to hemolysis. The mechanism for the loss of eNOS dimerization in sickle cell disease also remains to be explored, and in other conditions of vasculopathy such as diabetes mellitus, loss of eNOS dimerization has been attributed to S-nitrosylation,81 loss of tetrahydrobiopterin,82,83 or loss of NOS heme.84 Increased oxidative stress is implicated in several of these conditions, and the pathophysiology of sickle cell disease includes increased reactive oxygen species and heme oxygenase-1.85–88 Pulmonary hypertension studies in animal models can address these mechanistic possibilities under controlled conditions that are not possible in human studies. Future studies could also clarify the role of NO in pulmonary hypertension associated with hemolysis. For example, sickle bone marrow transplants into eNOS-deficient mice, hph-1 mice, or similar models might identify mechanisms and lead to new therapeutic insights.
In conclusion, transgenic mice expressing exclusively human sickle hemoglobin develop pulmonary arterial hypertension associated with functional, not structural, endothelial dysfunction characterized by global dysregulation of endothelial NO production and bioavailability. Chimeric BM-S mice and alloimmune hemolytic mice, like the sickle mice, have functional vasculopathy and reduced NO bioavailability. This is one of the few animal models to develop pulmonary hypertension without a chemical or hypoxic trigger. This model will facilitate pathophysiologic exploration of whether uncoupled eNOS is related to hemolysis, oxidant stress, or HbS polymerization and vasoocclusion. Mechanistic studies can also determine whether vascular dysfunction characterized by a global impairment in NO responsiveness and NO production is associated with other conditions of intravascular hemolysis.
Acknowledgments
This work was supported in part by federal funds from National Cancer Institute, NIH, under contract NO1-CO-12400; the Intramural Program of the Vascular Medicine Branch of the National Heart, Lung, and Blood Institute of the NIH; and grants from the American Heart Association and the Bernard Foundation. The content of the paper does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.L.H. and H.C.C. conceived the project, analyzed data, and wrote the paper; S.A.C.-L. contributed the alloimmunehemolytic mice; T.J.B., D.M.S., A.E.C., and X.W. collected data; E.A.M. and B.A.D. analyzed histology; A.N.S. and C.T.N. analyzed data; and M.T.G. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
L.L.H. and H.C.C. contributed equally to this work.
Correspondence: Lewis Hsu, Marian Anderson Sickle Cell Center at St Christopher's Hospital for Children, Erie Ave at Front St, Drexel University College of Medicine, Philadelphia, PA 19134; e-mail: lhsu@mail.nih.gov.
References
- 1.Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74:626–628. doi: 10.1016/0002-9149(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 2.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Sood N, De Gent G, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol. 2005;129:449–464. doi: 10.1111/j.1365-2141.2005.05432.x. [DOI] [PubMed] [Google Scholar]
- 6.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 7.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 8.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ataga KI, Moore CG, Jones S, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–115. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 11.Francis RB. Large-vessel occlusion in sickle cell disease: pathogenesis, clinical consequences, and therapeutic implications. Med Hypotheses. 1991;35:88–95. doi: 10.1016/0306-9877(91)90029-x. [DOI] [PubMed] [Google Scholar]
- 12.Lakkireddy DR, Patel R, Basarakodu K, Vacek J. Fatal pulmonary artery embolism in a sickle cell patient: case report and literature review. J Thromb Thrombolysis. 2002;14:79–83. doi: 10.1023/a:1022074507216. [DOI] [PubMed] [Google Scholar]
- 13.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125:1436–1441. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- 14.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 15.Vichinsky EP. Pulmonary hypertension in sickle cell disease. N Engl J Med. 2004;350:857–859. doi: 10.1056/NEJMp038250. [DOI] [PubMed] [Google Scholar]
- 16.Homi J, Levee L, Higgs D, Thomas P, Serjeant G. Pulse oximetry in a cohort study of sickle cell disease. Clin Lab Haematol. 1997;19:17–22. doi: 10.1046/j.1365-2257.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 17.Needleman JP, Franco ME, Varlotta L, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:418–422. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131:129–134. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batra AS, Acherman RJ, Wong WY, et al. Cardiac abnormalities in children with sickle cell anemia. Am J Hematol. 2002;70:306–312. doi: 10.1002/ajh.10154. [DOI] [PubMed] [Google Scholar]
- 20.Leong CS, Stark P. Thoracic manifestations of sickle cell disease. J Thorac Imaging. 1998;13:128–134. doi: 10.1097/00005382-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JF, Maron BJ, Castro O, Moosa YA. Left ventricular diastolic filling abnormalities identified by Doppler echocardiography in asymptomatic patients with sickle cell anemia. J Am Coll Cardiol. 1991;17:1473–1478. doi: 10.1016/0735-1097(91)90634-l. [DOI] [PubMed] [Google Scholar]
- 22.Tsironi M, Aessopos A. The heart in sickle cell disease. Acta Cardiol. 2005;60:589–598. doi: 10.2143/AC.60.6.2004929. [DOI] [PubMed] [Google Scholar]
- 23.Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 24.Nagel RL, Fabry ME. The panoply of animal models for sickle cell anaemia. Br J Haematol. 2001;112:19–25. doi: 10.1046/j.1365-2141.2001.02286.x. [DOI] [PubMed] [Google Scholar]
- 25.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of “Berkeley” sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107:1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Embury SH, Mohandas N, Paszty C, Cooper P, Cheung AT. In vivo blood flow abnormalities in the transgenic knockout sickle cell mouse. J Clin Invest. 1999;103:915–920. doi: 10.1172/JCI5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kean LS, Brown LE, Nichols JW, Mohandas N, Archer DR, Hsu LL. Comparison of mechanisms of anemia in mice with sickle cell disease and beta-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp Hematol. 2002;30:394–402. doi: 10.1016/s0301-472x(02)00780-4. [DOI] [PubMed] [Google Scholar]
- 28.Aslan M, Ryan TM, Adler B, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard KA, Jr, Ou J, Ou Z, et al. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L705–L714. doi: 10.1152/ajplung.00288.2002. [DOI] [PubMed] [Google Scholar]
- 31.Kaul DK, Liu XD, Choong S, Belcher JD, Vercellotti GM, Hebbel RP. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2004;287:H293–H301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- 32.Holtzclaw JD, Jack D, Aguayo SM, Eckman JR, Roman J, Hsu LL. Enhanced pulmonary and systemic response to endotoxin in transgenic sickle mice. Am J Respir Crit Care Med. 2004;169:687–695. doi: 10.1164/rccm.200302-224OC. [DOI] [PubMed] [Google Scholar]
- 33.Le Cras TD, Kim DH, Gebb S, et al. Abnormal lung growth and the development of pulmonary hypertension in the Fawn-Hooded rat. Am J Physiol. 1999;277:L283–L291. doi: 10.1152/ajplung.1999.277.4.L709. [DOI] [PubMed] [Google Scholar]
- 34.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 35.Schultze AE, Roth RA. Chronic pulmonary hypertension—the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev. 1998;1:271–346. doi: 10.1080/10937409809524557. [DOI] [PubMed] [Google Scholar]
- 36.Goto J, Ishikawa K, Kawamura K, et al. Heme oxygenase-1 reduces murine monocrotaline-induced pulmonary inflammatory responses and resultant right ventricular overload. Antioxid Redox Signal. 2002;4:563–568. doi: 10.1089/15230860260220058. [DOI] [PubMed] [Google Scholar]
- 37.Wu KH, Chang JS, Su BH, Peng CT. Tricuspid regurgitation in patients with beta-thalassemia major. Ann Hematol. 2004;83:779–783. doi: 10.1007/s00277-004-0954-8. [DOI] [PubMed] [Google Scholar]
- 38.Aessopos A, Farmakis D, Deftereos S, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–1530. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 39.Morris CR, Kuypers FA, Kato GJ, et al. Hemolysis-associated pulmonary hypertension in thalassemia. Ann N Y Acad Sci. 2005;1054:481–485. doi: 10.1196/annals.1345.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer ST, Kuypers FA, Styles L, Vichinsky EP, Foote D, Rosenfeld H. Pulmonary hypertension in thalassemia: association with platelet activation and hypercoagulable state. Am J Hematol. 2006;81:670–675. doi: 10.1002/ajh.20640. [DOI] [PubMed] [Google Scholar]
- 41.Noguchi CT, Gladwin M, Diwan B, et al. Pathophysiology of a sickle cell trait mouse model: human alpha(beta)(S) transgenes with one mouse beta-globin allele. Blood Cells Mol Dis. 2001;27:971–977. doi: 10.1006/bcmd.2001.0469. [DOI] [PubMed] [Google Scholar]
- 42.Campbell-Lee S, Liu J, Velliquette RW, et al. The production of red blood cell alloantibodies in mice transfused with blood from transgenic Fyb-expressing mice. Transfusion. 2006;46:1682–1688. doi: 10.1111/j.1537-2995.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 43.Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council. Washington, DC: National Academy Press; Guide for the Care and Use of Laboratory Animals. p. 1996. [Google Scholar]
- 44.Champion HC, Villnave DJ, Tower A, Kadowitz PJ, Hyman AL. A novel right-heart catheterization technique for in vivo measurement of vascular responses in lungs of intact mice. Am J Physiol Heart Circ Physiol. 2000;278:H8–H15. doi: 10.1152/ajpheart.2000.278.1.H8. [DOI] [PubMed] [Google Scholar]
- 45.Hyman AL, Hao Q, Tower A, et al. Novel catheterization technique for the in vivo measurement of pulmonary vascular responses in rats. Am J Physiol. 1998;274:H1218–H1229. doi: 10.1152/ajpheart.1998.274.4.H1218. [DOI] [PubMed] [Google Scholar]
- 46.Gumusel B, Hao Q, Hyman AL, et al. Analysis of responses to adrenomedullin-(13-52) in the pulmonary vascular bed of rats. Am J Physiol. 1998;274:H1255–H1263. doi: 10.1152/ajpheart.1998.274.4.H1255. [DOI] [PubMed] [Google Scholar]
- 47.Hsu LL, Schimel DM. Computed Tomography Imaging of Lungs in Mouse Models of Human Disease – Advancing the Computing Interfaces with Physiology:. Proceedings of 17th IEEE Symposium on Computer-Based Medical Systems; June 24-25 2004; Bethesda, MD. Los Alamitos, CA: IEEE Computer Society; 2004. pp. 385–391. [Google Scholar]
- 48.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 49.Berkowitz DE, White R, Li D, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 50.White AR, Ryoo S, Li D, et al. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 51.Takimoto E, Champion HC, Belardi D, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 52.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–1666. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang Y, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res. 2004;94:657–663. doi: 10.1161/01.RES.0000119323.79644.20. [DOI] [PubMed] [Google Scholar]
- 55.Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952;14:413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillespie MN, Hartsfield CL, O'Connor WN, Cohen DA. Pulmonary hypertension in a murine model of the acquired immunodeficiency syndrome. Am J Respir Crit Care Med. 1994;150:194–199. doi: 10.1164/ajrccm.150.1.8025749. [DOI] [PubMed] [Google Scholar]
- 57.Diwan BA, Gladwin MT, Noguchi CT, Ward JM, Fitzhugh AL, Buzard GS. Renal pathology in hemizygous sickle cell mice. Toxicol Pathol. 2002;30:254–262. doi: 10.1080/019262302753559597. [DOI] [PubMed] [Google Scholar]
- 58.Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 59.Nath KA, Shah V, Haggard JJ, et al. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 60.Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 61.Champion HC, Bivalacqua TJ, Toyoda K, Heistad DD, Hyman AL, Kadowitz PJ. In vivo gene transfer of prepro-calcitonin gene-related peptide to the lung attenuates chronic hypoxia-induced pulmonary hypertension in the mouse. Circulation. 2000;101:923–930. doi: 10.1161/01.cir.101.8.923. [DOI] [PubMed] [Google Scholar]
- 62.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19:989–991. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- 63.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gladwin MT, Shelhamer JH, Schechter AN, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 67.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aessopos A, Farmakis D. Pulmonary hypertension in beta-thalassemia. Ann N Y Acad Sci. 2005;1054:342–349. doi: 10.1196/annals.1345.041. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Qui Y, Zhu J, Gao D. Pulmonary hypertension associated with autoimmune hemolytic anemia: a case report. Int J Cardiol. 2007;115:e1–e2. doi: 10.1016/j.ijcard.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 72.Michelakis ED. The role of the NO axis and its therapeutic implications in pulmonary arterial hypertension. Heart Fail Rev. 2003;8:5–21. doi: 10.1023/a:1022150819223. [DOI] [PubMed] [Google Scholar]
- 73.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eberhardt RT, McMahon L, Duffy SJ, et al. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- 75.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278:H1799–H1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- 76.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 77.Nandi M, Miller A, Stidwill R, et al. Pulmonary hypertension in a GTP-cyclohydrolase 1-deficient mouse. Circulation. 2005;111:2086–2090. doi: 10.1161/01.CIR.0000163268.32638.F4. [DOI] [PubMed] [Google Scholar]
- 78.Dorfmuller P, Humbert M, Capron F, Muller KM. Pathology and aspects of pathogenesis in pulmonary arterial hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:9–19. [PubMed] [Google Scholar]
- 79.Voelkel NF, Cool C. Pathology of pulmonary hypertension. Cardiol Clin. 2004;22:343–351. doi: 10.1016/j.ccl.2004.04.010. v. [DOI] [PubMed] [Google Scholar]
- 80.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal?. Circ Res. 2005;97:95–98. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- 81.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 83.Bendall JK, Alp NJ, Warrick N, et al. Stoichiometric relationships between endothelial tetrahy-drobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 84.Cardaropoli S, Silvagno F, Morra E, Pescarmona GP, Todros T. Infectious and inflammatory stimuli decrease endothelial nitric oxide synthase activity in vitro. J Hypertens. 2003;21:2103–2110. doi: 10.1097/00004872-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 85.Hebbel RP. Auto-oxidation and a membrane-associated ‘Fenton reagent': a possible explanation for development of membrane lesions in sickle erythrocytes. Clin Haematol. 1985;14:129–140. [PubMed] [Google Scholar]
- 86.Nath KA, Grande JP, Haggard JJ, et al. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aslan M, Ryan TM, Townes TM, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 88.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132:108–113. doi: 10.1111/j.1365-2141.2005.05834.x. [DOI] [PubMed] [Google Scholar]