Abstract
The mTOR complex 2 (mTORC2) containing mTOR and rictor is thought to be rapamycin insensitive and was recently shown to regulate the prosurvival kinase AKT by phosphorylation on Ser473. We investigated the molecular effects of mTOR inhibition by the rapamycin derivatives (RDs) temsirolimus (CCI-779) and everolimus (RAD001) in acute myeloid leukemia (AML) cells. Unexpectedly, RDs not only inhibited the mTOR complex 1 (mTORC1) containing mTOR and raptor with decreased p70S6K, 4EPB1 phosphorylation, and GLUT1 mRNA, but also blocked AKT activation via inhibition of mTORC2 formation. This resulted in suppression of phosphorylation of the direct AKT substrate FKHR and decreased transcription of D-cyclins in AML cells. Similar observations were made in samples from patients with hematologic malignancies who received RDs in clinical studies. Our study provides the first evidence that rapamycin derivatives inhibit AKT signaling in primary AML cells both in vitro and in vivo, and supports the therapeutic potential of mTOR inhibition strategies in leukemias.
Introduction
The mammalian target of rapamycin (mTOR) pathway regulates cell growth, proliferation, and survival.1 mTOR, the central component of this pathway, partitions between 2 scaffold proteins, raptor and rictor. Upon activation, the rapamycin-sensitive raptor/mTOR protein complex (mTORC1) increases mRNA translation via activation of p70S6-kinase and inhibition of eIF4E-binding protein 4EPB1.2 The rictor/mTOR protein complex (mTORC2) was discovered only recently, is thought to be rapamycin insensitive, and phosphorylates AKT in the hydrophobic Ser473 site. It is therefore essential for AKT activity.3 Despite activity in model systems, the clinical antitumor activity of rapamycin derivatives in patients has been modest,1,4 and only a fraction of patients responds (reviewed in Thomas5). This has been attributed to the unanticipated ability of rapamycin to increase AKT activity via release of feedback inhibition of growth signaling pathways, both in cell systems and in tumor biopsies from patients.6 However, in certain cell types, prolonged inhibition of mTOR by rapamycin may impair mTORC2 assembly and hence AKT activation.7 In this study, we investigated the molecular consequences of mTOR inhibition in leukemic cells, both in vitro and in a clinical trial in vivo. Our results demonstrate that rapamycin derivatives suppress assembly of mTORC2, resulting in marked inhibition of AKT signaling. We propose that rapamycin-induced functional blockade of AKT in leukemic cells may define a subset of hematologic malignancies that is likely to respond favorably to mTOR inhibition, and that inhibition of AKT signaling may serve as a valuable biomarker of mTOR inhibition in vivo
Materials and methods
Acute myeloid leukemia (AML) cell lines were cultured under standard conditions8 with rapamycin derivatives CCI-779 and RAD001. Bone marrow or peripheral blood samples for the in vitro studies were obtained from patients with newly diagnosed or recurrent acute myeloid leukemia (AML) after informed consent. Peripheral blood samples were obtained from relapsed or refractory patients with hematologic malignancies treated with CCI-779 (temsirolimus; Wyeth Pharmaceuticals, Pearl River, NY) or RAD001 (everolimus; Novartis Pharmaceuticals, East Hanover, NJ)9 after obtaining written informed consent. Approval was obtained from the M. D. Anderson Cancer Center institutional review board for these studies. Clinical characteristics of patients are summarized in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Expression of total and phosphorylated AKT (Ser473), p70S6K (Thr389), 4EBP1 (Thr70), FoxO1 (Ser256), and PTEN was detected by Western blot analysis as previously reported.7 mTOR was immunoprecipitated using a specific anti-mTOR antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and protein-A/G agarose (Santa Cruz Biotechnology). Immune complexes were washed with CHAPS buffer3 and analyzed by Western blot as described.7
Real-time polymerase chain reaction (PCR) was carried out to detect the transcriptional level of CCND1, CCND2, and GLUT1 (for details, please refer to Document S1).
Results and discussion
We first investigated the effects of prolonged (24 hours) CCI-779 treatment on mTOR/raptor and mTOR/rictor complexes in U937 cells by immunoprecipitation/immunoblotting. CCI-779, without affecting the expression levels of mTOR, raptor, or rictor, interrupted the mTORC1 and mTORC2 formation at concentrations of 1.25 μg/mL and higher (Figure 1A). However, incubation of cell lysates with CCI-779 resulted in reduced raptor binding to mTOR with little effect on rictor/mTOR assembly (Figure 1B), consistent with the recent observation that prolonged rapamycin treatment in certain cell types can inhibit the assembly of mTORC2 in vivo, but interferes with raptor-mTOR interaction only in vitro.7 Functionally, mTORC1/mTORC2 inhibition in leukemic cells resulted in decreased phosphorylation of p70S6K and 4EBP1, well-established mTORC1 downstream targets. Additionally, we observed decreased phosphorylation of AKT (Ser473) and of its substrate FoxO1, indicating that the ability of CCI-779 to disrupt rictor/mTOR association in leukemic cells results in the blockade of AKT signaling (Figure 1C). Further, TaqMan (Applied Biosystems, Foster City, CA) PCR revealed inhibition on the transcription of the mTOR/HIF-1α target GLUT1,10 and transcriptional down-regulation of D-type cyclins, conceivably through mTOR-mediated inhibition of AKT signaling and restoration of the activity of forkhead transcription factors11 (Figure 1D). Similar results were observed in OCI-AML3 cells treated with CCI-779 or with the other rapamycin derivative everolimus (RAD001) (Figure 1E-G).
Figure 1.
Rapamycin derivatives inhibit mTORC1 and mTORC2 signaling in AML cell lines and in primary AML samples. (A-B) U937 cells (A) or cell lysates (B) were treated with different concentrations of CCI-779 for 24 hours. Cell lysates and mTOR immunoprecipitates prepared from the lysates were analyzed by Western blot for the levels of mTOR, rictor, and raptor. (C) U937 cells were treated with indicated concentrations of CCI-779 for 24 hours, and cell lysates were analyzed by immunoblotting for the indicated proteins and phosphorylation states. (D) The effects of mTOR inhibition on transcriptional level of CCND1/CCND2 and GLUT1 were assessed via real-time PCR. Error bars denote half the difference between the maximum and minimum values that arose on substituting ΔCt − SD or ΔCt + SD, respectively, for ΔCt in the formula RE = 100 × 2 exp [−ΔCt]. (E) OCI-AML3 cells were treated with indicated concentrations of CCI-779 and RAD001 for 24 hours. The level of mTOR, rictor, and raptor from cell lysates and mTOR immunoprecipitates was evaluated by Western blot. (F-G) The effect of mTOR inhibition on mTOR upstream regulators (Akt) and downstream targets was detected by Western blot (F) and real-time PCR (G). (H) Primary AML blasts or cell lysates from 2 patients' samples were treated with CCI-779 for 24 hours, and immunoprecipitation of mTOR was carried out as described in panel A. Representative results of 2 of the 8 primary samples yielded similar results. (I-J) Effects of mTOR inhibition on downstream mTOR and AKT substrates were examined by immunoblotting of cell lysates from patient no. 1 (I), and on the transcriptional levels of CCND1/CCND2 and glut-1 by real-time PCR (J).
Further, in 8 primary AML samples treated with CCI-779 in vitro for 24 hours, mTORC1 and mTORC2 formation was decreased by approximately 80% and 50%, respectively, in all samples tested, without affecting the expression of mTOR, raptor, and rictor (see examples in Figure 1H). Expression of wild-type PTEN protein was detected in 6 of 6 samples tested, consistent with previously published reports.12 Incubation of cell lysates from 5 of these 8 samples with CCI-779 resulted in reduced raptor binding to mTOR with little effect on rictor/mTOR assembly, consistent with the results obtained in U937 and OCI-AML3 cell lysates. Further, mTORC1 inhibition translated into dephosphorylation of p70S6K and 4EBP1, while CCI-779–induced inhibition of mTORC2 resulted in blockade of phosphorylation of AKT and FoxO1 (Figure 1I). TaqMan PCR demonstrated that CCI-779 down-regulated CCND1 and GLUT1 gene transcriptional levels in a concentration-dependent fashion (Figure 1J). These data indicate that mTOR inhibition by CCI-779 suppresses AKT signaling in AML cell lines and primary samples.
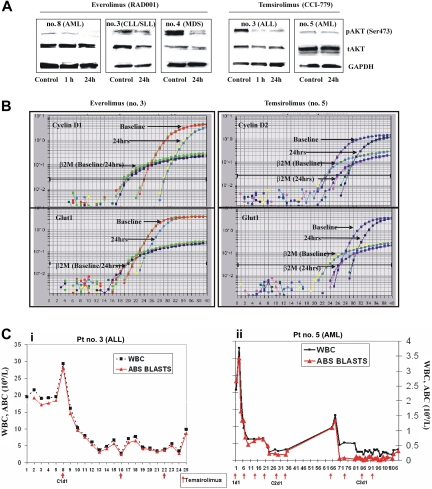
To determine whether the observations in Figure 1 are clinically relevant, we obtained peripheral blood samples from patients with hematologic malignancies treated on phase 1/2 protocols of the rapamycin derivatives temsirolimus (25 mg intravenously every week) and everolimus (continuously at 5 or 10 mg orally daily).9 The levels of Ser473-phosphorylated AKT decreased in 3 of 5 patient samples at 1 or 24 hours(s) of temsirolimus treatment, and in 6 of 8 patient samples treated with everolimus (Figure 2A; Table S1). In the 9 samples in which AKT was inhibited, a 2-fold or higher decrease in CCND1 mRNA was observed in 5 patients; CCND2, in 3; both CCND1 and CCND2, in 1; and GLUT1, in 4 (Figure 2B and not shown). In 9 samples in which rapamycin derivatives inhibited AKT phosphorylation, statistically significant decreases in CCND1 and CCND2 levels were observed (P = .028 and P = .039, respectively, Wilcoxon pairwise test), while changes in GLUT1 did not reach statistical significance (Table S2). In contrast, no statistically significant modulation of these genes was observed in a group of patients without AKT dephosphorylation (Table S2). In 7 of the 9 patients in whom AKT was inhibited, a more than 50% decrease in peripheral blood absolute blast count (3 AML, 1 ALL) or absolute lymphocyte count (1 chronic lymphocytic leukemia [CLL]) for more than 1-week duration was documented (Figure 2C), and 2 patients with RAEB-1 (no. 5 and no. 6) had improvements in platelet counts, with 1 fulfilling the criteria for hematologic improvement.9 No changes in peripheral blood counts or progression of leukemias were seen in 6 patients. Of these, decrease in pAKT was observed in 2, no change in 3, and increase in 1 (Table S2, Fisher exact 2-tailed, P = .021). These results suggest that the suppression of AKT signaling by mTOR inhibitors observed in vitro in leukemia cell lines and in primary clinical samples may be clinically important.
Figure 2.
Rapamycin derivatives inhibit AKT signaling in leukemic cells in vivo. (A) Peripheral blood mononuclear cells from patients treated with either everolimus or temsirolimus were subjected to immunoblotting analyses of pAKT, total AKT, and GAPDH, and (B) quantitative real-time PCR analysis of CCND1/CCND2 and GLUT1 transcription. The data shown are derived from TaqMan PCR analyses of these genes. (C) Changes in white blood cell count (WBC, 109/L) and absolute blast count (ABC, 109/L) during temsirolimus treatment. (i) Patient with relapsed refractory pre-B-cell acute lymphoblastic leukemia (pre-B-ALL) received 3 doses of temsirolimus at a dose of 25 mg intravenously every week (indicated by arrows). (ii) Patient with primary refractory AML has completed 2 courses of temsirolimus (4 weekly injections each, at a dose of 25 mg intravenously every week) and received 2 doses of temsirolimus in course 3.
In summary, our observations suggest that rapamycin derivatives potently inhibited AKT activity in leukemic cells via suppression of mTORC2 assembly, in addition to its well-characterized ability to suppress the mTORC1 pathway. Further, the data reported here provide the first in vivo evidence that rapamycin derivatives are capable of suppressing AKT signaling in patients treated with these inhibitors. We propose that this unforeseen mechanism of action of these agents defines hematologic tumors as malignancies, which are likely to respond favorably to pharmacological inhibition of mTOR signaling, in contrast to certain solid tumors in which the negative feedback loop from p70S6K to AKT may instead exacerbate cancer progression. Since dysregulation of components of the PI3K/AKT/mTOR pathway is a common event in hematologic malignancies,13,14 inhibition of mTOR signaling by rapamycin-like molecules may be a useful therapeutic strategy. Based on the results presented in this report, we speculate that the ability of rapamycin to inhibit AKT and its downstream prosurvival pathways may serve as a valuable biomarker in elucidating biologically effective doses and schedules of mTOR inhibitors in leukemia and cancer patients.
Supplementary Material
Acknowledgments
Supported in part by National Cancer Institute grants CA55164, CA16672, and CA49639 (M.A.); American Cancer Society grant RSG-06-054-01-LIB (M.K.) and the Paul and Mary Haas Chair in Genetics (M.A.).
Footnotes
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Z.Z. and D.D.S. conducted experiments; I.J.S. provided technical assistance and modified the experimental design; K.W.L.Y. conducted clinical trials and procured samples; M.F.M. served as statistical data analyst; C.E.J. served as research nurse and analyzed clinical trial data; F.J.G. conducted clinical trials and procured samples; D.M.S. served as collaborator, first described rictor function, and assisted with experimental design; M.A. assisted in conceptualization, and assisted in analysis of clinical trial data and assessment of lab data; M.K. assisted in conceptualization, and supervised conduct of experiments and data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Konopleva, Section of Molecular Hematology and Therapy, Department of Stem Cell Transplantation and Cellular Therapy, Unit 448, 1515 Holcombe Blvd, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; e-mail: mkonople@mdanderson.org
References
- 1.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 4.Panwalkar A, Verstovsek S, Giles F. Nuclear factor-kappaB modulation as a therapeutic approach in hematologic malignancies. Cancer. 2004;100:1578–1589. doi: 10.1002/cncr.20182. [DOI] [PubMed] [Google Scholar]
- 5.Thomas GV. mTOR and cancer: reason for dancing at the crossroads? Curr Opin Genet Dev. 2006;16:78–84. doi: 10.1016/j.gde.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarbassov dD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Konopleva M, Tsao T, Ruvolo P, et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–335. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 9.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 10.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 12.Aggerhom A, Grønbæk K, Guldberg P, Hokland P. Mutational analysis of the tumour suppressor gene MMAC1/PTEN in malignant myeloid disorders. Eur J Haematol. 2000;65:109–113. doi: 10.1034/j.1600-0609.2000.90181.x. [DOI] [PubMed] [Google Scholar]
- 13.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 14.Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004;100:657–666. doi: 10.1002/cncr.20026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.