Abstract
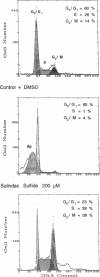
Nonsteroidal antiinflammatory drugs (NSAIDs), have cancer preventive and tumor regressive effects in the human colon. They lower the incidence of and mortality from colorectal cancer and sulindac reduces the number and size of polyps in patients with familial adenomatous polyposis. We studied the effect of sulindac, and its metabolite sulindac sulfide, on the proliferation of HT-29 colon adenocarcinoma cells. Both compounds reduced the proliferation rate of these cells, changed their morphology, and caused them to accumulate in the G0/G1 phase of the cell cycle. These responses were time- and concentration-dependent and reversible. In addition, these compounds reduced the level and activity of several cyclin-dependent kinases (cdks), which regulate cell cycle progression. Sulindac and sulindac sulfide also induced apoptosis in these cells at concentrations that affected their proliferation, morphology, and cell cycle phase distribution. Sulindac sulfide was approximately sixfold more potent than sulindac in inducing these cellular responses. Our results indicate that inhibition of cell cycle progression and induction of apoptotic cell death contribute to the anti-proliferative effects of sulindac and sulindac sulfide in HT-29 cells. These findings may be relevant to the cancer preventive and tumor regressive effects of these compounds in humans.
Full text
PDF



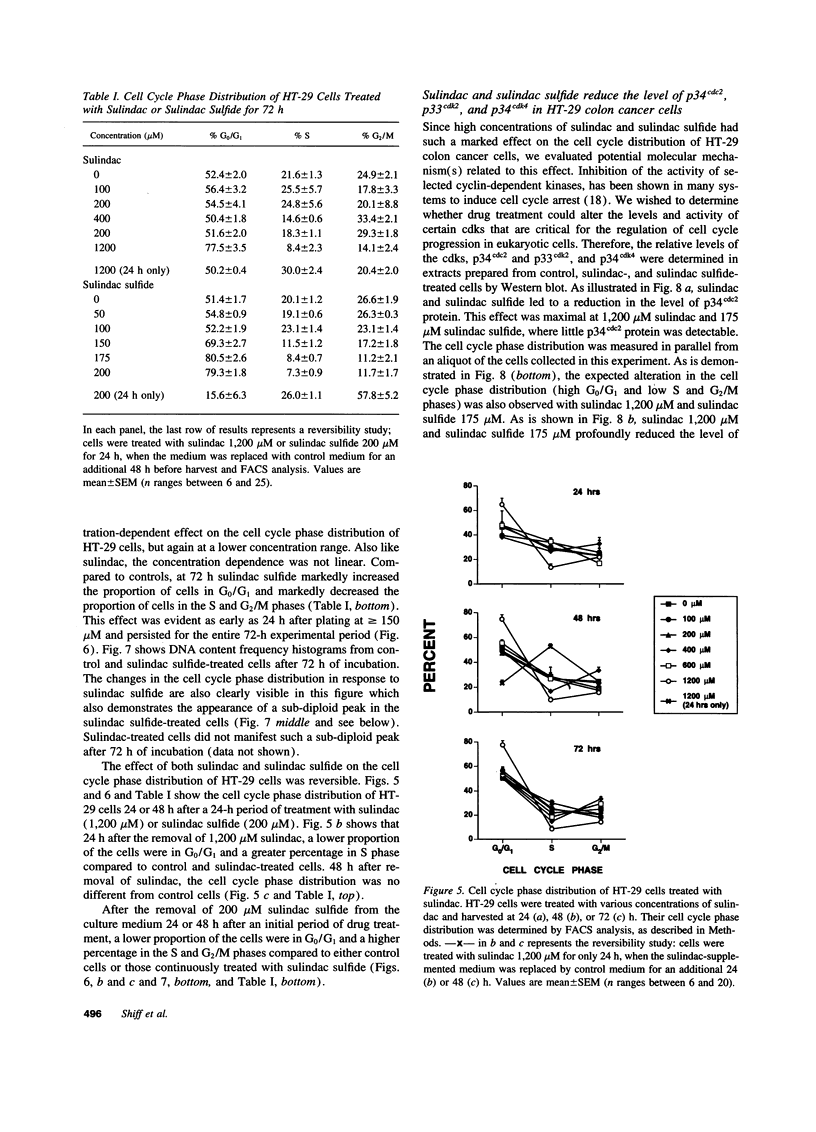
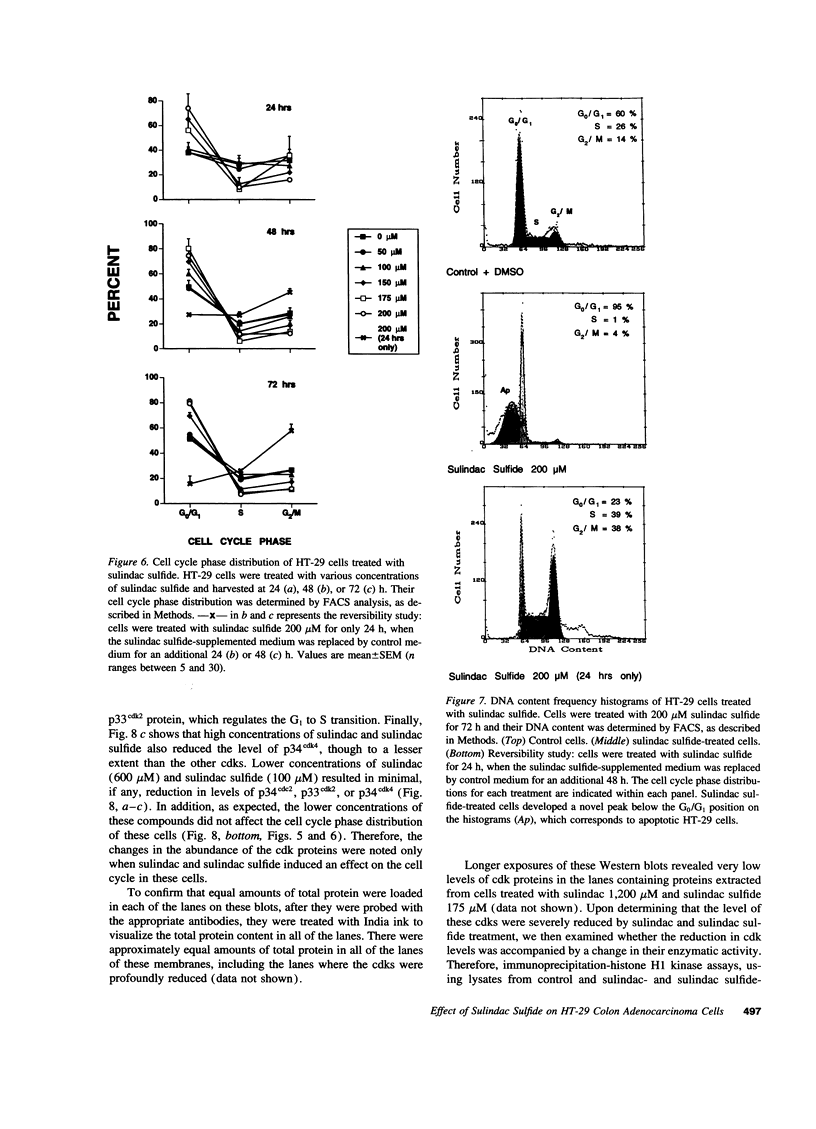
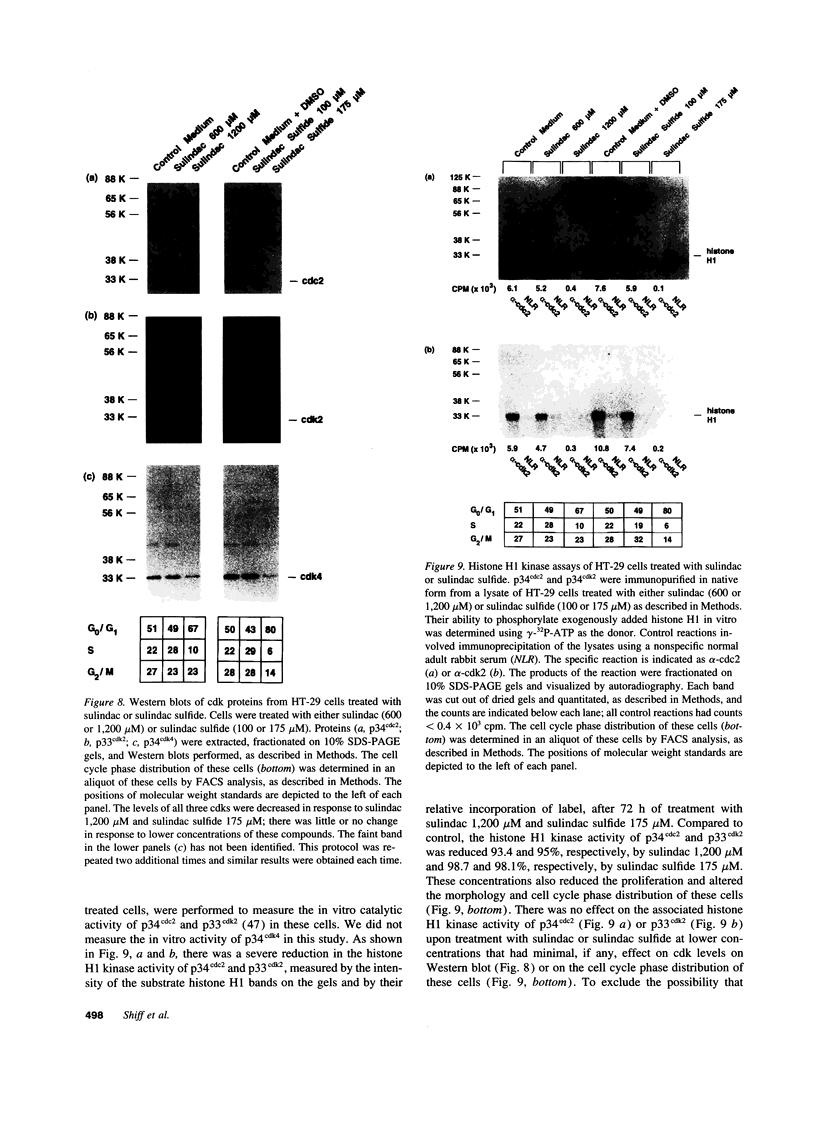

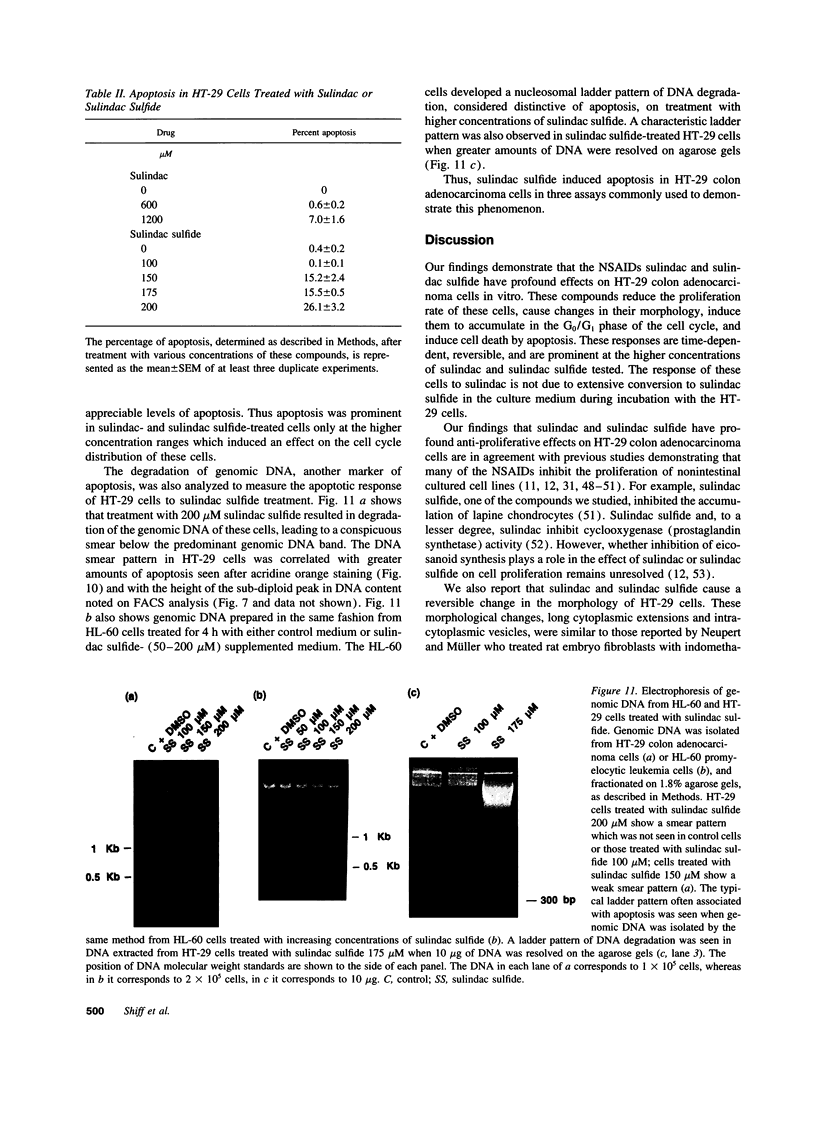



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolphe M., Deysson G., Lechat P. Action of some steroid and non-steroid anti-inflammatory agents on the cell cycle: cytophotometric study of the DNA content. Rev Eur Etud Clin Biol. 1972 Mar;17(3):320–323. [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Bayer B. M., Beaven M. A. Evidence that indomethacin reversibly inhibits cell growth in the G1 phase of the cell cycle. Biochem Pharmacol. 1979;28(3):441–443. doi: 10.1016/0006-2952(79)90112-6. [DOI] [PubMed] [Google Scholar]
- Bayer B. M., Kruth H. S., Vaughan M., Beaven M. A. Arrest of cultured cells in the G1 phase of the cell cycle by indomethacin. J Pharmacol Exp Ther. 1979 Jul;210(1):106–111. [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Cummings J. H. Short chain fatty acids in the human colon. Gut. 1981 Sep;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z. Differential staining of DNA and RNA in intact cells and isolated cell nuclei with acridine orange. Methods Cell Biol. 1990;33:285–298. doi: 10.1016/s0091-679x(08)60532-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Hare L. E., Ditzler C. A., Lei B. W., Kwan K. C. The disposition of sulindac. Clin Pharmacol Ther. 1977 Mar;21(3):326–335. doi: 10.1002/cpt1977213326. [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Hooke K. F., Hwang S. S. Kinetics of the tissue distributions of sulindac and metabolites. Relevance to sites and rates of bioactivation. Drug Metab Dispos. 1980 Jul-Aug;8(4):241–246. [PubMed] [Google Scholar]
- Dulić V., Lees E., Reed S. I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992 Sep 25;257(5078):1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- Elstein K. H., Zucker R. M. Comparison of cellular and nuclear flow cytometric techniques for discriminating apoptotic subpopulations. Exp Cell Res. 1994 Apr;211(2):322–331. doi: 10.1006/excr.1994.1094. [DOI] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P., Booker S. V., Robinson C. R., Offerhaus G. J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993 May 6;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Rimm E. B., Stampfer M. J., Colditz G. A., Ascherio A., Willett W. C. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994 Aug 15;121(4):241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- Gridley G., McLaughlin J. K., Ekbom A., Klareskog L., Adami H. O., Hacker D. G., Hoover R., Fraumeni J. F., Jr Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993 Feb 17;85(4):307–311. doi: 10.1093/jnci/85.4.307. [DOI] [PubMed] [Google Scholar]
- Hague A., Manning A. M., Hanlon K. A., Huschtscha L. I., Hart D., Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int J Cancer. 1993 Sep 30;55(3):498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- Hial V., De Mello M. C., Horakova Z., Beaven M. A. Antiproliferative activity of anti-inflammatory drugs in two mammalian cell culture lines. J Pharmacol Exp Ther. 1977 Aug;202(2):446–454. [PubMed] [Google Scholar]
- Karzel K., Aulepp H., Hack G. Effects of recently developed antiphlogistic drugs on viability, reduplication, mean volume and volume distribution of mammalian cells cultured in vitro. Pharmacology. 1973;10(5):272–290. doi: 10.1159/000136448. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. J., Mohr W., Mildfeuer A., Haferkamp O. Influence of nonsteroidal anti-inflammatory agents on lapine articular chondrocyte growth in vitro. Z Rheumatol. 1983 Mar-Apr;42(2):58–65. [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee F. D. Importance of apoptosis in the histopathology of drug related lesions in the large intestine. J Clin Pathol. 1993 Feb;46(2):118–122. doi: 10.1136/jcp.46.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Meyerson M., Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994 Mar;14(3):2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorghen M., Ince P., Finney K. J., Sunter J. P., Appleton D. R., Watson A. J. A protective effect of sulindac against chemically-induced primary colonic tumours in mice. J Pathol. 1988 Dec;156(4):341–347. doi: 10.1002/path.1711560411. [DOI] [PubMed] [Google Scholar]
- Neupert G., Müller P. Growth inhibition and morphological changes caused by indomethacin in fibroblasts in vitro. Exp Pathol (Jena) 1975;11(1-2):1–9. doi: 10.1016/s0014-4908(75)80066-4. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Roberts J. M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993 Mar 26;259(5103):1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Pines J. Arresting developments in cell-cycle control. Trends Biochem Sci. 1994 Apr;19(4):143–145. doi: 10.1016/0968-0004(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Effect of indomethacin on intestinal tumors induced in rats by the acetate derivative of dimethylnitrosamine. Science. 1981 Oct 30;214(4520):558–559. doi: 10.1126/science.7291992. [DOI] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H., Schmidt M. A. The suppressive effect of piroxicam on autochthonous intestinal tumors in the rat. Cancer Lett. 1983 Nov;21(1):57–61. doi: 10.1016/0304-3835(83)90082-4. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Li Y. Q., O'Connor P. J., Winton D. J. A possible explanation for the differential cancer incidence in the intestine, based on distribution of the cytotoxic effects of carcinogens in the murine large bowel. Carcinogenesis. 1992 Dec;13(12):2305–2312. doi: 10.1093/carcin/13.12.2305. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Rao C. V., Rivenson A., Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993 Aug;14(8):1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J., Gu Y., Morgan D. O. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Strong H. A., Warner N. J., Renwick A. G., George C. F. Sulindac metabolism: the importance of an intact colon. Clin Pharmacol Ther. 1985 Oct;38(4):387–393. doi: 10.1038/clpt.1985.192. [DOI] [PubMed] [Google Scholar]
- Swanson B. N., Boppana V. K., Vlasses P. H., Holmes G. I., Monsell K., Ferguson R. K. Sulindac disposition when given once and twice daily. Clin Pharmacol Ther. 1982 Sep;32(3):397–403. doi: 10.1038/clpt.1982.178. [DOI] [PubMed] [Google Scholar]
- Thorson A. G., Lynch H. T., Smyrk T. C. Rectal cancer in FAP patient after sulindac. Lancet. 1994 Jan 15;343(8890):180–180. doi: 10.1016/s0140-6736(94)90974-1. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [PubMed] [Google Scholar]
- Vaux D. L., Haecker G., Strasser A. An evolutionary perspective on apoptosis. Cell. 1994 Mar 11;76(5):777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Waddell W. R., Ganser G. F., Cerise E. J., Loughry R. W. Sulindac for polyposis of the colon. Am J Surg. 1989 Jan;157(1):175–179. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- Waddell W. R., Loughry R. W. Sulindac for polyposis of the colon. J Surg Oncol. 1983 Sep;24(1):83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S., Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993 Dec 24;262(5142):2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]