Abstract
Crystallographic and electron microscopy studies revealed genuflexed (bent) integrins in both unliganded (inactive) and physiologic ligandbound (active) states, suggesting that local conformational changes are sufficient for activation. Herein we have explored the role of local changes in the contact region between the membrane-proximal β-tail domain (βTD) and the ligand-binding βA domain of the bent conformation in regulating interaction of integrin CD11b/CD18 (αMβ2) with its physiologic ligand iC3b. We replaced the βTD CD loop residues D658GMD of the CD18 (β2) subunit with the equivalent D672SSG of the β3 subunit, with AGAA or with NGTD, expressed the respective heterodimeric receptors either transiently in epithelial HEK293T cells or stably in leukocytes (K562), and measured their ability to bind iC3b and to conformation-sensitive mAbs. In the presence of the physiologic divalent cations Ca2+ plus Mg2+ (at 1 mM each), the modified integrins showed increased (in HEK293) or constitutive (in K562) binding to iC3b compared with wild-type receptors. K562 expressing the βTD-modified integrins bound in Ca2+Mg2+ to the βA-directed high-affinity reporter mAb 24 but not to mAb KIM127, a reporter of the genu-straightened state. These data identify a role for the membrane proximal βTD as an allosteric modulator of integrin activation.
Introduction
Integrins are αβ heterodimeric receptors normally expressed in an inactive state on the cell surface but can switch rapidly and reversibly to the active physiologic ligand-binding state in response to inside-out activation signals generated from within cells (reviewed by Hynes1). The integrin ectodomain consists of a “head” segment on top of 2 “leg” segments2,3 (Figure 1). The head segment is formed of a 7-bladed β propeller from the α subunit that associates noncovalently with a von Willebrand factor type A (VWFA) domain (βA or I-like) from the β subunit. The α-subunit leg is formed of an Ig-like “thigh” domain followed by 2 large β-sandwich domains, calf-1 and calf-2. The β-subunit leg is formed of an Ig-like “hybrid” domain inserted into the N-terminal PSI domain,4,5 followed by 4 EGF-like domains and a novel beta tail domain (βTD)2 (Figure 1). In native integrins, each leg terminates in a single membrane-spanning segment and a short cytoplasmic tail. The prototypical ligand Arg-Gly-Asp binds to the head segment such that the ligand aspartate engages βA through a metal ion coordinated at the metal ion–dependent adhesion site (MIDAS) and the ligand arginine fits an adjacent pocket in the propeller.3 One half of the integrin α subunits have an additional VWFA domain (αA or I) inserted between blades 2 and 3 of the propeller. αA exists in inactive and active conformations, and the α1 helix, the βF-α7 (F/α7) loop, and α7 helix are known to be involved in this transition.6–8 In the active conformation of αA-containing integrins, a C-terminal glutamate from active αA ligates the βA MIDAS, stabilizing the high-affinity state.9
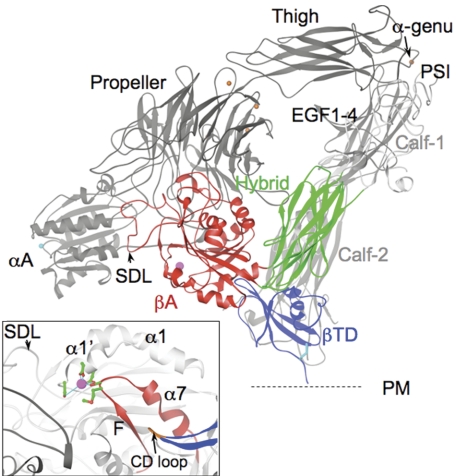
Figure 1.
Spatial relationships of the βTD domain. A ribbon diagram of a model of CD11b/CD18 based on the crystal structure of unliganded αVβ3 ectodomain.2 The 8 β2-subunit domains are labeled. The 5 CD11b-subunit domains αA (MIDAS ion in cyan), propeller (with 4 metal ions, orange circles), thigh, and calf-1 and -2 (light gray) are labeled. The metal ion in the α-genu (arrow) is in orange. The specificity-determining loop (SDL) in βA is indicated by an arrowhead. The C663-C687 disulfide bridge found at the bottom of the CD loop is shown in cyan. The position of the plasma membrane (PM) is indicated by a dotted line for orientation. (Inset) An enlarged image of the βTD's CD loop (brown, arrow) and F/α7 region (red) in the unliganded αVβ3 ectodomain.2 The ADMIDAS (adjacent to MIDAS) ion (magenta) links the α1′-α1 helix and F/α7 loop through D126D127 and M335 (equivalent to D119D120, E325 in the β2 subunit, respectively) (Cα is in green; oxygens, red). S123 in β3 (and S116 in β2) completes the metal coordination sphere.
Integrins assume a compact conformation bent at their αβ “knees” (located between the thigh and calf-1 domains of the α subunit and presumably EGF1 and EGF2 of the β subunit) such that the head contacts the lower legs of the same molecule.2 Earlier electron microscopy (EM) images showing genu-straightened integrins10 led to the early suggestion that inside-out activation (induction of physiologic ligand competency) takes place as a result of a switch from the bent to the linear state.11 Additional studies have shown, however, that such a global change is not required for switching to high-binding affinity,12–15 suggesting that local changes may be sufficient to enable physiologic ligand binding in the bent state (reviewed by Ginsberg et al16). In the crystal structure of the unliganded αVβ3 ectodomain,2 the βA and hybrid domains make discontinuous intramolecular contacts with the βTD, covering a combined approximately 33.4 nm2 of surface area. In this article, we examine the role of βTD's CD loop, which contacts βA, in physiologic ligand binding to integrin CD11b/CD18. The results reveal an important allosteric function for the βTD in regulating physiologic ligand binding by this integrin.
Materials and methods
Reagents and antibodies
Restriction and modification enzymes were obtained from New England Biolabs (Beverly, MA), Gibco BRL (Gaithersburg, MD), or Fisher Scientific (Pittsburgh, PA). All cell culture reagents were from Invitrogen (San Diego, CA). The anti-CD11b monoclonal antibody (mAb) 44a (IgG2a),17 the anti-CD18 mAb TS1/18,18 the heterodimer-specific mAb IB4 (IgG2a),19,20 and a polyclonal anti-CD18 antibody21 have been described previously. mAbs KIM127 and 24 (both IgG1) were kindly provided by M. Robinson22 and N. Hogg,23 respectively. Isotype control antibodies MOPC-21 (IgG1) and MOPC-173 (IgG2a) and FITC-conjugated mAbs A85-1 (rat anti–mouse IgG1), R19-15 (rat anti–mouse IgG2a), and goat anti–mouse Ig were from BD PharMingen (San Diego, CA).
DNA constructs
Site-directed CD18 mutants were created using standard recombinant DNA protocols24 in pcDNA3 expression vectors.25 The following forward (For) and reverse (Rev) oligonucleotides were used, each followed by the newly introduced restriction site in parentheses: For: 5′-CACGCTGGAGCAGCAGGACtcGtccGgCCGCTACCTCATCTATGTGGATG-3′ (Eag); Rev: 5′-CATCCACATAGATGAGGTAGCGGcCggaCgaGTCCTGCTGCTCCAGCGTG-3′ (Eag); For: 5′-CTACACGCTGGAGCAGCAGaACGGtAccGACCGCTACCTCATCTATGTGG-3′ (Kpn); Rev: 5′-CCACATAGATGAGGTAGCGGTCggTaCCGTtCTGCTGCTCCAGCGTGTAG-3′ (Kpn); For: 5′CACGCTGGAGCAGCAGGcCGGGgcGGcCCGgTACCTCATCTATGTGGATGAGAGCC-3′ (Kpn); Rev: 5′-GGCTCTCATCCACATAGATGAGGTAcCGGgCCgcCCCGgCCTGCTGCTCCAGCGTG-3′ (Kpn).
Polymerase chain reaction (PCR)–amplified DNA was transferred back into the original CD18-containing expression vector using Eco47III and AvrII restriction sites. Each mutation was confirmed by the presence of the introduced restriction site and by DNA sequencing.
Cell culture and transfection
Transient transfection assays were carried out in HEK293 cells (American Type Culture Collection [ATCC], Manassas, VA) as described.9 Briefly, HEK293 cells were plated on tissue culture–treated p-10 plates (Clontech, Palo Alto, CA) and maintained in complete medium consisting of Dulbecco modified Eagle medium ([DMEM] Gibco BRL) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL) and 50 IU/mL penicillin and streptomycin (Gibco BRL) at 37°C. The cells were detached with 0.05% Trypsin-EDTA solution (Gibco BRL) and plated in wells of 6-well plates at a concentration of 1 × 106 cells per well. Cells at about 70% confluence were transfected with supercoiled cDNA encoding wild-type (WT) and mutant CD18 together with WT CD11b using Lipofectamine2000 reagent (Invitrogen) and following the manufacturer's protocol. Transfected cells were grown for 24 hours at 37°C in 5% CO2. Cells were then carefully washed and detached with the 0.05% Trypsin-EDTA solution, counted, and seeded in replicates for 24 hours onto poly-l-lysine (Sigma Chemical, St Louis, MO)–coated 48-well plates (Clontech). Confluent monolayers in the 48-wells were then used for integrin cell surface expression and ligand-binding assays.
Stable transfection of WT and mutant integrins was carried out in K562 cells (ATCC) using published electroporation protocols.20 Briefly, K562 cells were grown to log phase in Iscove modified Dulbecco medium ([IMDM] CellGro; Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum and 50 IU/mL penicillin and streptomycin at 37°C and resuspended in serumfree IMDM at about 1 × 107/mL. A total of 0.5 mL of cells were transferred into a 0.4 cm cuvette (Fisher Scientific), and 10 μg WT CD11b and 10 μg WT or mutant CD18 cDNA were added. Electroporation was carried out at 960 μF and 320 volts (Gene-Pulser; Bio-Rad Laboratories, Hercules, CA). Transfectants were allowed to recover in serum-containing media for 48 hours and were then selected with 1 mg/mL G418 (Gibco BRL) for up to 2 weeks. CD11b/CD18-expressing cells were enriched by fluorescence-activated cell sorter (FACS) (on FACS Sort; BD Biosciences, San Jose, CA) using the heterodimer-specific mAb IB4.20,26 Sorted cells were cloned by limiting dilution, and clones with similar levels of integrin expression were identified by flow cytometry for further analyses. WT and mutant K562 were maintained in IMDM supplemented with 10% heat-inactivated fetal bovine serum, 50 IU/mL penicillin and streptomycin, and 1 mg/mL G418. K562 cells stably transfected with WT CD11b/CD18 using the expression plasmid EE6 hCMV carrying a G418 resistance marker were also provided by Dr D. Simon27 with similar results.
Preparation of complement iC3b-coated sheep erythrocytes (EiC3b)
Sheep erythrocytes (E) coated with complement iC3b (EiC3b) were prepared using human serum following published protocols.28 A slight modification was made in that Hanks balanced buffer solution ([HBSS] Gibco BRL) was used instead of the VBSG buffer. Coated and biotinylated erythrocytes (EiC3b and E) were diluted to a concentration of 1.5 × 107/mL to 6 × 107/mL for the ligand-binding assay.
Antibody and ligand binding to HEK293 cells
Binding of mAbs and EiC3b to transiently transfected HEK293 cells was performed in parallel in 48-well plates as described.9 Integrin cell surface expression was analyzed using anti-CD11b and anti-CD18 mAbs. Briefly, triplicate wells containing confluent monolayers of transfected cells, approximately 48 hours after transfection, were incubated with primary mAbs (10 μg/mL) in DMEM buffer containing 0.1% gelatin and 0.02% sodium azide for 1 hour at 4°C. Binding assays with mAb KIM127 were performed for 30 minutes at 37°C as described.22,29 Cells were washed and incubated with biotin-labeled rabbit antimouse antibody (Vectastain ABC-kit; Vector Laboratories, Burlingame, CA) for 1 hour at 4°C, washed 3 times, and subsequently fixed with 1% glutaraldehyde overnight at 4°C. After blocking, wells were developed using streptavidin–alkaline phosphatase ([AP] Vectastain ABC-kit; Vector Laboratories) and p-nitrophenyl phosphate ([PNPP] Sigma Chemical) to generate a colored product. The enzymatic reaction was quenched with equal volumes of 2N NaOH, 100 μL of this solution was transferred to 96-well plates (Costar, Corning, NY), and OD405 was measured. Binding results, reported as histograms representing mean ± SD of triplicate wells, were normalized such that binding of each antibody to the WT receptor was considered 100.
Ligand binding was assessed by adding 150 μL EiC3b (60 × 106/mL) to triplicate confluent wells in a total volume of 300 μL followed by a 15-second spin at 60g. After 35 minutes of incubation at 37°C, wells were washed and subsequently fixed with 1% glutaraldehyde. Wells were developed using AP and PNPP and binding quantitated. Specific binding was obtained by subtracting background binding of mock-transfected cells and expressed as percentage of binding to WT obtained in the presence of Ca2++Mg2+ after correcting for expression using mAb IB4 as previously described.30
Immunoprecipitations
Immunoprecipitation followed by a Western blot analysis was carried out to confirm heterodimerization of WT and mutant CD11b/CD18 integrins on the surface of transfected cells. Confluent, transfected HEK 293 cells in 6-well plates were extracted using RIPA buffer (Boston Bioproducts, Worcester, MA) supplemented with 1 mg/mL DNase and a cocktail of protease inhibitors (Complete; Roche Diagnostics, Basel, Switzerland). The detergent-soluble fraction was harvested upon centrifugation and immunoprecipitated using the anti-CD11b mAb 44a and protein G–Sepharose-4 (Amersham Pharmacia Biotech, Uppsala, Sweden). Washed immunoprecipitates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 4% to 15% gradient Tris-HCl gel (Bio-Rad Laboratories) under reducing conditions and electroblotted onto PVDF membranes (Bio-Rad Laboratories). After blocking with 10% nonfat milk in 25 mM Tris-HCl (pH 7.4), 137 mM NaCl, 2.7 mM KCl (TBS; Boston Bioproducts), the membrane was incubated with a polyclonal anti-CD18 antibody as described.9 Detection of proteins was performed using horseradish peroxidase (HRP)–linked antirabbit antibody (Amersham Pharmacia Biotech) and SuperSignal Chemiluminescent kit (Pierce Chemical, Rockford, IL). The luminescent signal was detected using BioMax x-ray films (Eastman Kodak, Rochester, NY).
Flow cytometry of K562
Flow cytometric analysis of K562 cells was performed using published protocols.31,32 Briefly, cells were counted and washed twice with TBS. Cells (5 × 105) were incubated with primary mAb (10 μg/mL) in 100 μL TBS on ice for 30 minutes, except for mAbs 24 and KIM127 and the isotype-matched control mAb MOPC-21, where incubations were performed at 37°C and in the presence of 1 mM each of Ca2+ and Mg2+ or 1 mM Mn2+.31 Cells were then washed with TBS and incubated with FITC-conjugated secondary mAbs on ice for 30 minutes. Stained cells were washed and resuspended in cold TBS and analyzed using FACS Scan flow cytometer (BD Biosciences, San Jose, CA) counting 10 000 events. Data were analyzed using the CellQuest software (BD Biosciences).
K562 binding to iC3b in suspension
K562 cells were washed twice in TBS and resuspended to 1 × 106/mL, of which 40 μL (4 × 104 cells) was incubated in suspension with EiC3b (0.75 × 106) in a total volume of 100 μL at 37°C for 25 minutes in the presence of 1 mM each of Ca2+ and Mg2+, 1 mM Mn2+, varying concentrations of Ca2+, or 5 mM EDTA. In preliminary studies, we found that the about 20:1 EiC3b/K562 ratio falls on the steep portion of the dose-response curve and is thus sensitive to changes in ligand-binding activity of the integrin (not shown). Binding, detected in the form of “rosettes” (at least 3 EiC3b/K562, more than 200 cells examined in multiple fields), was scored using phase-contrast microscopy. Binding results are reported as histograms representing mean ± SD of triplicate experiments.
K562 binding to iC3b immobilized on microtiter plates
Maxisorp 384-well microtiter plates (Nalge Nunc, Rochester, NY) were coated with 5 μg/mL monomeric iC3b ligand25 in PBS containing 1 mM Ca2+ and 1 mM Mg2+ (PBS++) overnight at 4°C. The concentration of iC3b used for these experiments fell on the steep portion of the dose-response curve (not shown). After coating with iC3b, the wells were washed with TBS and nonspecific sites were blocked by incubation with 2% nonfat milk in TBS at room temperature for 30 minutes. The wells were washed 3 times with TBS, and the plates were immediately used in adhesion assays. K562 cells (30 000) were transferred to each well and incubated at room temperature for 30 minutes in the presence of 1 mM each of Ca2+ and Mg2+, 1 mM Mn2+, or 5 mM EDTA in a total volume of 100 μL. Following a washing step, the adherent cells were fixed with formaldehyde (1.1% vol/vol final concentration), washed 3 more times with TBS, fluorescently labeled with DAPI (0.5 μM final in TBS with 0.1% Triton X-100), and quantitated using an automated microscope (CellWorx automated microscope; Cellomics, Pittsburgh, PA) set at 0.3-second exposure using a DAPI filter set to capture 1 to 3 images per well. Digitized photomicrographs were then analyzed using MetaXpress image analysis software (Molecular Devices, Sunnyvale, CA) using the built-in cell count module to quantify nuclear staining. Data output files were analyzed using MS Excel.
Cell spreading assay
Maxisorp 384-well microtiter plates coated with 5 μg/mL iC3b ligand were prepared as described in the previous section. K562 cells (20 000) were transferred to each well and incubated at room temperature for 1.5 hours in serumfree IMDM, washed, and cell spreading visualized (more than 200 cells examined in multiple fields) using phase-contrast microscopy. Spreading results are reported as histograms representing mean ± SD of triplicate determinations from 3 separate experiments.
Results
Expression of mutant forms of CD11b/CD18 in HEK293 cells
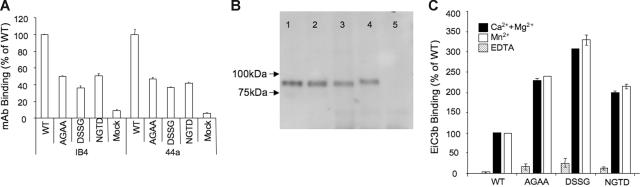
We modified the βTD's CD loop residues D658GMD of WT CD18 (Figure 1, inset) by swapping them with the equivalent D672SSG residues of the β3 subunit. The D658GMD sequence was also replaced with AGAA or with NGTD (to fashion a neo–N-glycan at N658). We assessed transient expression of mutant CD11b/CD18 integrins on the surface of HEK293 cells, which lack endogenous CD11b/CD18, using the heterodimer-specific mAb IB4 and the CD11b-specific mAb 44a. Figure 2A shows that all 3 CD loop mutants were expressed on the surface of HEK293 cells but at reduced levels (about 50% of that of the WT integrin). Both the 44a and IB4 mAbs registered a similar level of integrin expression, which suggests that the expressed integrins are in their heterodimeric form. We further verified integrin heterodimer formation by immunoprecipitation from detergent HEK293 cell lysates with mAb 44a followed by Western blotting using polyclonal anti-CD18 antibody (Figure 2B). Autoradiograph of the Western blot shows the presence of comparable amounts of CD18 in immunoprecipitates from HEK293 cells expressing WT or mutant integrins (Figure 2B, lanes 1-4), suggesting that equivalent amounts are being synthesized. The slower mobility of the NGTD CD18 mutant (Figure 2B, lane 4) is consistent with the attachment of a neo–N-glycan at the introduced site.13 No CD18 was seen in anti-CD11b immunoprecipitates from mock-transfected HEK293 cells (Figure 2B, lane 5).
Figure 2.
Surface expression and iC3b binding of WT and mutant CD11b/CD18 in HEK293 cells. (A) Histograms showing the relative binding of the heterodimer-specific mAb IB4 and the anti-CD11b mAb 44a to HEK293 cells expressing WT and βTD mutant CD11b/CD18 receptors. Binding of the respective antibody to the WT receptor was considered 100. Each histogram represents mean ± SD of triplicate determinations from a representative experiment (1 of 3 performed). (B) Western blots following 4% to 15% gradient SDS-PAGE showing the presence of CD18 in anti-CD11b (using mAb 44a) immunoprecipitates from HEK293 cells expressing WT (lane 1), AGAA (lane 2), DSSG (lane 3), NGTD CD11b/CD18 mutants (lane 4), and mock-transfected cells (lane 5). Approximately equivalent amounts of the immunoprecipitated receptors were loaded in each lane. The shift in CD18 in the NGTD mutant (lane 4) is consistent with attachment of a neo–N-glycan. Arrowheads indicate molecular weight markers at 100 kDa and 75 kDa (High MW Markers; Boston Bioproducts). No CD18 was seen in anti-CD11b immunoprecipitates from mock-transfected HEK293 cells (lane 5). (C) Histograms showing the relative binding of EiC3b to WT and βTD mutant CD11b/CD18 in buffer containing EDTA (5 mM), Ca2+ and Mg2+ (1 mM each), or Mn2+ (1 mM) and expressed as percentage of binding to WT obtained in the presence of 1 mM each of Ca2+ and Mg2+ after correcting for expression using mAb IB4 as described.9 Similar results were obtained when the activation-insensitive mAb 44a was used as reference (not shown). Each bar represents mean ± SD of triplicate determinations from a representative experiment (1 of 3 performed).
The βTD-modified CD11b/CD18 integrins are active in HEK293
Binding of CD11b/CD18 to its physiologic ligands including iC3b is divalent cation and activation dependent.30,33 We therefore used the iC3b ligand to assess the functional impact of modifying the βTD CD loop in the CD11b/CD18 mutants expressed on the surface of HEK293 in the physiologic divalent cations (Ca2+ plus Mg2+, each at 1 mM). A portion of the WT CD11b/CD18 expressed on HEK293 assumes the active state,34 explaining its intrinsic binding to EiC3b (Figure 2C). Binding of the βTD mutants to EiC3b increased by 2- to 3-fold above that of the WT receptor (set empirically at 100%) (Figure 2C). This level of binding did not increase further by the activating cation Mn2+ (at 1 mM) (Figure 2C), commonly used as a mimic of inside-out integrin activation (reviewed by Hynes1), suggesting that most of the expressed receptors are already activated.
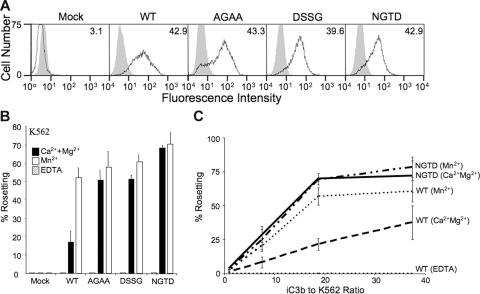
The βTD-modified CD11b/CD18 is constitutively active in K562
The nature of the conformational changes that may occur in parallel with the high-affinity (physiologic ligand-binding) state were next examined in more detail in the βTD mutants expressed stably in K562 cells. K562 cells express WT integrins in a default low-affinity state as in normal leukocytes,35 which can be activated by inside-out signals primarily through a change in integrin affinity rather than avidity,36 thereby providing a more relevant context to observe changes in the level of integrin activation.35 K562 cells expressing equivalent levels of heterodimeric WT and mutant CD11b/CD18 (Figure 3A) were examined for their ability to bind iC3b (covalently bound to the surface of erythrocytes using fresh serum, as in the physiologic state) or adsorbed to microtiter plastic plates. In the presence of the physiologic divalent cations, binding (rosette formation) of the mutant CD11b/CD18 receptors to EiC3b in suspension was constitutive, unlike that of the WT, which was minimal (20% or less). Mn2+ increased the level of binding of the WT to the same degree seen in the mutants in physiologic cations but did not induce a further increase in EiC3b binding by the mutants (Figure 3B), indicating that the respective integrin mutants are maximally activated in this assay, as in HEK293. We also performed a titration with increasing numbers of EiC3b to a constant concentration of WT, NGTD, or mock K562 cells. Results presented in Figure 3C show that even at very low numbers of EiC3b (and thus a low EiC3b/K562 cell ratio), the mutant NGTD-expressing K562 cells showed higher binding to EiC3b in physiologic cations compared with WT-expressing cells. These differences were most pronounced at an EiC3b/K562 cell ratio of about 18. While Mn2+ produced the expected increase in binding of WT-expressing K562 to EiC3b, no increase in EiC3b binding to the NGTD mutant took place at any EiC3b/K562 cell ratio tested.
Figure 3.
Physiologic ligand binding of K562 stably expressing WT and βTD mutant CD11b/CD18. (A) Cell surface expression of CD11b/CD18 on K562 cells using flow cytometry. K562 cells were immunostained with the primary heterodimer-specific mAb IB4 (thick lines, unshaded area) or isotype-matched control mAb MOPC-173 (gray shaded area), washed, developed with FITC-rat anti–mouse IgG2a, and analyzed counting 10 000 events. Median fluorescence intensity (MFI) for mAb IB4 staining is shown in each panel. (B) Histograms showing the relative binding of iC3b to WT and mutant CD11b/CD18 in buffer containing 5 mM EDTA, 1 mM each of Ca2+ and Mg2+, or 1 mM Mn2+ expressed as the number of cells showing rosettes (more than 3 EiC3b bound per K562 cell) as a percentage of total number of cells counted in a rosetting assay. Each histogram represents mean ± SD of triplicate determinations from a representative experiment (1 of 3 performed). No binding was observed in EDTA and with mock-transfected cells. The methods are detailed in “Materials and methods.” (C) A titration ligand binding curve showing ligand binding (% rosetting) of an increasing number of EiC3b to a constant number of WT or NGTD mutant CD11b/CD18-expressing K562 cells in buffer containing 1 mM each of Ca2+ and Mg2+ or 1 mM Mn2+ (as labeled). Each data point represents mean ± SD of triplicate determinations. Not shown are rosetting results from mock-transfected cells (0 rosettes under all 3 buffer conditions). The graph shows that NGTD-expressing cells have a higher percentage of rosetting in physiologic buffer cells under all conditions tested as compared with WT cells. The difference is greatest when ligand is limiting (at EiC3b/K562 cell ratio of about 18). No difference is seen in mutant integrin binding to iC3b in the presence of Ca2++Mg2+ or Mn2+ at all ratios used.
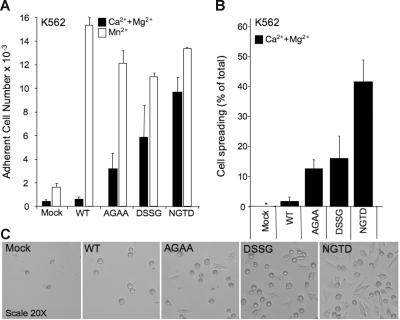
We also evaluated ligand binding under conditions in which CD11b/CD18-expressing K562 cells were allowed to bind ligand adsorbed onto microtiter plastic plates. The specificity of the interaction was again reflected in the lack of binding of mock K562 or WT CD11b/CD18-expressing K562 cells in the presence of the physiologic divalent cations (Figure 4A), while 1 mM Mn2+ was sufficient to induce maximal binding by the WT receptor. K562 cells expressing the βTD mutant CD11b/CD18 receptors all showed significant binding to immobilized iC3b in the presence of physiologic cations. However and in contrast to the rosetting assay and the binding assay in HEK293, there were small but significant differences in the magnitude of the response to the addition of Mn2+; in the case of the NGTD mutant, Mn2+ produced only a modest increase in iC3b binding above that seen in Ca2++Mg2+, but the increase was more in the case of the DSSG and even higher in the case of the AGAA mutant (Figure 4A).
Figure 4.
Binding and spreading by WT and βTD mutant CD11b/CD18 to iC3b-coated plates. (A) Histograms showing the relative binding of mock, WT, and mutant CD11b/CD18-expressing cells to iC3b-coated plates in buffer containing 1 mM each of Ca2+ and Mg2+ or 1 mM Mn2+ and expressed as the absolute number of adherent cells per well. Each histogram represents mean ± SD of triplicate determinations from a representative experiment (1 of 2 performed). The methods are detailed in “Materials and methods.” No iC3b binding was seen with mock, WT, or mutant CD11b/CD18 in the presence of EDTA (not shown). (B) Histograms showing the relative level of spreading by mock, WT, and mutant CD11b/CD18 cells to iC3b-coated plates in buffer containing 1 mM each of Ca2+ and Mg2+ and expressed as a percent of spread cells in the total field (more than 200 cells examined in multiple fields using phase-contrast microscopy). Spreading results are reported as histograms representing mean ± SD of triplicate experiments. The methods are detailed in “Materials and methods.” (C) Phase-contrast microscopy images (visualized using a Nikon Eclipse E800 microscope [Nikon, Melville, NY] equipped with a 20×/0.50 numerical aperture objective in TBS) showing the morphology of the cells (mock, WT, and mutant CD11b/CD18) after adhering to iC3b-coated plates for about 1.5 hours in buffer containing 1 mM each of Ca2+ and Mg2+. Images were acquired using SPOT version 4.5 (Diagnostic Instruments, Sterling Heights, MI) and were cropped using Adobe Photoshop version 5.0 (Adobe Systems, San Jose, CA).
The CD11b/CD18 βTD mutants show increased cell spreading
To determine whether the activating βTD mutations also modify outside-in signaling, we measured cell spreading on iC3b-coated plates in the presence of Ca2+ and Mg2+ (1 mM each). Both mock and WT CD11b/CD18-expressing K562 cells showed minimal cell spreading (Figures 4B-C). All 3 βTD mutants showed increased cell spreading, with the NGTD mutant presenting the maximal level of spreading, followed by DSSG and AGAA.
Reactivity of βTD-modified CD11b/CD18 with conformationally sensitive mAbs
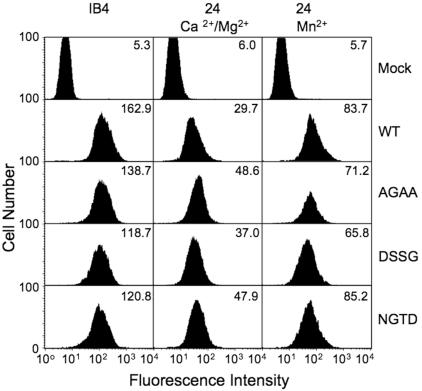
We next used mAbs to probe the conformational state of the expressed mutants in K562. mAb 24 is widely used as a reporter of the high-affinity state in β2 integrins.23 It binds to an activation- and cation-dependent epitope in βA, which includes residues Arg122 of the α1′ helix and Glu175 in the specificity-determining loop (SDL)31 (Figure 1). We assessed the binding of this mAb to WT and mutant CD11b/CD18 expressed on K562 cells. AGAA, DSSG, and NGTD mutants showed a significant increase in binding to mAb 24 compared with WT (Figure 5). The magnitude of this increase was similar to that observed in maximally activated β2 integrins expressed in K562 37 or Jurkat cells.38 In the presence of Mn2+, the reactivity of mAb 24 with WT increased by about 3-fold, compared with the 1.5- to 1.8-fold increase seen in the case of the mutant integrins (Figure 5).
Figure 5.
Reactivity of WT and βTD mutant CD11b/CD18 with conformation-sensitive mAb 24. FACS analysis of the reactivity of mAb 24 with WT and mutant CD11b/CD18-expressing K562 cells. Level of CD11b/CD18 surface expression was analyzed using mAb IB4. Mean fluorescence intensity (MFI) values for mAb staining are shown in each panel from a representative experiment (1 of 3 performed), except that MFI of mAb 24 staining is shown after subtraction of the background mock fluorescence staining levels and after normalization to the IB4 expression values.
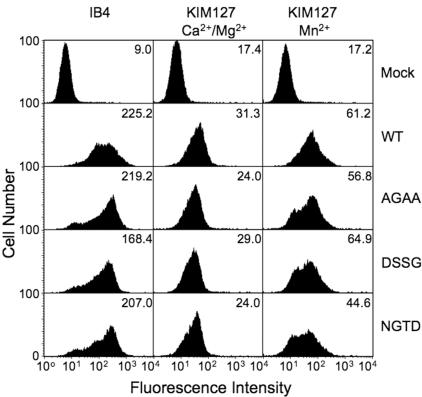
mAb KIM127 binds an epitope in the EGF2 domain in genu-straightened β2-integrins39 and has therefore been used as a marker of this state.40 Because WT CD11b/CD18 is largely inactive when expressed on K562, whereas all 3 mutants are constitutively active as judged by the iC3b-binding assays, we tested mAb KIM127 binding to WT and βTD mutant receptors in physiologic Ca2++Mg2+. There was no significant difference in the level of binding of mAb KIM127 to the constitutively active βTD mutants versus the WT receptor (Figure 6). Reactivity of mAb KIM127 to the WT integrin was doubled in the presence of 1 mM Mn2+, a level of increase consistent with previous reports.38 Interestingly, Mn2+ produced an equivalent increase in binding of mAb KIM127 to each of the 3 βTD mutants (Figure 6).
Figure 6.
Reactivity of WT and βTD mutant CD11b/CD18 with genu extension–dependent mAb KIM127. FACS analysis of the reactivity of mAb KIM127 with WT and mutant CD11b/CD18-expressing K562 cells. Level of CD11b/CD18 surface expression was analyzed using mAb IB4. MFI values for mAb staining are shown in each panel from a representative experiment (1 of 3 performed), except that mean fluorescence intensity of KIM127 staining is shown after subtraction of the background mock fluorescence staining levels and after normalization to the IB4 expression values. No binding was observed with isotype control mAb (not shown).
Discussion
Previous studies in the ligand-binding integrin αA domain have shown that the transition from the inactive to the active state involves coordinated changes in the α1 helix, the F/α7 loop, and α7 helix (reviewed by Arnaout et al41). Small molecules or certain mAbs that bind in the F/α7 region allosterically block ligand binding.42,43 The membrane-proximal βTD in the bent integrin contacts βA in the F-α7 region; replacing the contact residues S674 (from the βTD CD loop) and V332 (from the βA F-strand) with cysteines creates a disulfide bridge that locks the membranebound integrin in the low-affinity state.11,44 Here we show that modifications of the CD loop in the β2 integrin CD11b/CD18 are activating, suggesting that the βTD acts as an allosteric regulator of ligand binding in this integrin. Constitutive activation of the integrin by the CD loop mutations also led to a proportional increase in cell spreading on iC3b-coated surfaces, an outside-in signaling response.
The crystal structure of the bent integrin shows that the βA F/α7 loop is also stabilized in the low-affinity state, through an ADMIDAS metal ion-mediated bridge to the α1 helix, which prevents the inward movement of this helix and stabilizes the F/α7 loop (Figure 1, inset) (reviewed by Arnaout et al41). This link is unique to βA domains (versus the αA domains) and is broken in the crystal structure of the RGD-bound bent integrin ectodomain.3 Mutational removal of this link also generates constitutively active bent β2 integrins but impairs cell spreading (data not shown and Chen et al45), the latter effect perhaps reflective of the need for the ADMIDAS ion in stabilizing the liganded (signaling) integrin state as well.41 Other studies have shown that swapping of the βA's F/α7 loop residues in β2 with those in the β1 subunit or replacing certain residues with alanine induced the high-affinity state in the respective integrin.46,47 Taken together, these data argue that local conformational changes taking place within βA or its contact with the βTD are sufficient to switch the integrin to high binding affinity.
Binding of K562-expressing βTD mutants to EiC3b in suspension was equivalent in the presence of physiologic Ca2+ + Mg2+ or Mn2+ over a broad range of ligand concentrations (Figure 3B-C). This was also the case when EiC3b was allowed to bind to adherent HEK293 cells (Figure 2C). However, only in the case of the NGTD-expressing K562 did binding to the adsorbed iC3b in Ca2+ + Mg2+ approach that seen in the presence of the activating Mn2+. Mn2+ produced a significant increase in binding of AGAA- and to a lesser degree DSSG-expressing K562 to iC3b above that obtained in Ca2+ + Mg2+ (Figure 4A). Also, Mn2+ further increased the constitutive binding of the affinity reporter mAb 24 to all 3 βTD mutants (Figure 5). One interpretation of these findings is that the βTD mutations increase both receptor avidity as well as affinity. It is known, however, that the change in physiologic ligand binding to integrins expressed in K562 reflects an affinity rather than an avidity switch.36 If a change in avidity is also operational, it is more likely to impact integrin binding to the plastic-adsorbed purified ligand. A second interpretation is that the AGAA and DSSG mutants may exert a priming effect, enhancing the transition from the low- to the high-affinity state, short of the full activation state induced by Mn2+. This priming effect is more likely detected when receptor-carrying cells are in suspension, as in the adsorbed ligand-binding assay, explaining the selective enhancing effect of Mn2+ in this case. The nearly equivalent ligand-binding activity of the NGTD mutant in Ca2+ + Mg2+ or Mn2+ in all 3 ligand-binding assays performed indicate, however, that this mutation is most effective in inducing the activation state. Why then is its binding to mAb 24 incomplete unless Mn2+ is present? It is likely that the incremental increase in mAb 24 binding to NGTD-expressing K562 by Mn2+ reflects the small but significant enhancement observed in ligand binding by this cation (Figure 4A). It is also known that mAb 24 reports ligand-occupied βA48-in αA-containing integrins, the endogenous high-affinity αA domain engages the βA MIDAS through the MIDAS cation, which allows αA to then bind exogenous physiologic ligands like iC3b (the ligand relay model).9 Thus, the Mn2+-induced further increase in binding of mAb 24 (versus iC3b ligand) to the NGTD mutant may reflect the preferential coordination of αA-occupied βA by Mn2+ at MIDAS, in comparison with Mg2+ or Ca2+.
It could be argued that more complete activation of CD11b/CD18 by the NGTD mutation may be the result of the bulky sugar moiety breaking the additional contacts the βTD makes with the hybrid domain2 (Figure 1), resulting in head-leg separation, genu straightening, and the opening of the βA/hybrid hinge, all believed to be features of the high-affinity state.13,39 However, mAb KIM127, used as the reporter of the genu-straightened conformation of the integrin,31 did not bind to the βTD mutants, particularly NGTD, in the presence of Ca2+ + Mg2+ (Figure 6). Mn2+ exposed the KIM127 eiptope in both WT and the βTD mutants to the same degree. This finding together with the physiologic ligand binding data suggests that integrin activation can take place in the absence of genu straightening, which has been linked to the opening of the βA/hybrid hinge. Consistently, the high-binding affinity ADMIDAS-defective CD11a/CD18 integrin was also found to be poorly reactive with KIM127,45 and fluorescence lifetime imaging (FLIM) analysis in intact K562 cells in the presence of physiologic cations shows that the ligand-binding site in WT and the NGTD mutant CD11b/CD18 is unchanged relative to the lipid bilayer, arguing against genu straightening induced by this mutation (V. G., Alexandra Makarova, Bradley T. Hyman, and M. A. A., manuscript in preparation). Finally, a recent molecular dynamics study49 found that the activating inward shift of the βA α1 helix can be achieved with a very modest approximately 20 degree opening of the βA/hybrid hinge; an increase of this magnitude can be accommodated in the ligand-bound bent conformation.14
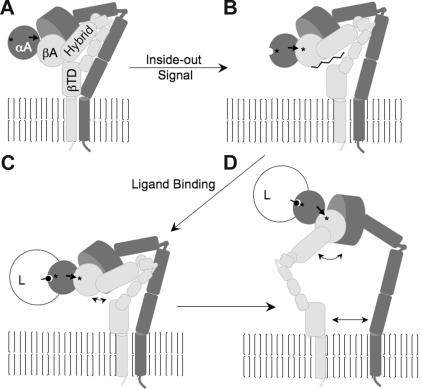
The results presented here add new insights into the allosteric pathways resulting in the integrin-activated state (Figure 7). In the low-affinity state, the integrin is likely expressed on the cell surface in a bent conformation (Figure 7A), stabilized by head-to-leg contacts, inclusive of the membrane-proximal βTD. Conformational changes in the cytoplasmic tails and transmembrane helices, generated by inside-out activation, likely produce a βTD/βA hinge opening (Figure 7B), which switches the integrin βA to the high-affinity binding state, manifested by the restructuring of the F/α7 loop, disrupting ADMIDAS, which permits the activating inward movement of the α1 helix needed to create a stable ligand-binding MIDAS. We cannot exclude a modest (20 degrees or less)14 opening of the βA/hybrid hinge in the bent integrin as part of this process. The strain build-up induced by the binding of physiologic ligand to the bent high-affinity integrin is released by various degrees of opening of the βA/hybrid hinge (Figure 7C-D) that likely extend to those seen in the crystal structure of the ligand-bound αIIbβ3 head segment,5 eliciting genuextension and leg separation, which likely characterize the outside-in signaling state.
Figure 7.
A mechanistic model for the conformational changes during integrin activation and signaling. (A) Schematic of the domain structure of low-affinity leg-bent CD11b/CD18 (CD11b and CD18 in dark and light gray, respectively). βTD contacts the βA and hybrid domains, and the 2 legs are in close proximity. The αA is occupied by a metal ion in the low-affinity MIDAS (asterisk); the bottom of α7 helix of inactive αA (thick black arrow) is not engaged by βA. (B) Inside-out activation disrupts the βTD contact with βA, which unlocks the βA F/α7 loop and allows the inward shift of the α1 helix leading to the formation of a stable αA-bound βA MIDAS (asterisk), which in turn stabilizes αA in the open (high-affinity) state (open semicircle). (C-D) Ligand (L) (black circle on a stick) bound at αA MIDAS stabilizes the occupancy of βA MIDAS by endogenous αA, opening the βA/hybrid hinge to different degrees (double-headed arrows), with a larger hinge opening forcing genu extension and leg separation (D), resulting in outside-in signaling. See text for additional details.
In conclusion, we have shown that the βTD CD loop in the bent CD11b/CD18 integrin is functionally relevant, playing an important role in inside-out integrin activation, as proposed in the deadbolt model.32 Additional accumulating evidence may now be explained in this light (reviewed by Arnaout et al41 and Kamata et al50). For example, creating a disulfide linkage between the βTD and calf-2 has been shown to block activation44; this may prevent the βTD/βA hinge movement. Similarly, mutation of each of the 4 cysteine pairs present in the βTD domain revealed that only C663-C687 in the β3 subunit, located at the base of the CD loop 5 residues away from the TM segment (Figure 1), is activating when interrupted.50 Of interest in this regard is that the βTD's CD loop and the strands, inclusive of the stabilizing disulfide at its base, are naturally absent in the β8 subunit in vertebrates51; the present data suggest that these modifications may prime or activate the respective β8 integrin.
Acknowledgments
This work was supported by grants DK48549, DK50305, and DK068253 from the National Institutes of Health.
We thank Dr Martyn Robinson for providing mAb KIM127, Dr Nancy Hogg for providing mAb 24, and Dr Jun Park for help with the plate adhesion assays.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Amin Arnaout, Nephrology Division, Massachusetts General Hospital, 149 13th St, Charlestown, MA 02129; e-mail: arnaout@receptor.mgh.harvard.edu.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Xiong JP, Stehle T, Diefenbach B, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong JP, Stehle T, Zhang R, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 4.Xiong JP, Stehle T, Goodman SL, Arnaout MA. A novel adaptation of the integrin PSI domain revealed from its crystal structure. J Biol Chem. 2004;279:40252–40254. doi: 10.1074/jbc.C400362200. [DOI] [PubMed] [Google Scholar]
- 5.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JO, Rieu P, Arnaout MA, Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18). Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee JO, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 8.Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 9.Alonso JL, Essafi M, Xiong JP, Stehle T, Arnaout MA. Does the integrin alphaA domain act as a ligand for its betaA domain? Curr Biol. 2002;12:R340–R342. doi: 10.1016/s0960-9822(02)00852-7. [DOI] [PubMed] [Google Scholar]
- 10.Nermut MV, Green NM, Eason P, Yamada SS, Yamada KM. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7:4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 12.Calzada MJ, Alvarez MV, Gonzalez-Rodriguez J. Agonist-specific structural rearrangements of integrin alpha IIbbeta 3. Confirmation of the bent conformation in platelets at rest and after activation. J Biol Chem. 2002;277:39899–39908. doi: 10.1074/jbc.M205886200. [DOI] [PubMed] [Google Scholar]
- 13.Luo BH, Springer TA, Takagi J. Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc Natl Acad Sci U S A. 2003;100:2403–2408. doi: 10.1073/pnas.0438060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adair BD, Xiong JP, Maddock C, Goodman SL, Arnaout MA, Yeager M. Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J Cell Biol. 2005;168:1109–1118. doi: 10.1083/jcb.200410068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas A, Shimaoka M, Phan U, Kim M, Springer TA. Transition from rolling to firm adhesion can be mimicked by extension of integrin alphaLbeta2 in an intermediate affinity state. J Biol Chem. 2006;281:10876–10882. doi: 10.1074/jbc.M512472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Arnaout MA, Todd RF, 3rd, Dana N, Melamed J, Schlossman SF, Colten HR. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983;72:171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Madrid F, Simon P, Thompson S, Springer TA. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright SD, Rao PE, Van Voorhis WC, et al. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983;80:5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogg N, Stewart MP, Scarth SL, et al. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J Clin Invest. 1999;103:97–106. doi: 10.1172/JCI3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dana N, Clayton LK, Tennen DG, et al. Leukocytes from four patients with complete or partial Leu-CAM deficiency contain the common beta-subunit precursor and beta-subunit messenger RNA. J Clin Invest. 1987;79:1010–1015. doi: 10.1172/JCI112868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson MK, Andrew D, Rosen H, et al. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- 23.Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch EF, Maniatis T. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 25.Li R, Arnaout MA. Functional analysis of the beta 2 integrins. Methods Mol Biol. 1999;129:105–124. doi: 10.1385/1-59259-249-X:105. [DOI] [PubMed] [Google Scholar]
- 26.Tan SM, Hyland RH, Al-Shamkhani A, Douglass WA, Shaw JM, Law SK. Effect of integrin beta 2 subunit truncations on LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) assembly, surface expression, and function. J Immunol. 2000;165:2574–2581. doi: 10.4049/jimmunol.165.5.2574. [DOI] [PubMed] [Google Scholar]
- 27.Simon DI, Xu H, Ortlepp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler Thromb Vasc Biol. 1997;17:528–535. doi: 10.1161/01.atv.17.3.528. [DOI] [PubMed] [Google Scholar]
- 28.Michishita M, Videm V, Arnaout MA. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72:857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- 29.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Rieu P, Griffith DL, Scott D, Arnaout MA. Two functional states of the CD11b A-domain: correlations with key features of two Mn2+-complexed crystal structures. J Cell Biol. 1998;143:1523–1534. doi: 10.1083/jcb.143.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu C, Ferzly M, Takagi J, Springer TA. Epitope mapping of antibodies to the C-terminal region of the integrin beta 2 subunit reveals regions that become exposed upon receptor activation. J Immunol. 2001;166:5629–5637. doi: 10.4049/jimmunol.166.9.5629. [DOI] [PubMed] [Google Scholar]
- 32.Xiong JP, Stehle T, Goodman SL, Arnaout MA. New insights into the structural basis of integrin activation. Blood. 2003;102:1155–1159. doi: 10.1182/blood-2003-01-0334. [DOI] [PubMed] [Google Scholar]
- 33.Xiong JP, Li R, Essafi M, Stehle T, Arnaout MA. An isoleucine-based allosteric switch controls affinity and shape shifting in integrin CD11b A-domain. J Biol Chem. 2000;275:38762–38767. doi: 10.1074/jbc.C000563200. [DOI] [PubMed] [Google Scholar]
- 34.Shimaoka M, Shifman JM, Jing H, Takagi J, Mayo SL, Springer TA. Computational design of an integrin I domain stabilized in the open high affinity conformation. Nat Struct Biol. 2000;7:674–678. doi: 10.1038/77978. [DOI] [PubMed] [Google Scholar]
- 35.Ortlepp S, Stephens PE, Hogg N, Figdor CG, Robinson MK. Antibodies that activate beta 2 integrins can generate different ligand binding states. Eur J Immunol. 1995;25:637–643. doi: 10.1002/eji.1830250302. [DOI] [PubMed] [Google Scholar]
- 36.Beals CR, Edwards AC, Gottschalk RJ, Kuijpers TW, Staunton DE. CD18 activation epitopes induced by leukocyte activation. J Immunol. 2001;167:6113–6122. doi: 10.4049/jimmunol.167.11.6113. [DOI] [PubMed] [Google Scholar]
- 37.Annenkov A, Ortlepp S, Hogg N. The beta 2 integrin Mac-1 but not p150,95 associates with Fc gamma RIIA. Eur J Immunol. 1996;26:207–212. doi: 10.1002/eji.1830260132. [DOI] [PubMed] [Google Scholar]
- 38.Cherry LK, Li X, Schwab P, Lim B, Klickstein LB. RhoH is required to maintain the integrin LFA-1 in a nonadhesive state on lymphocytes. Nat Immunol. 2004;5:961–967. doi: 10.1038/ni1103. [DOI] [PubMed] [Google Scholar]
- 39.Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Activation of leukocyte beta(2) integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–594. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Shimaoka M, Salas A, Takagi J, Springer TA. Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc Natl Acad Sci U S A. 2004;101:2906–2911. doi: 10.1073/pnas.0307340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 42.Kallen J, Welzenbach K, Ramage P, et al. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Haruta I, Rieu P, Sugimori T, Xiong JP, Arnaout MA. Characterization of a conformationally sensitive murine monoclonal antibody directed to the metal ion-dependent adhesion site face of integrin CD11b. J Immunol. 2002;168:1219–1225. doi: 10.4049/jimmunol.168.3.1219. [DOI] [PubMed] [Google Scholar]
- 44.Kamata T, Handa M, Sato Y, Ikeda Y, Aiso S. Membrane-proximal {alpha}/{beta} stalk interactions differentially regulate integrin activation. J Biol Chem. 2005;280:24775–24783. doi: 10.1074/jbc.M409548200. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Yang W, Kim M, Carman CV, Springer TA. Regulation of outside-in signaling and affinity by the beta2 I domain of integrin alphaLbeta2. Proc Natl Acad Sci U S A. 2006;103:13062–13067. doi: 10.1073/pnas.0605666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehirchiou D, Xiong YM, Li Y, Brew S, Zhang L. Dual function for a unique site within the beta2I domain of integrin alphaMbeta2. J Biol Chem. 2005;280:8324–8331. doi: 10.1074/jbc.M413525200. [DOI] [PubMed] [Google Scholar]
- 47.Hato T, Yamanouchi J, Yakushijin Y, Sakai I, Yasukawa M. Identification of critical residues for regulation of integrin activation in the beta6-alpha7 loop of the integrin beta3 I-like domain. J Thromb Haemost. 2006;4:2278–2280. doi: 10.1111/j.1538-7836.2006.02137.x. [DOI] [PubMed] [Google Scholar]
- 48.Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA. Small molecule integrin antagonists that bind to the beta2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity. 2003;19:391–402. doi: 10.1016/s1074-7613(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 49.Puklin-Faucher E, Gao M, Schulten K, Vogel V. How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J Cell Biol. 2006;175:349–360. doi: 10.1083/jcb.200602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamata T, Ambo H, Puzon-McLaughlin W, et al. Critical cysteine residues for regulation of integrin alphaIIbbeta3 are clustered in the epidermal growth factor domains of the beta3 subunit. Biochem J. 2004;378:1079–1082. doi: 10.1042/BJ20031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jannuzi AL, Bunch TA, West RF, Brower DL. Identification of integrin beta subunit mutations that alter heterodimer function in situ. Mol Biol Cell. 2004;15:3829–3840. doi: 10.1091/mbc.E04-02-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]