Abstract
The onset of hematopoiesis in the mouse embryo and in the embryonic stem (ES) cell differentiation model is defined by the emergence of the hemangioblast, a progenitor with both hematopoietic and vascular potential. While there is evidence for the existence of a hemangioblast in the mouse, it is unclear if this progenitor develops during the establishment of the human hematopoietic system. In this report, we have mapped hematopoietic development in human ES cell (hESC) differentiation cultures and demonstrated that a comparable hemangioblast population exists. The human hemangioblasts were identified by their capacity to generate blast colonies that display both hematopoietic and vascular potential. These colony-forming cells express the receptor tyrosine kinase KDR (VEGF receptor 2) and represent a transient population that develops in BMP-4–stimulated embryoid bodies (EBs) between 72 and 96 hours of differentiation, prior to the onset of the primitive erythroid program. Two distinct types of hemangioblasts were identified, those that give rise to primitive erythroid cells, macrophages, and endothelial cells and those that generate only the primitive erythroid population and endothelial cells. These findings demonstrate for the first time the existence of the human hemangioblast and in doing so identify the earliest stage of hematopoietic commitment.
Introduction
The earliest stage of hematopoietic development in the mouse has been historically associated with the appearance of yolk sac blood islands that consist of clusters of primitive erythroblasts surrounded by maturing endothelial cells.1–3 The close temporal and spatial development of the hematopoietic and endothelial cells in these blood islands provided the basis for the hypothesis that these lineages share a common ancestor, a progenitor known as the hemangioblast.4,5 While expression analyses6–10 and gene targeting approaches11–14 have provided circumstantial evidence in support of this concept, it was studies using the embryonic stem (ES) cell differentiation model that first identified a progenitor with properties of the hemangioblast.15,16 When cultured in methylcellulose, these progenitors generate immature blast colonies that display both hematopoietic and vascular potential.15 The cell that initiates these colonies, the blast colony–forming cell (BL-CFC) or hemangioblast, expresses the receptor tyrosine kinase Flk-1 and the mesodermal gene T (brachyury), indicating that it represents a population undergoing mesoderm specification to the hematopoietic and vascular lineages.17
A progenitor comparable with the BL-CFC has also been identified in the early gastrulating embryo.18 Similar to the BL-CFC, the embryo-derived progenitor displays primitive erythroid and myeloid potential and expresses both Flk-1 and T. Mapping studies revealed that the embryo hemangioblast is found at highest numbers in the posterior region of the primitive streak and not in the yolk sac, an observation that further supports the interpretation that this progenitor represents a transient mesodermal population undergoing hematopoietic and vascular commitment. The identification of the hemangioblast in the primitive streak of the early embryo demonstrates that hematopoietic commitment initiates earlier than previously described, prior to the establishment of the yolk sac blood islands. The striking similarities between the ES cell– and embryo-derived hemangioblasts add strong support to a growing body of evidence indicating that lineage commitment in this in vitro model accurately recapitulates the stages of development in the early embryo.19
Hematopoiesis in the human embryo is also initiated in the yolk sac, within blood islands comprised of developing primitive erythroblasts surrounded by endothelial cells.20–23 Primitive erythrocytes, identified as large nucleated erythroid cells that produce embryonic globins, represent the predominant hematopoietic population early in human development. Mature macrophages are the only other hematopoietic cell type that can be detected at this stage. Primitive erythropoiesis in the human, as that of the mouse, is transient and appears to be restricted to the yolk sac stage of hematopoiesis. The close association of the erythroblasts with the developing endothelial cells in the human yolk sac strongly suggests that human hematopoiesis is also initiated by the development of a hemangioblast. Identification of a putative yolk sac hemangioblast in the human has not been possible to date, however, as the earliest stages of human development are inaccessible for experimentation.
The human ES cell (hESC) differentiation system provides an outstanding alternative to the early embryo for the identification of the hemangioblast as it allows easy access to large number of cells representing the earliest stages of hematopoietic commitment.24–27 Previous studies have demonstrated that the primitive erythroid lineage is the first to develop in hESC differentiation cultures, suggesting that as in the mouse, this model does recapitulate yolk sac hematopoiesis.25 In addition, several groups have identified hESC-derived populations that display both hematopoietic and endothelial potential, observations that support the existence of a human hemangioblast.25,26 To determine if human hematopoiesis is initiated with the development of a hemangioblast, we analyzed early-stage hESC-derived embryoid bodies (EBs) and identified a progenitor that gives rise to blast colonies with both hematopoietic and vascular potential. This colony-forming cell expresses KDR and represents a transient population that precedes the development of the primitive erythroid lineage, demonstrating that, as in the mouse, the hemangioblast marks the onset of human hematopoietic commitment.
Materials and methods
Maintenance of human ES cells
The 2 hESC lines used in this study, H127 (NIH code WA01) obtained from WiCell Research Institute (Madison, WI) and HES228 (NIH code ES02) obtained from ESI International (Singapore), were maintained on irradiated mouse embryonic feeder cells in hESC media consisting of DMEM/F12 (50:50; Mediatech, Herndon, VA) supplemented with 20% knock-out serum replacement (SR), 100 μM nonessential amino acids, 2 mM glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin (Invitrogen, Grand Island, NY), 10−4 M β-mercaptoethanol (Sigma, St Louis, MO), and 20 ng/mL hbFGF (R&D Systems, Minneapolis, MN) in 6-well tissue culture plates.29 Cells were passaged to new feeders as single-cell suspensions, following dissociation with trypsin-EDTA (Mediatech).
Differentiation of ES cells
Prior to differentiation, the hESCs were feeder-depleted by culturing on a thin layer of matrigel (BD Biosciences, Bedford, MA) in hESC media for 24 to 48 hours. To generate EBs, cells were dissociated to small clusters (∼ 10-20 cells) using collagenase B (1 mg/mL; Roche, Indianapolis, IN) for 20 minutes followed by trypsin-EDTA (0.05%) for approximately 2 minutes. The clusters were washed and then plated in 6-well low cluster plates at a concentration of 1 well equivalent to 1 well (Corning, Corning, NY) in 2 mL aggregation media, consisting of StemPro-34 (Invitrogen) supplemented with penicillin/streptomycin, 10 ng/mL BMP-4 (R&D Systems), 2 mM glutamine, 4 × 10−4 M monothioglycerol (MTG), and 50 μg/mL ascorbic acid (Sigma). The aggregates were cultured at 37°C in an environment of 5% CO2, 5% O2, and 90% N2. Twenty-four hours later, 1 mL of the media was carefully removed and replaced with 1 mL aggregation media supplemented with 10 ng/mL hbFGF, resulting in a final concentration of 5 ng/mL hbFGF (induction media 1). The aggregates were incubated in this media for 72 hours, harvested, and resuspended in induction media 2, which consisted of the induction media 1 supplemented with 10 ng/mL VEGF for an additional 3 to 4 days.
EB harvest and dissociation
On the day of assay, EBs were harvested and dissociated to single cells by a 5-minute treatment with trypsin (0.25% trypsin-EDTA). Following trypsinization, 1 mL media with serum was added and the EBs were dissociated to single cells by passaging 6 times through a 20-gauge needle.
Colony growth and expansion
For the generation of blast colonies, unfractionated EB cells (5 × 104 cells/mL) or KDR+ cells (1-2.5 × 104 cells/mL) were plated in IMDM with 1% methylcellulose (Sigma) supplemented with 10% FCS (Atlas, Fort Collins, CO), glutamine (2 mM), ascorbic acid (50 μg/mL), MTG (4 × 10−4M), transferrin (150 μg/mL; Roche Diagnostics, Mannheim, Germany), 20% D4T endothelial cell–conditioned medium, hbFGF (1 ng/mL), hVEGF (10 ng/mL), hSCF (100 ng/mL), hIL-6 (20 ng/mL), hEPO (2 U/mL), hIL-11 (5 ng/mL), hIL-3 (40 ng/mL), and hIGF-1 (25 ng/mL). Cultures were maintained at 37°C in an environment of 5% CO2, 5% O2, and 90% N2. For hematopoietic colony growth, cells were plated in IMDM with 1% methylcellulose, 10% plasma-derived serum (PDS; Animal Technologies, Tyler, TX), protein-free hybridoma medium (5%, PFHM-II; Invitrogen), glutamine (2 mM), hSCF (100 ng/mL), hEPO (2 U/mL), hIL-6 (5 ng/mL), hIL-3 (40 ng/mL), hTPO (40 ng/mL), hIL-11 (5 ng/mL), hIGF-1 (25 ng/mL), hVEGF (10 ng/mL), and hGM-CSF (1 ng/mL). Cultures were maintained at 37°C in a 5% CO2 environment. For blast colony expansion, individual 6-day-old colonies were harvested using a mouth pipette and deposited into matrigel-coated 96-well plates containing IMDM media supplemented with 10% PDS, 10% horse serum (Invitrogen), glutamine (2 mM), 4 × 10−4M MTG, transferrin (150 μg/mL), hbFGF (5 ng/mL), hVEGF (10 ng/mL), hSCF (100 ng/mL), hIL-6 (20 ng/mL), hEPO (2 U/mL), hIL-11 (5 ng/mL), and hIGF-1 (25 ng/mL). Blast and hematopoietic colonies were visualized and recorded using a Leica DM IRB microscope (Leica Microsystems, Bannockburn, IL). Images were taken with a Magnafire digital camera (Optronics, Goleta, CA) using either a 20× (numeric aperture 0.30) or 40× (numeric aperture 0.55) objective lens. Cells from the colonies were visualized and recorded with a Leica DMLB microscope. Images were taken with a SPOT Insight™ 2 Megapixel Color Mosaic digital camera (Diagnostic Instruments, Sterling Heights, MI) using a 100× (numeric aperture 1.25) objective. Cultures were maintained at 37°C in an environment of 5% CO2, 5% O2, and 90% N2. All cytokines were purchased from R&D Systems.
Immunohistochemistry
Blast colonies were plated onto fibronectin-coated glass coverslips (BD Biosciences) in IMDM with 20% FCS, hIL-6 (20 ng/mL), hIGF-1 (50 ng/mL), hVEGF (50 ng/mL), hbFGF (20 ng/mL), and 4 × 10−4 M MTG and cultured for 2 to 3 days. Following the development of distinct adherent populations, DiI-Ac-LDL (5 μg/mL; Biomedical Technologies, Stoughton, MA) was added to the media for 2 hours. The cultures were then washed in 1 × PBS, and the cells were fixed with 4% PFA, 3% sucrose in 1 × PBS for 20 minutes at room temperature and blocked with protein block (Dako Cytomation, Carpenteria, CA) for 45 minutes at room temperature. The cultures were then incubated for 1 hour with mouse anti–human CD31 antibody (BD Pharmingen, San Diego, CA) in staining buffer containing 1 × PBS supplemented with 10% FCS, 2% BSA, 0.2% Tween, and 0.02% NaN3 (Sigma), and then washed 5 times in 1 × PBS containing 0.2% Tween and 0.02% NaN3 and incubated with anti–mouse FITC (Biosource International, Camarillo, CA) for 1 hour in the dark at room temperature. The coverslips were washed 5 times and then inverted onto a drop of DAPI mounting medium (Vector Laboratories, Burlingame, CA). Fluorescence was visualized using a Leica DMRA2 fluorescence microscope (Leica, Wetzlar, Germany), and images were recorded using a digital Hamamatsu CCD camera (Hamamatsu City, Japan). Images were taken with a 40× (numeric aperture 0.85) objective lens.
PCR
RNA was extracted from the different samples using the Qiagen RNeasy Kit (Valencia, CA). Gene expression analyses were carried out using a modified version30 of the protocol described by Brady et al.31 The modifications include the use of a shorter X(dT) oligonucleotide (5′-GTTAACTCGAGAATTC(T)24-3′) and the use of 2 instead of 4 μL polymerase chain reaction (PCR) mix for every 1 μL cDNA. The amplified cDNA was then subjected to semiquantitative gene-specific PCR using the primers and conditions described in Table 1. All annealing reactions were carried out at 59°C.
Table 1.
Sequence of oligonucleotides
| Gene | Forward primer | Reverse primer | Cycle no. | Product size, bp |
|---|---|---|---|---|
| CD31 | atc att tct agc gca tgg cct ggt | att tgt gga ggg cga ggt cat aga | 36 | 159 |
| CD34 | aaa tcc tct tcc tct gag gct gga | aag agg cag ctg gtg ata agg gtt | 36 | 216 |
| C-FMS | aca gga cct ctt agt ctc tgc cct at | agt ttg tgc ttc ctg ctt ggt gtg | 34 | 196 |
| FOXA2 | cca ttg ctg ttg ttg cag gga agt | cac cgt gtc aag att ggg aat gct | 28 | 196 |
| GATA1 | tta gcc acc tca tgc ctt tcc ct | cca gag act tgg gtt gtc cag aat | 36 | 197 |
| KDR | cct cta ctc cag taa acc tga ttg gg | tgt tcc cag cat ttc aca cta tgg | 32 | 219 |
| Neuro D | ccc atg gtg ggt tgt cat ata ttc atg t | cca gca tca cat ctc aaa cag cac | 40 | 200 |
| OCT4 | aac ctg gag ttt gtg cca ggg ttt | tga act tca cct tcc ctc caa cca | 30 | 123 |
| P1H12 | ggg agc aga caa aga tga ggt cta ca | aca tag aca gac aca cac acc cgt | 32 | 184 |
| RUNX1 | atg tgg tcc tat tta agc cag ccc | tca tct ggc tga aga cac cag ctt | 34 | 170 |
| SCL | aag ggc aca gca tct gta gtc a | aag tct tca gca gag ggt cac gta | 34 | 104 |
| SOX7 | tga caa ctt gtt gcc aac tcc ctg | ttc agc agt gga gga aga gca gaa | 28 | 216 |
| T | tgt ccc agg tgg ctt aca gat gaa | ggt gtg cca aag ttg cca ata cac | 32 | 144 |
| VE-cadherin | tgg aga agt ggc atc agt caa cag | tct aca atc cct tgc agt gtg ag | 30 | 118 |
| β-Actin | ttt gaa tga tga gcc ttc gtc ccc | ggt ctc aag tca gtg tac agg taa gc | 25 | 129 |
| β-Globin | tgt cca ctc ctg atg ctg tta tgg | agc tta gtg ata ctt gtg ggc cag | 26 | 302 |
| ε-Globin | cac tag cct gtg gag caa gat gaa | aat cac cat cac gtt acc cag gag | 26 | 304 |
| γ-Globin | cgc ttc tgg aac gtc tga ggt tat | cca gga gct tga agt tct cag gat | 26 | 370 |
Flow cytometry
Analyses were carried out using a LSRII flow cytometer (Becton Dickinson, San Jose, CA) or Facscalibur flow cytometer (Becton Dickinson). Cells were sorted using either a MoFlo (Dako Cytomation, Fort Collins, CO), a FACSVantage SE (Becton Dickinson), or an Influx (Cytopeia, Seattle, WA) cell sorter. Flow cytometric data were analyzed using the CELLQuest (Becton Dickinson) and FlowJo (Treestar, San Carlos, CA) software programs. Anti–KDR-PE, anti–KDR-APC, and anti–PECAM-1 (CD31)–FITC were purchased from R&D Systems; anti–CD31-PE, from BD Pharmingen; anti–CD117-APC and anti–CD34-APC, from Caltag (South San Francisco, CA); and anti–CD117-PE, from BD Immunocytometry Systems (San Jose, CA).
Results
Kinetics of primitive erythroid development in BMP-4–induced EBs
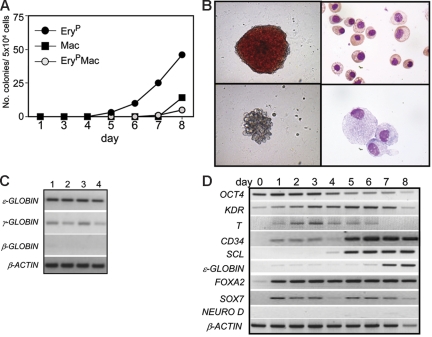
To eliminate the unknown effects of serum in the hESC differentiation cultures, we developed a serum-free protocol that includes 2 3-day induction steps. The first step uses BMP-4 and bFGF, while the second step contains BMP-4, bFGF, and VEGF (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). To define the onset of hematopoiesis in the BMP-4–induced serum-free cultures, EBs were harvested daily between days 1 and 8 of differentiation and assayed for hematopoietic progenitors. Erythroid progenitors were detected as early as day 5 of differentiation (Figure 1A), and their numbers increased during the following 3 days of culture. Colonies generated from the erythroid progenitors consisted predominantly of primitive erythrocytes (EryP) as demonstrated by the large size of the cells and their expression of embryonic (ϵ) and fetal (γ) but not adult (β) globin (Figure 1B-C). In addition to the erythroid colonies, day-8 EBs also contained progenitors that gave rise to small colonies of macrophages (Mac) (Figure 1B) as well as to colonies that contained erythrocytes and macrophages (ErypMacs).
Figure 1.
Kinetics of primitive hematopoietic development in human EBs. (A) Kinetics of hematopoietic development in H1 hESC-derived EBs. EBs were harvested on the indicated days, and the cells were dissociated and plated in methylcellulose supplemented with hematopoietic cytokines. EryP indicates colonies of primitive erythrocytes; Mac, colonies of macrophages; and EryPMac, mixed primitive erythroid/macrophage colonies. Bars indicating standard error of the mean number of colonies from 3 cultures are not visible. (B) The top row shows a primitive erythroid colony (left) and primitive erythrocytes (right), whereas the bottom row shows a macrophage colony (left) and macrophages (right). The primitive erythrocytes are from a pool of 10-day-old erythroid colonies generated from day-8 EBs. The macrophages are from the colony shown. Original magnification: for the colonies, × 400; for the cells, × 1000. (C) PCR analyses of 4 individual primitive erythroid colonies from day 8-EBs showing ϵ- and γ- but not β-globin expression. (D) Expression analyses of EBs at different stages of development. Numbers on top represent day of differentiation.
Molecular analyses showed that OCT4 expression declined during the 8 days of differentiation, indicating a loss of ES and epiblast cells in the EBs (Figure 1D). KDR transcripts were detected in the undifferentiated ES cells, and the levels of expression appeared to increase following the onset of differentiation. T, a marker of primitive streak cells and nascent mesoderm,32 was detected by day 1 of differentiation, increased by day 3, and then declined to undetectable levels by day 7. This temporal pattern of differentiation is consistent with the emergence of a transient primitive streak/mesoderm population. Expression of the hematopoietic genes CD3433 and SCL34 was up-regulated between days 4 and 5 and then persisted throughout the remainder of the time course. ϵ-Globin was expressed by day 6, a finding consistent with the maturation of the primitive erythroid lineage at this stage of differentiation. Neuro D, a gene expressed in early neuroectoderm progenitors,35 was not detected at any stage of EB development, indicating that this lineage was not induced under these culture conditions. FOXA2, a marker of visceral and definitive endoderm,36,37 and SOX7,38,39 a marker of visceral endoderm, were expressed by day 1 of differentiation, and expression was maintained throughout the 8-day time course. These patterns likely reflect the induction of visceral endoderm as Sox7 expression has been reported in primitive but not definitive endoderm,40 and previous studies have shown that BMP signaling will induce this population in hESC cultures.41 Collectively, the findings from these molecular analyses reveal temporal changes indicative of mesoderm induction and hematopoietic specification within the first 5 days of EB differentiation.
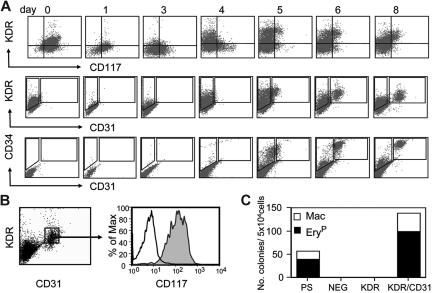
In the mouse ES cell differentiation cultures, hematopoietic commitment is defined by the sequential up-regulation of specific cell surface markers including FLK-1, CD117 (c-KIT), CD31, CD34, and CD41.17,42,43 Studies with serum-induced human ES cell differentiation cultures have shown that the earliest stages of hematopoietic development are also associated with expression of CD31 and CD34.24 Our analyses revealed that CD117 and KDR are coexpressed on the undifferentiated ES cells (Figure 2A). Expression of both receptors dropped significantly within one day of differentiation and increased again on separate populations of cells between days 3 and 4 of differentiation. A KDR+/CD117+ population emerged between days 5 and 8 of differentiation. CD31 was first detected on a subpopulation of KDR+ cells at day 5. This KDR+CD31+ population increased in size by day 6 and then persisted through day 8. Expression of CD34 emerged slightly earlier than CD31, with a small population detectable at day 4 and a significant population present at day 5 of differentiation. All CD31+ cells expressed CD34 and CD117 at day 8 of differentiation (Figure 2B). To determine if these markers defined the beginning of primitive hematopoiesis, CD31+KDR+, CD31−KDR+ (KDR), and CD31−KDR− (NEG) populations were isolated from day-8 EBs and assayed for progenitor potential (Figure 2C). All progenitors segregated to the CD31+KDR+ fraction. Taken together, the findings from these kinetic analyses establish day 5 as the onset of the primitive erythroid stage of EB differentiation, as characterized by the up-regulation of SCL expression and the emergence of primitive erythroid progenitors that express CD31 and KDR.
Figure 2.
Surface marker expression on developing EBs. (A) Flow cytometric analyses of different aged EBs showing expression patterns of KDR, CD117, CD31, and CD34. (B) Coexpression of KDR, CD31, and CD117 on a subpopulation of day-8 EBs. Shaded histogram represents population stained with anti-CD117 antibody; open histogram is unstained control. (C) Hematopoietic progenitor potential of the presorted population (PS), and the CD31−KDR− (NEG), CD31−KDR+ (KDR), and CD31+KDR+ (KDR/CD31) fractions isolated from day-8 EBs.
Identification of a hemangioblast in EBs from hESCs
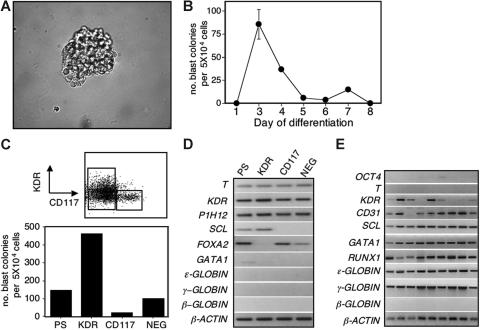
In the mouse ES cell model, the hemangioblast develops prior to the onset of primitive erythropoiesis and is defined as a FLK-1+ progenitor that can generate blast colonies with hematopoietic and vascular potential.15,17 To determine if hematopoiesis in the human ES cell differentiation cultures is initiated by a hemangioblast, cells from early-stage EBs were cultured in conditions known to support the growth of blast colonies from mouse ES cell–derived BL-CFCs/hemangioblasts. As shown in Figure 3A-B, colonies with the morphology of mouse blast colonies were detected in cultures of day-3 and -4 EB-derived cells. With the H1 hESC line, the number of these colonies peaked at day 3 of differentiation and then declined to undetectable levels by day 5, preceding the beginning of primitive erythropoiesis and the onset of CD31 expression (Figure 3B). A comparable kinetics was observed with the HES2 hESC line, although the peak of colony-forming activity was detected on day 4 rather than day 3 (Figure S2). To determine if the human blast colonies developed from a KDR+ cell, day-3 EBs were fractionated into KDR+, CD117+, and KDR−CD117− (NEG) populations and assayed for blast colony–forming activity. As shown in Figure 3C, the majority of blast colonies were generated by the KDR+ cells. Approximately 2.4% (range, 1%-5%) of the cells from this population gave rise to a colony. Expression analyses revealed that T, KDR, and the endothelial marker P1H12 were present in all 3 fractions (Figure 3D). SCL was detected only in the KDR+ fraction, consistent with its blast colony–forming potential. In contrast, FOXA2 was expressed in the CD117+ and NEG fractions but not in the KDR+ population. Neither GATA1 nor the globin genes were expressed in any of the populations at this early progenitor stage of development.
Figure 3.
Blast cell colony development from human EBs. (A) Photograph of a 6-day-old blast colony generated from day-3 EBs. Original magnification × 400. (B) Kinetics of blast colony development in EBs generated from H1 ES cells. Bars, where visible, indicate standard error of the mean number of colonies from 3 cultures. (C) Blast colony–forming potential of the presorted population (PS) and the KDR+CD117− (KDR), KDR−CD117+ (CD117), and KDR−CD117− (NEG) populations isolated from day-3 EBs. Top figure shows the gates used to isolate the different fractions. Bottom figure indicates the blast colony potential of each of the fractions as well as of the PS population. Bars indicating standard error of the mean number of colonies from 3 cultures are not visible. (D) Expression analyses of the different fractions isolated in panel C. (E) Expression analyses of 9 individual 6-day-old blast colonies generated from KDR+ cells isolated from day-4 EBs.
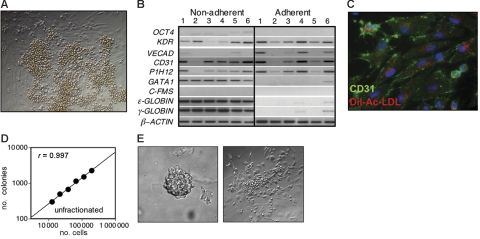
Six-day-old blast colonies generated from day-3 KDR+ cells expressed the hematopoietic genes SCL,34 GATA1,6 RUNX1,44,45 ϵ-globin, and γ-globin as well as genes associated with vascular development including KDR46 and CD31 (Figure 3E). This pattern of expression indicates that these colonies contain the potential to generate these 2 different lineages. None of the colonies analyzed expressed T, a finding consistent with the interpretation that they represent a stage of development beyond mesoderm. Mouse blast colonies are distinguished by their ability to generate distinct adherent vascular progeny and nonadherent hematopoietic cells when cultured on a thin layer of matrigel in media containing hematopoietic and endothelial cytokines.15 Human blast colonies displayed a similar potential, as more than 90% of 6-day-old colonies gave rise to adherent and nonadherent outgrowths under comparable culture conditions (Figure 4A). The adherent cells generated from most colonies expressed a number of different vascular genes including KDR, VE-cadherin, CD31, and P1HI2.47 Most did not express the hematopoietic gene GATA1 or any of the globin genes (Figure 4B). Of significance was the finding that the adherent populations did not express the macrophage marker C-FMS, indicating that they were not contaminated with macrophages. The nonadherent populations expressed ϵ-globin, γ-globin, and GATA1, as well as some of the endothelial genes. Expression of the endothelial genes in the nonadherent populations is likely due to contamination by adherent cells. Immunostaining of the blast colony–derived adherent population revealed the presence of CD31+ cells that displayed the potential to take up acetylated LDL,48 further supporting the interpretation that they represent the endothelial lineage (Figure 4C).
Figure 4.
Developmental potential of human blast colonies. (A) Photograph showing the adherent and nonadherent population generated from an individual 6-day-old blast colony plated on a thin layer of matrigel in media containing both hematopoietic and endothelial cytokines. (B) Expression analyses of the nonadherent and adherent populations generated from 6 individual blast colonies. The colonies were grown from day-4 EB-derived KDR-positive cells. (C) Immunostaining and DiI-Ac-LDL uptake of adhesive cells generated from a single blast colony. CD31 expression is indicated by green fluorescence and LDL uptake by red fluorescence. Original magnification: × 400. (D) Cell dose-response showing the relationship between the number of day-4 EB cells plated and the number of blast colonies that develop. (E) Photograph of a blast colony generated from a single KDR+ cell and of the adherent and nonadherent populations generated from it. Single KDR+ cells from day-3 EBs were deposited into microtiter wells containing methylcellulose. After 6 days of culture, the colony was picked from the microtiter well and cultured on a thin layer of matrigel for an additional 6 days. Original magnification for the colony and expanded populations: × 400.
The hematopoietic and endothelial potential of the 6-day-old blast colonies suggests that they represent the progeny of the human hemangioblast. To demonstrate the clonality of the blast colonies, 2 different strategies were used. The first was a cell dose-response experiment, in which different numbers of unfractionated day-4 EB cells were plated in blast colony conditions. As shown in Figure 4D, the relationship between the number of colonies and the number of cells plated was linear, with a slope approaching 1. These findings indicate that each colony was generated from a single colony-forming unit. As a second approach, we plated single day-4 EB-derived KDR+ cells into microtiter wells containing hemangioblast methylcellulose. In 3 independent experiments using both hESC lines, blast colonies developed at a frequency of 0.4% (H1), 0.7% (HES2), and 1.4% (HES2). When picked and expanded on matrigel, colonies derived from single cells could generate both adherent and nonadherent populations (Figure 4E). The above frequencies are based on colonies that generated both populations. These findings show that the blast colonies with hematopoietic and endothelial potential are derived from single KDR+ cells.
Development of 2 populations of hemangioblasts
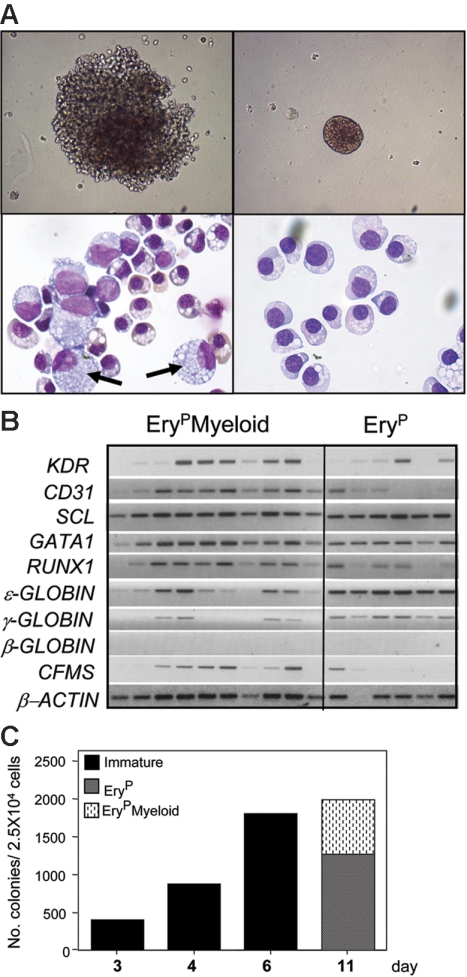
By maintaining the blast colonies in methylcellulose cultures for longer than 6 days, 2 distinct types of colonies were observed: small- to medium-sized erythroid colonies and larger colonies consisting of erythroid and nonerythroid cells (Figure 5A). Morphologic analyses demonstrated that the erythroid colonies consisted predominantly of primitive erythrocytes, whereas the larger colonies contained macrophages and erythroid cells. Molecular analyses confirmed the morphologic assessment of these 2 types of colonies and demonstrated that both expressed ϵ- and γ- but not β-globin, consistent with the presence of primitive erythroid cells (Figure 5B). Most of the large blast colonies expressed C-FMS, indicative of the presence of macrophages. Only one of the erythroid colonies expressed C-FMS. To distinguish the various stages of blast colony development, colonies at days 6 to 7 of culture will be referred to as immature blast colonies and those beyond day 10 of culture, as primitive erythroid (EryP) and primitive erythroid/myeloid (EryPMyeloid) blast colonies. Kinetic analyses revealed that the total number of blast colonies increased between days 3 and 6 of culture and then remained constant to day 11. The lack of increase in number beyond day 6 reflects the maturation of the immature colonies to the EryP and EryPMyeloid blast colonies (Figure 5C).
Figure 5.
Development of 2 populations of hemangioblasts. (A, top row) Photograph of an 11-day-old EryPMyeloid–restricted (left) and EryP-restricted (right) blast colony (original magnification, × 200). (A, bottom row, left) Photograph of erythrocytes and macrophages (arrows) from an individual EryPMyeloid blast colony, and (right) erythroid cells from a pool of EryP blast colonies. Original magnification: × 1000. (B) Expression analyses of 10 individual EryPMyeloid and 6 EryP blast colonies. (C) Kinetics of blast colony development from KDR+ cells isolated from day-4 EBs.
The hematopoietic potential of the EryP and EryPMyeloid blast colonies was further evaluated through analyses of their replating potential. Pools of colonies were picked at day 10 of growth, and the cells were dissociated and replated into methylcellulose cultures containing a broad spectrum of hematopoietic cytokines. The replated EryP blast colonies did not generate secondary colonies, indicating that they represent populations of maturing erythrocytes, beyond the progenitor stage of development. In contrast, the pools of larger EryPMyeloid blast colonies did give rise to secondary erythroids, macrophages, and low numbers of mast colonies. In a representative experiment, a pool of 50 EryPMyeloid blast colonies generated 200 primitive erythroid, 230 macrophage, 130 mixed erythroid/macrophage, and 50 mast cells colonies. The appearance of the mast cell colonies is not surprising, as this lineage does develop within the EBs between 8 and 10 days of culture (M. K. and G. K., unpublished data, May 2006). Definitive erythroid cells, defined as those that express β-globin, were not detected in these replated cultures.
Growth factor regulation of hemangioblast-derived blast colony development
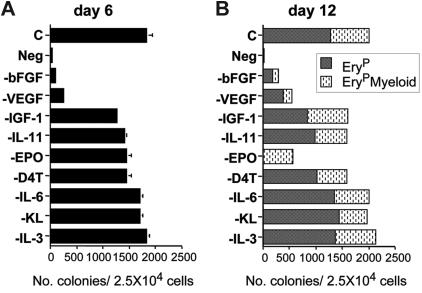
The methylcellulose cultures used for blast colony growth consist of a mixture of cytokines that we have previously shown to impact the growth of these colonies in mouse ES cell– and embryo-derived cultures. To further investigate their role in the development of human blast colonies, each was eliminated from the mixture in a sequential fashion. Blast colonies did not develop in the absence of factors (Neg, Figure 6A). Elimination of bFGF or VEGF had the most dramatic effect on colony growth, as in their absence few blast colonies developed. The removal of either IGF1-, IL-11–, EPO-, or D4T-conditioned media had a modest effect on the number of colonies that developed, whereas elimination of IL-6, KL, and IL-3 did not significantly impact colony development. The patterns of colony development at day 12 under these different conditions were similar to those at day 6, with the exception of the group lacking EPO (Figure 6B). No EryP blast colonies developed in these cultures, indicating that EPO is required for the maturation of this type of colony. Taken together, these findings demonstrate that the development of blast colonies from the human hemangioblast is dependent on the presence of bFGF and VEGF. While EPO is not required for the development of the immature blast colonies, it is required for the maturation of the EryP-restricted blast colonies.
Figure 6.
Cytokine regulation of hemangioblast growth and differentiation. (A) The effect of removing individual factors from the blast colony methylcellulose cultures on the number of immature blast colonies that develop at day 6. (B) The numbers of EryP and EryPMyeloid blast colonies that develop on day 12 of culture.
BMP-4 is essential for hemangioblast development
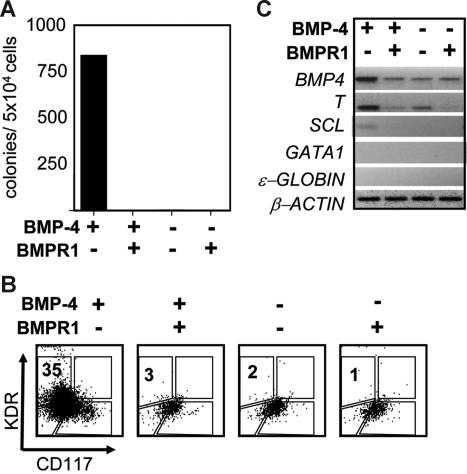
Our differentiation protocol that includes BMP-4 was optimized based on the development of hematopoietic progenitors in day-8 and -14 EBs (M. K. and G. K., manuscript in preparation). To determine if hemangioblast development was dependent on BMP-4, EB differentiation was established in the presence and absence of the inducer as well as in the presence and absence of soluble BMP receptors (sBMPR-IA and sBMPR-IB), inhibitors of BMP signaling. As shown in Figure 7A, no blast colonies developed in the absence of BMP-4 or when the inhibitors were added together with the factor. FACS analyses were consistent with the colony data and demonstrated that the typical single KDR+ and CD117+ populations did not develop in the absence of BMP signaling (Figure 7B). Expression analyses of the EBs generated in the different conditions showed that mesoderm induction, as measured by T expression, was dependent on BMP signaling as the levels diminished in the presence of the inhibitors. Of interest, T mRNA was detected in the EBs that were not exposed to BMP-4 (Figure 7C). This was likely due to expression of endogenous BMP-4 as mRNA was detected in all of the populations and T expression was reduced by addition of the inhibitors. Taken together, these findings demonstrate that the development of the human hemangioblast is dependent on BMP signaling.
Figure 7.
BMP-4 requirement for hemangioblast development. (A) Blast colony potential of day 4-EBs induced in the presence and absence of BMP-4 and soluble BMP-4 receptor (sBMP4R-lA, sBMP4R-lB, 250 ng/mL). The presence and absence of factor and receptor are indicated below the graph. (B) Flow cytometric analysis showing KDR and CD117 expression of the 4 different EB populations. (C) RT–PCR expression analyses of the same EB populations analyzed in panel A.
Discussion
Studies in the mouse have shown that development of the hemangioblast marks the onset of hematopoiesis in both the ES cell differentiation model as well as in the early embryo.15,18 Moreover, these studies revealed that the ES cell– and embryo-derived progenitors share many similarities including coexpression of Flk-1 and T, a temporal pattern of development preceding primitive erythropoiesis and the ability to grow and differentiate in methylcellulose cultures and generate blast colonies with hematopoietic and endothelial potential.15,17,18 These striking similarities support the interpretation that the ES cell model accurately recapitulates the developmental events observed in the embryo. Our current study shows that the hESC-derived hemangioblast expresses KDR and develops within 72 to 96 hours of EB differentiation, the stage during which KDR and CD117 are expressed on distinct populations, prior to the expression of CD31 and CD34 and the onset of primitive erythropoiesis. As observed with the mouse progenitors, the human hemangioblasts generate distinct blast colonies that display hematopoietic and endothelial potential. When extrapolated to the embryo, our findings provide strong evidence that human yolk sac hematopoiesis is initiated by a hemangioblast.
The developmental potential of the mouse ES cell–derived hemangioblast accurately reflects the potential of the early yolk sac prior to establishment of circulation.3,15,49 Based on morphologic assessment, 2 broad classes of human hemangioblasts were easily identified: those that generated primitive erythrocytes and those that gave rise to primitive erythrocytes and macrophages. Our replating studies revealed that the EryPMyeloid blast colonies also generate mast cell colonies in addition to macrophage and primitive erythroid colonies. While it is unclear if mast cell progenitors are generated in the early human yolk sac, they are found in EBs differentiated for more than 8 days, indicating that this lineage does develop with time in the differentiation cultures. As the earliest stages of human yolk sac development prior to the onset of circulation are not readily accessible, there is little information on the emergence of different progenitor populations prior to 3 weeks of gestation. Data that do exist on these early stages are based on histologic studies that show the development of blood islands consisting of primitive erythrocytes and endothelial cells between days 16 and 19 of gestation.20,21 Together with the primitive erythrocytes, which represent the predominant hematopoietic population in the yolk sac, infrequent macrophages were also detected. The primitive erythroid and macrophage potential of the hESC-derived hemangioblast is entirely consistent with these observations, and strongly supports the interpretation that it represents the progenitor for human yolk sac hematopoiesis.
Several previous studies have described the development of hESC-derived cell types with hemangioblast properties. Wang et al26 identified a CD31+VE-cadherin+KDR+CD45− population in day-10 EBs that displayed the potential to generate both hematopoietic and endothelial progeny. Clonal analyses revealed that a rare subset of progenitors within this population was able to give rise to CD45+ and VE-cadherin+ cells. As the hematopoietic potential of these CD45+ progenitors was not further characterized, it is difficult to determine their developmental status relative to the hemangioblasts described here. Given that these progenitors express CD31 and develop significantly later than the CD31− hemangioblasts we have identified, they most certainly represent a different population. In a more recent study, Zambidis et al25 described the development of mesodermal-hematoendothelial colonies from EBs differentiated for 6 to 15 days. These colonies formed as adherent cell masses that ultimately gave rise to endothelial and hematopoietic progeny. Such colonies may be similar in potential to the blast colonies described in this study. However, as the colony-forming cells were not characterized and clonality was not determined, it is unclear if these colonies develop from mesoderm or more committed populations or if they are of single-cell origin.
One significant difference between our study and those of Wang et al26 and Zambidis et al25 is the conditions used for mesoderm induction and hematopoietic specification. We used recombinant BMP-4 together with VEGF and bFGF to generate hematopoietic progeny, while their induction relied on serum. Given the mixture of cytokines, inducers, and inhibitors present in serum, it is quite possible that specific batches may delay the kinetics of EB differentiation or mask the appearance of subpopulations within the EBs. The primitive streak/mesoderm induction with BMP-4 appears to be quite efficient, as Neuro D, a gene indicative of neuroectoderm development, was not detected in the EBs. BMP-4 has been previously shown to augment hematopoietic development in hESC cultures.50,51 However, in these studies, it was included in the context of serum and/or other cytokines. Here, we show that BMP-4 plays a pivotal role in mesoderm induction and hemangioblast development, as no progenitors were detected when this pathway was inhibited by the addition of sBMPR. The requirement for BMP-4 for hemangioblast development in hESC cultures is in line with studies in the mouse ES cell model and other model systems that indicate that this signaling pathway is required for hematopoietic development.52–54
In summary, the findings reported here have identified a progenitor with characteristics of the human hemangioblast in hESC differentiation cultures and in doing so define the earliest stage of human hematopoietic development. The identification of the hemangioblast in defined hESC differentiation cultures provides the first insight into the earliest stages of human hematopoietic commitment. Access to the hemangioblast and different stages of blast colony development provides a unique opportunity to investigate the molecular mechanisms that regulate these early developmental processes.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 HL080627 and P20 GM075019.
We wish to thank members of the Keller lab for critically reading the paper.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.K. designed research, performed research, analyzed data, and wrote the paper; S.L.D. designed research, performed research, analyzed data, and wrote the paper; M.L.-K. performed research; S.S. performed research and analyzed data; G.K. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gordon Keller, Department of Gene and Cell Medicine, The Black Family Stem Cell Institute, Mount Sinai School of Medicine, New York, NY 10029; e-mail: gordon.keller@mssm.edu.
References
- 1.Haar JL, Ackerman GA. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec. 1971;170:199–224. doi: 10.1002/ar.1091700206. [DOI] [PubMed] [Google Scholar]
- 2.Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- 3.Palis J, Roberston S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 4.Sabin FR. Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Contrib Embryol. 1920;9:213–262. [Google Scholar]
- 5.Murray PDF. The development in vitro of the blood of the early chick embryo. Proc Royal Soc London. 1932;11:497–521. [Google Scholar]
- 6.Orkin S. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575–581. [PubMed] [Google Scholar]
- 7.Watt SM, Gschmeissner SE, Bates PA. PECAM-1: its expression and function as a cell adhesion molecule on hemopoietic and endothelial cells. Leuk Lymph. 1995;17:229–244. doi: 10.3109/10428199509056827. [DOI] [PubMed] [Google Scholar]
- 8.Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- 9.Fong GH, Klingensmith J, Wood CR, Rossant J, Breitman ML. Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn. 1996;207:1–10. doi: 10.1002/(SICI)1097-0177(199609)207:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Takakura N, Huang X, Naruse T, et al. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- 11.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 12.Robb L, Lyons I, Li R, et al. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci U S A. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivdasani R, Mayer E, Orkin SH. Absence of blood formation in mice lacking the T-cell leukemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 14.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1 deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 15.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies Flk+VE-cadherin+ cells at a diverging point of endothelial hematopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 17.Fehling HJ, Lacaud G, Kubo A, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 18.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 19.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 20.Kelemen E, Calvo W. New York, NY: Springer-Verlag; 1979. Atlas of human hematopoietic development. [Google Scholar]
- 21.Tavian M, Hallais MF, Peault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development. 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- 22.Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 23.Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 24.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 25.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 29.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 30.Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development. 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- 31.Brady B, Barbara M, Iscove N. Representative in vitro cDNA amplification from individual hematoietic cells and colonies. Methods Mol Cell Bio. 1990;2:17–25. [Google Scholar]
- 32.Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse [see comments]. Nature. 1990;343:617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- 33.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 34.Begley CG, Aplan PD, Denning SM, Haynes BF, Waldmann TA, Kirsch IR. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989;86:10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 36.Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Takash W, Canizares J, Bonneaud N, et al. SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res. 2001;29:4274–4283. doi: 10.1093/nar/29.21.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh M. Expression of human SOX7 in normal tissues and tumors. Int J Mol Med. 2002;9:363–368. [PubMed] [Google Scholar]
- 40.Kanai-Azuma M, Kanai Y, Gad JM, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 41.Pera MF, Andrade J, Houssami S, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 42.Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- 43.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 44.Wang SW, Speck NA. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992;12:89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa E, Inuzuka M, Maruyama M, et al. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 47.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 48.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive and definitive hematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 50.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 51.Tian X, Morris JK, Linehan JL, Kaufman DS. Cytokine requirements differ for stroma and embryoid body-mediated hematopoiesis from human embryonic stem cells. Exp Hematol. 2004;32:1000–1009. doi: 10.1016/j.exphem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Davidson AJ, Zon LI. Turning mesoderm into blood: the formation of hematopoietic stem cells during embryogenesis. Curr Top Dev Biol. 2000;50:45–60. doi: 10.1016/s0070-2153(00)50003-9. [DOI] [PubMed] [Google Scholar]
- 53.Nakayama N, Lee J, Chiu L. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 2000;95:2275–2283. [PubMed] [Google Scholar]
- 54.Park C, Afrikanova I, Chung YS, et al. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.