Abstract
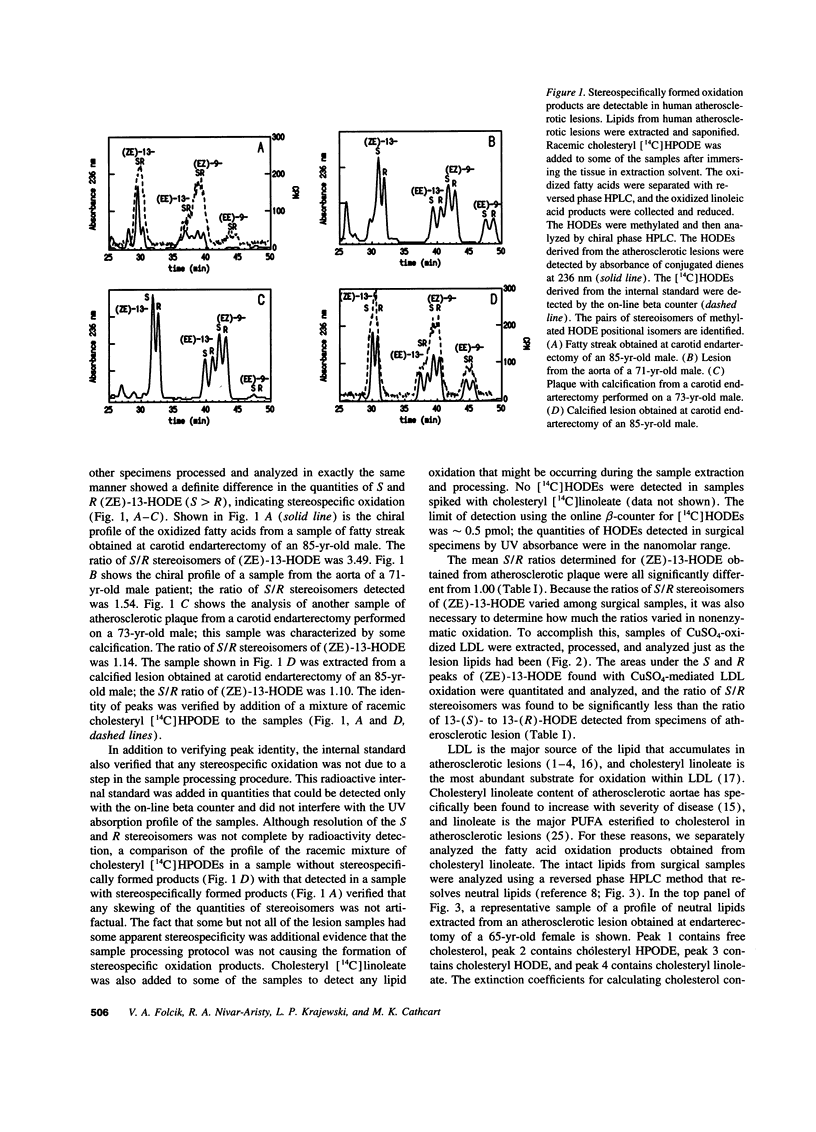
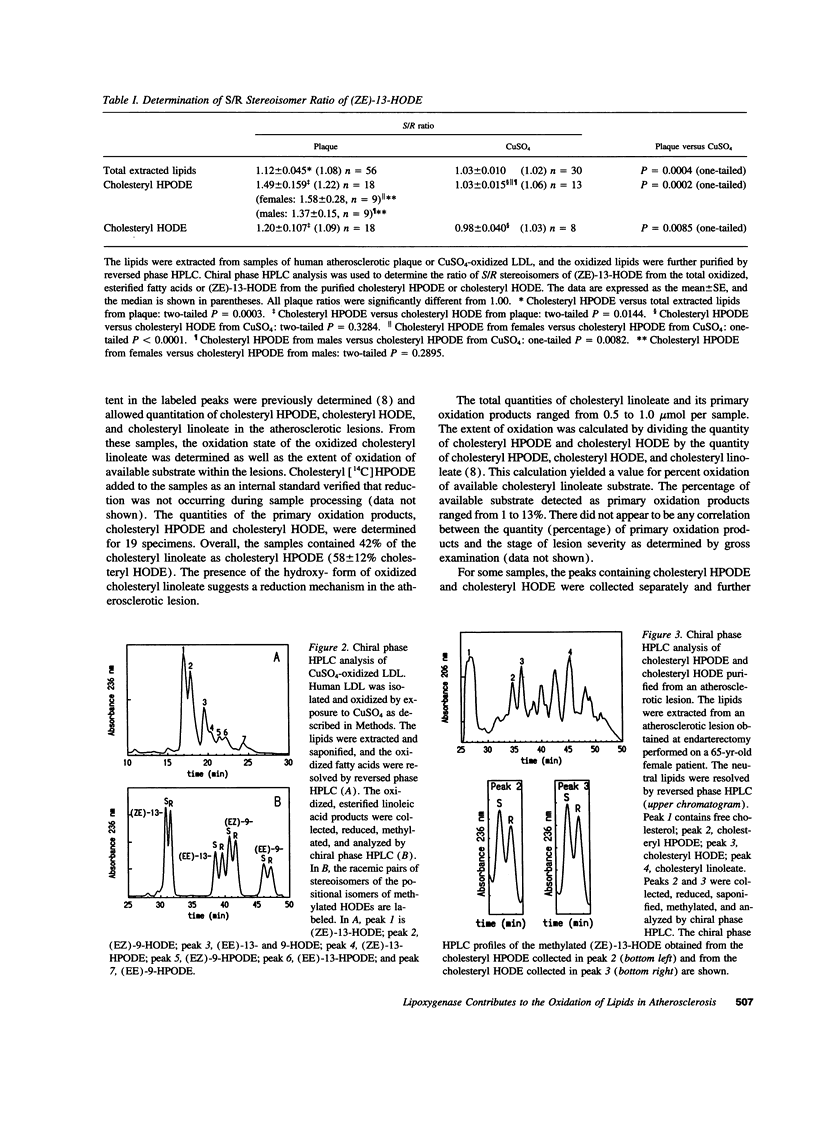
Oxidized LDL is present in human atherosclerotic lesions, but the mechanisms responsible for oxidation in vivo have not been definitively demonstrated. Circumstantial evidence has implicated the enzyme 15-lipoxygenase as a contributor to the formation of oxidized lipids in this disease. To assess whether oxidized lipids are indeed formed by the action of 15-lipoxygenase on polyunsaturated fatty acids (PUFAs) in vivo, we have used a sensitive and specific method (chiral phase HPLC) to analyze the lipid oxidation products present in human atherosclerotic lesions. Human 15-lipoxygenase is an omega-6 lipoxygenase that has previously been shown to oxidize esterified PUFA in a stereospecific manner, forming predominantly cholesteryl hydroperoxy-octadecadienoate (13(S)-HPODE) from cholesteryl linoleate substrate in LDL. This property allows its activity to be distinguished from nonenzymatic oxidation, which results in the formation of equal quantities of the S and R stereoisomers of the same oxidation product. A total of 80 specimens of human atherosclerotic plaque were analyzed. Esterified, oxidized linoleate was purified from human atherosclerotic lesions and from LDL oxidized by copper, and the chirality of these oxidation products was compared. There was significantly greater stereospecificity of oxidation in the oxidized linoleate from human atherosclerotic lesions. Even greater stereospecificity was detected in the HPODE derived from cholesteryl ester, purified from human lesions. Cholesteryl HPODE is the primary oxidation product from cholesteryl linoleate, the major esterified PUFA that accumulates in atherosclerotic vessels. Cholesteryl HPODE and its reduced form, cholesteryl hydroxy-octadecadienoate, were detected in all lesions analyzed. Neither the stereospecificity of oxidation nor the percentage of available substrate oxidized to primary oxidation products was correlated with the stage of disease of the lesions examined. We conclude that 15-lipoxygenase contributes to the formation of oxidized lipids in human atherosclerotic lesions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BOTTCHER C. J., WOODFORD F. P., ter HAAR ROMENY-WACHTER C., BOELSMA-VAN HOUTE E., van GENT C. Fatty-acid distribution in lipids of the aortic wall. Lancet. 1960 Jun 25;1(7139):1378–1383. doi: 10.1016/s0140-6736(60)91153-3. [DOI] [PubMed] [Google Scholar]
- Belkner J., Wiesner R., Kühn H. Identification of oxidatively modified lipids in atherosclerotic lesions of human aortas. Agents Actions Suppl. 1992;37:78–84. doi: 10.1007/978-3-0348-7262-1_12. [DOI] [PubMed] [Google Scholar]
- Belkner J., Wiesner R., Kühn H., Lankin V. Z. The oxygenation of cholesterol esters by the reticulocyte lipoxygenase. FEBS Lett. 1991 Feb 11;279(1):110–114. doi: 10.1016/0014-5793(91)80263-3. [DOI] [PubMed] [Google Scholar]
- Belkner J., Wiesner R., Rathman J., Barnett J., Sigal E., Kühn H. Oxygenation of lipoproteins by mammalian lipoxygenases. Eur J Biochem. 1993 Apr 1;213(1):251–261. doi: 10.1111/j.1432-1033.1993.tb17755.x. [DOI] [PubMed] [Google Scholar]
- Brooks C. J., Steel G., Gilbert J. D., Harland W. A. Lipids of human atheroma. 4. Characterisation of a new group of polar sterol esters from human atherosclerotic plaques. Atherosclerosis. 1971 Mar-Apr;13(2):223–237. doi: 10.1016/0021-9150(71)90025-6. [DOI] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Chisolm G. M. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991 Jan;32(1):63–70. [PubMed] [Google Scholar]
- Claeys M., Kivits G. A., Christ-Hazelhof E., Nugteren D. H. Metabolic profile of linoleic acid in porcine leukocytes through the lipoxygenase pathway. Biochim Biophys Acta. 1985 Oct 23;837(1):35–51. doi: 10.1016/0005-2760(85)90083-9. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994 Jul;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folcik V. A., Cathcart M. K. Assessment of 5-lipoxygenase involvement in human monocyte-mediated LDL oxidation. J Lipid Res. 1993 Jan;34(1):69–79. [PubMed] [Google Scholar]
- Folcik V. A., Cathcart M. K. Predominance of esterified hydroperoxy-linoleic acid in human monocyte-oxidized LDL. J Lipid Res. 1994 Sep;35(9):1570–1582. [PubMed] [Google Scholar]
- GLAVIND J., HARTMANN S., CLEMMESEN J., JESSEN K. E., DAM H. Studies on the role of lipoperoxides in human pathology. II. The presence of peroxidized lipids in the atherosclerotic aorta. Acta Pathol Microbiol Scand. 1952;30(1):1–6. doi: 10.1111/j.1699-0463.1952.tb00157.x. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967 Nov 25;242(22):5329–5335. [PubMed] [Google Scholar]
- Harland W. A., Gilbert J. D., Steel G., Brooks C. J. Lipids of human atheroma. 5. The occurrence of a new group of polar sterol esters in various stages of human atherosclerosis. Atherosclerosis. 1971 Mar-Apr;13(2):239–246. doi: 10.1016/0021-9150(71)90026-8. [DOI] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Härtel B., Ludwig P., Schewe T., Rapoport S. M. Self-inactivation by 13-hydroperoxylinoleic acid and lipohydroperoxidase activity of the reticulocyte lipoxygenase. Eur J Biochem. 1982 Aug;126(2):353–357. doi: 10.1111/j.1432-1033.1982.tb06787.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H., Belkner J., Wiesner R., Brash A. R. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem. 1990 Oct 25;265(30):18351–18361. [PubMed] [Google Scholar]
- Kühn H., Belkner J., Suzuki H., Yamamoto S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J Lipid Res. 1994 Oct;35(10):1749–1759. [PubMed] [Google Scholar]
- Kühn H., Belkner J., Wiesner R., Schewe T., Lankin V. Z., Tikhaze A. K. Structure elucidation of oxygenated lipids in human atherosclerotic lesions. Eicosanoids. 1992;5(1):17–22. [PubMed] [Google Scholar]
- Kühn H., Belkner J., Zaiss S., Fährenklemper T., Wohlfeil S. Involvement of 15-lipoxygenase in early stages of atherogenesis. J Exp Med. 1994 Jun 1;179(6):1903–1911. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Wiesner R., Lankin V. Z., Nekrasov A., Alder L., Schewe T. Analysis of the stereochemistry of lipoxygenase-derived hydroxypolyenoic fatty acids by means of chiral phase high-pressure liquid chromatography. Anal Biochem. 1987 Jan;160(1):24–34. doi: 10.1016/0003-2697(87)90609-9. [DOI] [PubMed] [Google Scholar]
- McNally A. K., Chisolm G. M., 3rd, Morel D. W., Cathcart M. K. Activated human monocytes oxidize low-density lipoprotein by a lipoxygenase-dependent pathway. J Immunol. 1990 Jul 1;145(1):254–259. [PubMed] [Google Scholar]
- Mukhin D. N., Orekhov A. N., Andreeva E. R., Schindeler E. M., Smirnov V. N. Lipids in cells of atherosclerotic and uninvolved human aorta. III. Lipid distribution in intimal sublayers. Exp Mol Pathol. 1991 Feb;54(1):22–30. doi: 10.1016/0014-4800(91)90040-5. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn M. S., Chisolm G. M. Oxidized lipoproteins, altered cell function and atherosclerosis. Atherosclerosis. 1994 Aug;108 (Suppl):S21–S29. doi: 10.1016/0021-9150(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- Rapp J. H., Connor W. E., Lin D. S., Inahara T., Porter J. M. Lipids of human atherosclerotic plaques and xanthomas: clues to the mechanism of plaque progression. J Lipid Res. 1983 Oct;24(10):1329–1335. [PubMed] [Google Scholar]
- Taylor R. G., Jerome W. G., Lewis J. C. Ultrastructural localization of peroxidase in atherosclerotic lesions of pigeons. Exp Mol Pathol. 1992 Dec;57(3):167–179. doi: 10.1016/0014-4800(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Geiger P. G., Maiorino M., Ursini F., Girotti A. W. Enzymatic reduction of phospholipid and cholesterol hydroperoxides in artificial bilayers and lipoproteins. Biochim Biophys Acta. 1990 Aug 6;1045(3):252–260. doi: 10.1016/0005-2760(90)90128-k. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]



