Abstract
The natural history of EBV and CMV reactivation and the potential for serious complications following antibody-based immunosuppressive treatment for bone marrow failure syndromes in the absence of transplantation is not known. We monitored blood for EBV and CMV reactivation by polymerase chain reaction (PCR) weekly in 78 consecutive patients (total of 99 immunosuppressive courses) with aplastic anemia. Four regimens were studied: (1) HC, horse ATG/cyclosporine; (2) HCS, horse ATG/CsA/sirolimus; (3) RC, rabbit ATG/CsA; and (4) CP, alemtuzumab. There were no cases of EBV or CMV disease, but EBV reactivation occurred in 82 (87%) of 94 and CMV reactivation in 19 (33%) of 57 seropositive patients after starting immunosuppression. The median peak EBV copies were higher in the RC group when compared with HC, HCS, and alemtuzumab (P < .001). The median duration of PCR positivity for EBV was higher in the RC group compared with HC, HCS, and alemtuzumab (P = .001). Subclinical reactivation of both EBV and CMV is common and nearly always self-limited in patients with bone marrow failure receiving immunosuppression; different regimens are associated with different intensity of immunosuppression as measured by viral load and lymphocyte count; and viral reactivation patterns differ according to immunosuppressive regimens.
Introduction
After primary infection, which usually occurs in childhood, Epstein-Barr virus (EBV) and cytomegalovirus (CMV) remain latent, EBV in B cells and CMV in monocytes, bone marrow, and other tissues.1–4 Infected persons develop lifelong humoral and cellular immunity to the viruses, but reactivation is only prevented in healthy persons through immunosurveillance by virus-specific CD8+ cytotoxic T lymphocytes and virus specific CD4+ T cells.5,6 When the cellular immune response is compromised by human immunodeficiency virus, or in patients receiving immunosuppressive therapies following solid-organ or hematopoietic stem cell transplantation (HSCT), both CMV and EBV can reactivate and cause clinical disease. Certain immunosuppressive agents, such as the monoclonal antibody to CD3, antithymocyte globulin (ATG), and alemtuzumab used in transplantation, are also associated with an elevated incidence of CMV and/or EBV reactivation and disease.7–9
Major complications from EBV and CMV reactivation can usually be avoided by regular monitoring of viral DNA or viral antigen, but these assays are so sensitive that they detect levels of viral reactivation below the threshold of clinical significance. Because it is common practice to promptly treat CMV or EBV reactivation in HSC transplant or organ transplant recipients, the natural history of EBV and CMV reactivation after immunosuppressive treatment is not known. Indeed, therapeutic immunosuppression outside the context of allogeneic stem cell or organ transplantation is only rarely complicated by CMV or EBV disease.10–13 For example, we have treated more than 1000 patients with severe aplastic anemia (SAA) with immunosuppressive regimens without encountering CMV disease and with only a single instance of EBV-induced lymphoproliferative disorder (genetic testing for X-linked lymphoproliferative disease in this case was negative). This latter event stimulated us to systematically search for EBV and CMV reactivation following several immunosuppressive regimens currently in use to treat SAA to better understand the dynamics of viral load increases. Here, we report that distinct patterns of reactivation in patients with SAA receiving various immunosuppressive regimens are common but without clinical consequence or need for treatment.
Patients, materials, and methods
Seventy-eight consecutive patients with aplastic anemia who were treated between January 2004 and April 2006 at the Warren Grant Magnuson Clinical Center and Mark O. Hatfield Clinical Research Center at the National Institutes of Health in Bethesda, MD, were studied. Patients signed informed consent for study protocols approved by the Institutional Review Board of the National, Heart, Lung, and Blood Institute, Bethesda, MD. Criteria for SAA in this study has been defined previously.14
Immunosuppressive regimens
Treatment-naive patients with SAA were randomly assigned to receive horse ATG/cyclosporine (HC) or horse ATG/cyclosporine/sirolimus (HCS). Intravenous horse ATG (ATGAM; Pharmacia & Upjohn Company, Kalamazoo, MI) was administered at a dose of 40 mg/kg daily for 4 days. Serum sickness prophylaxis with oral prednisone 1 mg/(kg · d) was given prior to the first dose of horse ATG and continued for 10 days and then tapered over the subsequent 7 days. Cyclosporine 10 mg/(kg · d) by mouth [15 mg/(kg · d) for children < 12 years] in divided doses every 12 hours was started on day 1 and continued for at least 6 months. Dosing was adjusted to maintain cyclosporine levels between 200 and 400 ng/mL. Oral sirolimus 2 mg/d in adults and 1 mg/(m2 · d) in children (< 40 kg) was given on day 1 of ATG and continued for 6 months; dose was adjusted to maintain serum levels between 5 and 15 ng/mL.
In patients who had no response to horse ATG, a second course of treatment was administered after random assignment between rabbit ATG/cyclosporine (RC) or alemtuzumab (Campath; CP). Rabbit ATG (Thymoglobulin) was given at a dose of 3.5 mg/(kg · d) for 5 consecutive days. Serum sickness prophylaxis and cyclosporine (for 6 months) was administered as described for horse ATG. After a test dose of 1 mg and premedication with oral diphenhydramine and acetaminophen, alemtuzumab was given by 2-hour intravenous infusion of 10 mg/d for 10 days. As prophylaxis for Pneumocystis carinii pneumonia all patients received aerosolized pentamidine for at least 6 months. Daily valacyclovir at a dose of 500 mg daily for at least 8 weeks was given for herpes simplex virus prophylaxis in the HCS and alemtuzumab recipients. A third course of immunosuppression was administered to patients with persistent pancytopenia and not suitable for HSCT. Three patients with relapsed SAA were treated with alemtuzumab with the same regimen as the refractory patients.
EBV and CMV monitoring
EBV and CMV molecular monitoring was performed at baseline, weekly for the first month after each treatment course, every 2 weeks in the second month, and monthly thereafter for another 6 months. More frequent testing was done at the discretion of the treating physician. EBV and CMV quantitative real-time polymerase chain reaction (Q-PCR) were done as previously described.15,16 Briefly, blood samples were collected in EDTA-treated tubes and the NucliSens kit reagents (bioMérieux, Durham, NC) were used according to the manufacturer's recommendations to extract total nucleic acid from the whole-blood (for CMV) and peripheral blood mononuclear cells (for EBV). To verify that impurities that might contribute to PCR inhibition were completely removed during the extraction procedure, an internal control that was amplifiable by the CMV and EBV primers were constructed. DNA was quantified using the LightCycler system (Roche Molecular Biochemicals, Indianapolis, IN). For CMV Q-PCR, positive and negative controls were included with each batch of patient specimens extracted. A plasmid that contained the amplification site was generated by cloning the target region, located on the CMV glycoprotein-B gene, into the pCR2.1 vector (Invitrogen, Carlsbad, CA). In each experiment, duplicates of the standards (5000, 500, 50, and 5 copies/reaction) were included to generate a standard curve for the quantification of positive patient samples. For EBV, amplichek EBV viral DNA control (Advanced Biotechnologies, Columbia, MD) was used as a quantitative reference standard. To determine the number of human cellular equivalents tested for the presence of EBV, another aliquot of patient DNA samples was also subjected to real-time PCR analysis for the human β-globin gene. The EBV viral load was expressed per 106 mononuclear cell genome equivalents and CMV load as copies per milliliter of blood.
A positive PCR was defined as more than 250 EBV copies/106 mononuclear cells genome equivalents (MNCs) or more than 250 CMV copies/mL blood. The duration of PCR positivity was defined as the time from the initial EBV reactivation to the first negative PCR.
Statistical analysis
Summary statistics, such as proportions, means, standard deviations, 95% confidence intervals, medians, and ranges, were used to describe the related variables. Log transformation of peak EBV copies were taken to be the log10 transformation whenever the peak EBV copies were positive, and set to zero when there was no EBV reactivation. The same log transformation was also applied to peak CMV copies. Multiple regression models were used to evaluate the relationship between duration of positive EBV reactivation and log peak EBV copies. Statistical tests based on t tests, chi square tests, likelihood ratio tests, and analysis of variance F tests were used to evaluate the statistical significance of treatment groups and the effects of covariates in multiple regression models. All numerical results were computed using Splus 6 (Insightful Corp, Seattle, WA) for Windows statistical software.
Results
Seventy-eight patients aged 4 to 79 years received a total of 99 courses of immunosuppression. At least 6 viral load measurements following treatment in a 6-month period were required for a patient to be considered evaluable for viral analysis. In one patient EBV serostatus was not determined prior to therapy. Overall, 34 patients were treated with HC, 25 with HCS, 14 with RC, and 26 with alemtuzumab. Seventy-six patients (97%) were seropositive for antibodies to EBV and 57 (73%) were seropositive for CMV prior to immunosuppressive therapy. No patient developed clinical disease from primary infection or reactivation of EBV or CMV, and no treatment for either virus was instituted. Among seropositive patients, EBV and CMV reactivations were frequent and varied in intensity and duration, according to the immunosuppressive regimen.
EBV reactivations
EBV reactivation occurred in 82 (87%) of 94 courses in seropositive patients. All EBV seropositive patients in the RC arm reactivated. The peak level of EBV reactivation usually occurred within the first 3 weeks of treatment, with the exception of HCS recipients, in whom peak EBV reactivation occurred beyond 3 weeks in approximately half the patients. Among patients who reactivated EBV, the median peak EBV copies was higher in the RC group when compared with the HC, HCS, and alemtuzumab groups (P < .001); and the median duration of PCR positivity for EBV was higher in the RC group compared with the HC, HCS, and alemtuzumab groups (P = .001) (Table 1)
Table 1.
Characteristics of EBV reactivations on all patients treated with immunosuppressive therapy
| HC | HCS | RC | CP | P* | |
|---|---|---|---|---|---|
| Courses, no. (%)† | 33 (33) | 25 (25) | 14 (14) | 26 (26) | |
| Courses in patients seropositive for EBV, no. (%) | 33 (100) | 24 (96) | 13 (93) | 24 (92) | NS |
| EBV reactivations in seropositive patients, no. (%) | 29 (88) | 22 (92) | 13 (100) | 19 (80) | NS |
| Peak EBV copies, median (range) | 4 380 (480-160 000) | 5 000 (260-466 000) | 270 000 (6 700-1 025 000) | 10 000 (620-655 000) | |
| Mean of log peak EBV copies (95% CI) | 3.8 (3.6-4.0) | 3.6 (3.2-4.0) | 5.2 (4.8-5.6) | 4.1 (3.7-4.6) | < .001 |
| Time to peak EBV | |||||
| Median, d (range) | 17 (4-55) | 21 (4-182) | 19 (7-27) | 6 (4-70) | |
| Mean, d (95% CI) | 18 (13-23) | 43 (21-66) | 17 (12-22) | 12 (5-20) | .003 |
| Positivity for EBV, wk | |||||
| Median (range) | 6 (1-25) | 5 (1-28) | 12 (4-24) | 2 (0-19) | |
| Mean (95% CI) | 8 (6-10) | 10 (6-14) | 13 (9-17) | 3 (1-5) | .001 |
HC indicates horse ATG/CsA; HCS, horse ATG/CsA/sirolimus; RC, rabbit ATG/CsA; CP, alemtuzumab; NS, not statistically significant.
P values are for the comparison among the 4 groups; P value is computed based on the ANOVA F test for the log-transformed peak EBV copies.
EBV serostatus was not determined in 1 patient in the HC arm (not tabulated) prior to immunosuppressive therapy.
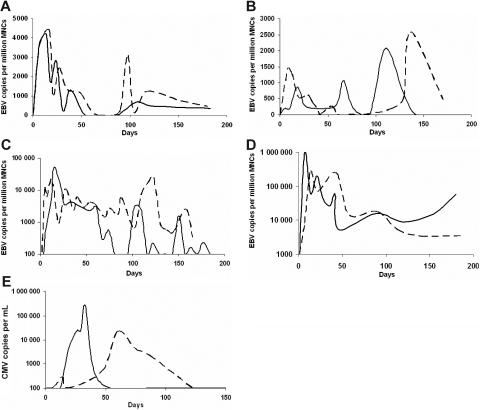
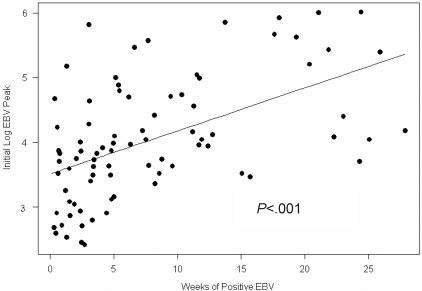
More than half of the initial EBV reactivations in the HC, HCS, and CP arms were at levels less than or equal to 104 copies/106 MNCs but were higher in the RC arm (> 105 copies) (Table 2). EBV reactivation generally followed 4 distinct patterns. Illustrative examples of each are shown in Figure 1A-D. In some patients, after an initial reactivation peak in the first 3 weeks, EBV PCR positivity usually decreased over 1 to 2 weeks (Figure 1A); in other cases the highest EBV PCR positivity was observed later in the course of immunosuppression (Figure 1B). Successive increase in EBV viral load often followed and in other cases, the EBV PCR remained positive for many months with a steady decline after initial reactivation or with fluctuating levels of viral loads (despite steady levels of CsA and sirolimus [Figure 1C]). Within the therapeutic range, a correlation between drug levels and viral load was not observed. In 10 patients (2 in the HC, 5 in the HCS, and 3 in the RC arm), the EBV PCR remained positive for 5 to 6 months following ATG (Figure 1D). Patients with the highest EBV copy numbers were more likely to have persistent PCR-positivity (Figure 2). During a period of more than 4700 days of EBV PCR positivity during this study, there were no cases of symptomatic EBV.
Table 2.
Range of peak EBV reactivation in EBV seropositive patients
| EBV copies per 106 MNCs/treatment | HC, no. (%) | HCS, no. (%) | RC, no. (%) | CP, no. (%) | All groups, no. (%) |
|---|---|---|---|---|---|
| Negative | 4 (12) | 2 (8) | 0 (0) | 6 (21) | 12 (12) |
| Greater than 250-103 | 3 (9) | 7 (29) | 0 (0) | 1 (4) | 12 (12) |
| Greater than 103-104 | 18 (55) | 7 (29) | 1 (8) | 9 (36) | 36 (37) |
| Greater than 104-105 | 7 (21) | 6 (18) | 4 (31) | 6 (21) | 23 (23) |
| Greater than 105 | 1 (3) | 2 (8) | 8 (62) | 3 (12) | 15 (15) |
HC indicates horse ATG/CsA; HCS, horse ATG/CsA/sirolimus; RC, rabbit ATG/CsA; CP, alemtuzumab; MNCs, mononuclear cells genome equivalents.
Figure 1.
Illustrative examples of viral reactivation patterns in individual patients treated with antibody-based immunosuppressive therapy. Dashed lines and solid lines represent separate patients. Following HC (A), EBV DNA positivity peaked at about 3 weeks, decreasing precipitously in the following weeks. Increases in EBV viral load were common in subsequent months (A). In more than half the patients who received HCS, the highest EBV viral load occurred later than 3 weeks (B). In some cases the EBV PCR remained positive for most of the course of immunosuppression with frequent fluctuations in viral load (C; HC, solid; HCS, dashed). Patients who received RC had the highest EBV copies of all 4 groups, often remaining positive for many months (D). CMV reactivation occurred more frequently after HC, and the highest CMV viral load of our cohort was in the CP arm (E; CP, solid line; HC, dashed line). HC and HCS were administered as a first course of immunosuppression, and RC and CP as a second course of immunosuppression. HC indicates horse ATG + cyclosporine; HCS, horse ATG + cyclosporine + sirolimus; RC, rabbit ATG + cyclosporine; CP, alemtuzumab.
Figure 2.
Number of weeks of positive PCR for EBV DNA following initial reactivation in relation to the initial EBV viral load peak for all treatment groups. Patients with a greater initial EBV reactivation remained positive for EBV PCR longer. Slope = 0.06 (SD of slope = 0.01).
CMV reactivations
CMV reactivations occurred in 19 (33%) of 57 courses in seropositive patients. The incidence of CMV reactivations in each treatment group is shown in Table 3Four patients did not reactivate following the initial course of immunosuppression but then reactivated CMV when re-treated with RC or alemtuzumab. One developed the highest CMV viral load of the cohort (268 000 CMV copies /mL) on day 34 following alemtuzumab (Figure 1E); CMV antigenemia at this time showed more than 100 cells positive per 400 000 cells. CMV copy numbers decreased to 550 in the following week and then to zero without treatment. CMV reactivations usually occurred in tandem with EBV; however, in some cases, the CMV peaked 1 month after the EBV peak as was observed in the HC arm (Tables 1 and 2). Although there were more than 700 days of documented CMV PCR positivity (> 250 CMV copies/mL blood) in patients in this study, none developed CMV disease.
Table 3.
Characteristics of CMV reactivations on all patients treated with immunosuppressive therapy
| HC | HCS | RC | CP | P* | |
|---|---|---|---|---|---|
| Courses, no. (%) | 34 (34) | 25 (25) | 14 (14) | 26 (26) | |
| Courses in patients seropositive for CMV, no. (%) | 26 (76) | 18 (72) | 12 (86) | 19 (73) | NS |
| CMV reactivations in seropositive patients, no. (%) | 9 (35) | 2 (11) | 4 (33) | 4 (21) | NS |
| Peak CMV copies | |||||
| Median (range) | 1 400 (300-39 500) | 750 (650-850) | 762 (450-1 875) | 2 313 (1 200-268 000) | |
| Mean of log peak CMV copies (95% CI) | 3.3 (2.7-3.9) | 2.9 (2.1-3.6) | 2.9 (2.5-3.3) | 3.8 (2.0-5.6) | NS |
| D to peak CMV | |||||
| Median (range) | 54 (26-95) | 26 (24-27) | 23 (13-54) | 26 (21-34) | |
| Mean (95% CI) | 54 (37-72) | 26 (6-45) | 28 (0-58) | 27 (18-35) | .049 |
| Wk of positivity for CMV | |||||
| Median (range) | 5 (2-17) | 3 (2-4) | 4 (2-7) | 3 (2-5) | |
| Mean (95% CI) | 7 (3-12) | 3 (0-15) | 4 (0-7) | 3 (1-5) | NS |
HC indicates horse ATG/CsA; HCS, horse ATG/CsA/sirolimus; RC, rabbit ATG/CsA; CP, alemtuzumab; NS, not statistically significant.
P values are for the comparison among the 4 groups; P value is computed based on the ANOVA F test for the log-transformed peak CMV copies.
Discussion
Immunosuppression with ATG and CsA is the standard initial treatment in patients with SAA who are not suitable candidates for HSCT.17 Patients with SAA almost never develop clinical complications from EBV or CMV reactivation with immunosuppressive regimens. For these reasons, and because antiviral chemotherapy can itself cause morbidity, including cytopenia, we considered it justifiable to monitor patients with SAA receiving immunosuppression for viral reactivation without intervening with antiviral therapy. We therefore had the opportunity to study the natural evolution of CMV and EBV reactivation under 4 immunosuppression protocols.
SAA is most often a T-cell–mediated organ-specific destruction of the hematopoietic stem cell compartment.18 Aside from infectious complications resulting from neutropenia, patients with aplastic anemia generally do not have cellular or humoral immunodeficiency and do not have an increased susceptibility to viral infections; with susceptibility to virus being related only to immunosuppressive therapy. Immunoglobulin levels and antibody titers to common viral antigens are typically normal, and some patients are anergic to a panel of common antigens, but lymphocyte proliferation in tissue culture is normal.19,20 Clinical and laboratorial associations have been made between EBV and aplastic anemia when it occurs following a mononucleosis-like syndrome21,22 or when there is evidence of EBV infection in the bone marrow cells.23 The association between CMV and aplastic anemia is weaker and is based on short reports of CMV-infected marrow cells in patients with aplastic anemia.24 Both EBV and CMV have been implicated in the pathogenesis of certain cases of aplastic anemia, but the vast majority of cases are idiopathic, with no prodrome or other evidence of a viral infection which precedes the onset of pancytopenia. Although the objective of this study was not to establish the causality of EBV infection and SAA, we did notice that 14 (18%) of 78 patients had EBV DNA positivity in the peripheral blood prior to receiving a first course of immunosuppression (range, 260-7700 copies/106 MNCs; median, 775 copies). Five patients with myelodysplasia (not included in the analysis) who were treated with alemtuzumab at our institution were monitored for viral reactivation. One patient reactivated CMV and 3 reactivated EBV with no clinical consequence. Thus, viral reactivation observed in our patients was most likely related to the treatment and not to the underlying disease, as is supported by the temporal relationship between immunosuppressive treatment and viral reactivation. Furthermore, our patients had not received other treatments liable to cause immune suppression prior to entry into our research studies.
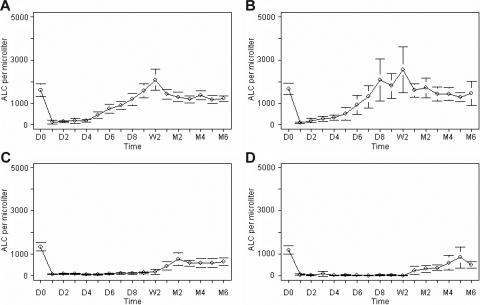
EBV viral loads were higher in patients receiving rabbit ATG compared with horse ATG, suggesting that rabbit ATG was more immunosuppressive than horse ATG. Indeed, lymphopenia was more prolonged with rabbit ATG compared with horse ATG in our study (Figure 3). Also, rabbit ATG has been reported to be more effective than horse ATG in preventing and reversing acute renal allograft rejection25,26 as well as being associated with a higher incidence of post-transplantation lymphoproliferative disease (PTLD) after HSCT.27,28 The enhanced clinical efficacy of rabbit ATG may be explained by higher affinity IgG subtype to human lymphocytes, less batch-to-batch variability, longer half-life, and more efficient lymphocyte depletion.29 We cannot exclude that the greater increase in EBV viral load following rabbit ATG is related to its administration as a second course of immunosuppression to refractory patients. Despite its ability to induce similar duration of lymphopenia, a similar increase in EBV viral load was not seen in patients receiving alemtuzumab (also as a second course of immunosuppression), probably because the anti-CD52 antibody also reduces the B cells which are the EBV reservoir.30,31 Of interest are 2 patients who experienced EBV reactivation following horse ATG but not after a second course of immunosuppression with alemtuzumab.
Figure 3.
Absolute peripheral blood lymphocyte count after immunosuppression. Mean absolute peripheral blood lymphocyte count (ALC; with 95% confidence interval) at baseline and following 95 courses of immunosuppression with (A) HC, (B) HCS, (C) RC, and (D) CP. D indicates day; W, week; M, month.
The degree and duration of EBV reactivation varied widely in our patients from no reactivation to DNA copy numbers in the region of 103 to 105, levels which in other clinical contexts are associated with PTLD.32 Although viral reactivations were self-limited in SAA, the same degree of reactivation often is preemptively treated following HSCT.33,34 The presence of a rising EBV viral load often precedes the onset of symptomatic EBV, and viral loads at the time of diagnosis of PTLD are usually very elevated. Because the temptation to intervene is high as a consequence of rising EBV viral loads following transplantation, few studies have monitored EBV viral loads prospectively with no interventions prior to the onset of PTLD. The risk of PTLD in the presence of an elevated EBV PCR after HSCT depends on the degree of CD8+ T-lymphocyte recovery and may also be affected by genetic variation in chemokines, chemokine receptors, and cytokine expression affecting the immune response.34,35 Because the goal of determining which patients with rising viral loads will likely develop PTLD remains elusive, understanding the different effects of immunosuppressive agents on EBV and CMV may help clarify this important clinical distinction. Our data show that after the initial EBV peak, the viral load precipitously decreases in subsequent weeks; the dynamics of EBV and CMV reactivation varied according to the immunosuppressive regimen; fluctuations in viral load are not uncommon following initial reactivation; and high EBV viral load correlates with persistently elevated PCR positivity for a longer period of time during immunosuppressive treatment. The study of the virus-specific immune response (with tetramers and/or interferon-γ secretion assays) during periods of reactivation in our cohort will be of value because no antivirals were instituted therapeutically with all reactivations being self-limited despite high levels of viremia. In particular patients who experienced frequent reactivations may assist in the understanding of loss of viral control.
CMV reactivations were less frequent than was EBV reactivations. After HSCT, ATG is not clearly associated with CMV reactivation and disease. In contrast, alemtuzumab used in HSCT and in the treatment of CLL has led to CMV reactivations and disease.7,36 The highest single CMV reactivation in our cohort was seen in a patient who received alemtuzumab. Notably, there was no reactivation of CMV in this patient during the earlier course of HCS. The patient remained asymptomatic during the CMV DNA positivity, and no treatment was instituted. Among the different ATG formulations, the RC arm often resulted in very high EBV viral loads but not in high CMV viral loads; conversely, antibody treatment with HC often resulted in a high CMV viral load and longer duration of PCR positivity for CMV but not EBV. Thus, other virus-specific factors may play a role in reactivation other than the pan-CD8 diminution that occurs following treatment with immunosuppressive agents or regimens.
Our findings highlight the existence of a subclinical level of DNA virus reactivation following immunosuppressive therapy outside the transplantation setting. The progression to clinical disease after EBV or CMV reactivation in transplant recipients therefore appears to correlate more with the inability to mount an effective immune response to the reactivating virus rather than to viral reactivation itself. Functional measures of viral-specific immunity may more accurately predict clinical disease than do simple quantitations of viral load. In a recent retrospective analysis, a higher incidence of PTLD was observed in patients undergoing umbilical cord cell transplantation who were conditioned with horse ATG-containing regimens.37 Although the highest EBV viral loads in our cohort were following RC, a recently treated patient who received HC (not included in the present analysis) developed EBV copy numbers of 2 800 000/106 MNCs 1 month following immunosuppressive treatment; one of the highest observed to date in our cohort. The viral load decreased by 2 logs in the following 10 days to 28 000 copies; the patient is remaining asymptomatic. Nevertheless, since this analysis, a patient reached 7 400 000/106 MNCs EBV copies 1 month following treatment with RC (the highest EBV viral load in our cohort). Our findings justify the continued practice of treating SAA with ATG without routinely monitoring viral reactivation. However, caution and attention to monitoring viral reactivation is appropriate when introducing new and more potent immunosuppressive regimens into clinical practice, because the threshold to the development of disease from CMV or EBV reactivation is not well understood.
Acknowledgments
We thank Vu Haphuong and Barbara Weinstein for being attentive and careful with the scheduling and handling of samples.
This work was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.S. is the principal investigator; provided primary conception, execution, and data analysis; and drafted the manuscript. E.M.S. contributed to patient enrollment, data collection, and manuscript preparation. J.I.C., N.S.Y., and A.J.B. were involved in the design, data analysis, interpretation of results, interim discussions, and manuscript preparation. S.H.F. and L.L. did the EBV and CMV Q-PCR. C.O.W. analyzed the data. O.N. was the research nurse and contributed to enrolling patients in the study, ensured adherence to viral monitoring, and attended to patients needs.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Phillip Scheinberg, Hematology Branch, NHLBI, 10 Center Dr, Bldg 10 CRC, Rm 3-5140, MSC 1202, Bethesda, MD 20892-1202; e-mail: scheinbp@nhlbi.nih.gov.
References
- 1.Williams H, Crawford DH. Epstein-Barr virus: the impact of scientific advances on clinical practice. Blood. 2006;107:862–869. doi: 10.1182/blood-2005-07-2702. [DOI] [PubMed] [Google Scholar]
- 2.Pignatelli S, Dal Monte P, Rossini G, et al. Latency-associated human cytomegalovirus glycoprotein N genotypes in monocytes from healthy blood donors. Transfusion. 2006;46:1754–1762. doi: 10.1111/j.1537-2995.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 3.Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76(pt 4):741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Hoy C, Torok-Storb B. Occult cytomegalovirus infection of marrow stroma. Clin Infect Dis. 1998;26:209–210. doi: 10.1086/517022. [DOI] [PubMed] [Google Scholar]
- 5.Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Landais E, Saulquin X, Houssaint E. The human T cell immune response to Epstein-Barr virus. Int J Dev Biol. 2005;49:285–292. doi: 10.1387/ijdb.041947el. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 8.Hibberd PL, Tolkoff-Rubin NE, Cosimi AB, et al. Symptomatic cytomegalovirus disease in the cytomegalovirus antibody seropositive renal transplant recipient treated with OKT3. Transplantation. 1992;53:68–72. doi: 10.1097/00007890-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Gandhi M, Naik P, et al. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br J Haematol. 2005;129:229–239. doi: 10.1111/j.1365-2141.2005.05439.x. [DOI] [PubMed] [Google Scholar]
- 10.Calistri E, Tiribelli M, Battista M, et al. Epstein-Barr virus reactivation in a patient treated with anti-thymocyte globulin for severe aplastic anemia. Am J Hematol. 2006;81:355–357. doi: 10.1002/ajh.20560. [DOI] [PubMed] [Google Scholar]
- 11.Dorr V, Doolittle G, Woodroof J. First report of a B cell lymphoproliferative disorder arising in a patient treated with immune suppressants for severe aplastic anemia. Am J Hematol. 1996;52:108–113. doi: 10.1002/(SICI)1096-8652(199606)52:2<108::AID-AJH7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Koo JY, Kadonaga JN, Wintroub BV, Lozada-Nur FI. The development of B-cell lymphoma in a patient with psoriasis treated with cyclosporine. J Am Acad Dermatol. 1992;26:836–840. doi: 10.1016/0190-9622(92)70117-x. [DOI] [PubMed] [Google Scholar]
- 13.Oosterveld M, Lesterhuis WJ, MacKenzie M, van Krieken JH. EBV-related lymphoproliferative disorders in immunocompetent patients. Leukemia. 2003;17:2537–2538. doi: 10.1038/sj.leu.2403155. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- 15.Feng WH, Cohen JI, Fischer S, et al. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst. 2004;96:1691–1702. doi: 10.1093/jnci/djh313. [DOI] [PubMed] [Google Scholar]
- 16.Cortez KJ, Fischer SH, Fahle GA, et al. Clinical trial of quantitative real-time polymerase chain reaction for detection of cytomegalovirus in peripheral blood of allogeneic hematopoietic stem-cell transplant recipients. J Infect Dis. 2003;188:967–972. doi: 10.1086/378413. [DOI] [PubMed] [Google Scholar]
- 17.Frickhofen N, Rosenfeld SJ. Immunosuppressive treatment of aplastic anemia with antithymocyte globulin and cyclosporine. Semin Hematol. 2000;37:56–68. doi: 10.1016/s0037-1963(00)90030-1. [DOI] [PubMed] [Google Scholar]
- 18.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elfenbein GJ, Kallman CH, Tutschka PJ, et al. The immune system in 40 aplastic anemia patients receiving conventional therapy. Blood. 1979;53:652–665. [PubMed] [Google Scholar]
- 20.Falcao RP, Voltarelli JC, Bottura C. Some immunological studies in aplastic anaemia. J Clin Lab Immunol. 1983;10:25–28. [PubMed] [Google Scholar]
- 21.van Doornik MC, van TV-KET, Wierenga H. Fatal aplastic anaemia complicating infectious mononucleosis. Scand J Haematol. 1978;20:52–56. doi: 10.1111/j.1600-0609.1978.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 22.Ahronheim GA, Auger F, Joncas JH, Ghibu F, Rivard GE, Raab-Traub N. Primary infection by Epstein-Barr virus presenting as aplastic anemia. N Engl J Med. 1983;309:313–314. [PubMed] [Google Scholar]
- 23.Baranski B, Armstrong G, Truman JT, Quinnan GV, Jr, Straus SE, Young NS. Epstein-Barr virus in the bone marrow of patients with aplastic anemia. Ann Intern Med. 1988;109:695–704. doi: 10.7326/0003-4819-109-9-695. [DOI] [PubMed] [Google Scholar]
- 24.Torok-Storb B, Bolles L, Iwata M, et al. Increased prevalence of CMV gB3 in marrow of patients with aplastic anemia. Blood. 2001;98:891–892. doi: 10.1182/blood.v98.3.891. [DOI] [PubMed] [Google Scholar]
- 25.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. doi: 10.1097/00007890-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Nash RA, Dansey R, Storek J, et al. Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant. 2003;9:583–591. doi: 10.1016/s1083-8791(03)00228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peres E, Savasan S, Klein J, Abidi M, Dansey R, Abella E. High fatality rate of Epstein-Barr virus-associated lymphoproliferative disorder occurring after bone marrow transplantation with rabbit antithymocyte globulin conditioning regimens. J Clin Microbiol. 2005;43:3540–3543. doi: 10.1128/JCM.43.7.3540-3543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas FT, Griesedieck C, Thomas J, et al. Differential effects of horse ATG and rabbit ATG on T cell and T cell subset levels measured by monoclonal antibodies. Transplant Proc. 1984;16:1561–1563. [PubMed] [Google Scholar]
- 30.Juliusson G, Theorin N, Karlsson K, Frodin U, Malm C. Subcutaneous alemtuzumab vs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transplant. 2006;37:503–510. doi: 10.1038/sj.bmt.1705263. [DOI] [PubMed] [Google Scholar]
- 31.Lundin J, Porwit-MacDonald A, Rossmann ED, et al. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukaemia. Leukemia. 2004;18:484–490. doi: 10.1038/sj.leu.2403258. [DOI] [PubMed] [Google Scholar]
- 32.van Esser JW, Niesters HG, van der Holt B, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–4369. doi: 10.1182/blood.v99.12.4364. [DOI] [PubMed] [Google Scholar]
- 33.Gruhn B, Meerbach A, Hafer R, Zell R, Wutzler P, Zintl F. Pre-emptive therapy with rituximab for prevention of Epstein-Barr virus-associated lymphoproliferative disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:1023–1025. doi: 10.1038/sj.bmt.1704061. [DOI] [PubMed] [Google Scholar]
- 34.Meij P, van Esser JWJ, Niesters HGM, et al. Impaired recovery of Epstein-Barr virus (EBV)—specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101:4290–4297. doi: 10.1182/blood-2002-10-3001. [DOI] [PubMed] [Google Scholar]
- 35.Ohshima K, Karube K, Hamasaki M, et al. Differential chemokine, chemokine receptor and cytokine expression in Epstein-Barr virus-associated lymphoproliferative diseases. Leuk Lymphoma. 2003;44:1367–1378. doi: 10.1080/1042819031000082984. [DOI] [PubMed] [Google Scholar]
- 36.Laurenti L, Piccioni P, Cattani P, et al. Cytomegalovirus reactivation during alemtuzumab therapy for chronic lymphocytic leukemia: incidence and treatment with oral ganciclovir. Haematologica. 2004;89:1248–1252. [PubMed] [Google Scholar]
- 37.Brunstein CG, Weisdorf DJ, Defor T, et al. Marked increased risk of epstein-barr virus-related complications with the addition of anti-thymocyte globulin to a non-myeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]