Abstract
The unique immunoglobulin (Ig) idiotype on the surface of each B-cell lymphoma represents an ideal tumor-specific antigen for use as a therapeutic vaccine. We have used an Escherichia coli—based, cell-free protein-expression system to produce a vaccine within hours of cloning the Ig genes from a B-cell tumor. We demonstrated that a fusion protein consisting of an idiotypic single chain Fv antibody fragment (scFv) linked to a cytokine (GM-CSF) or to an immunostimulatory peptide was an effective lymphoma vaccine. These vaccines elicited humoral immune responses against the native Ig protein displayed on the surface of a tumor and protected mice against tumor challenge with efficacy equal to that of the conventional Ig produced in a mammalian cell and chemically coupled to keyhole limpet hemocyanin. The cell-free E coli system offers a platform for rapidly generating individualized vaccines, thereby allowing much more efficient application in the clinic.
Introduction
B lymphocytes, their clonal progeny, and the malignant lymphomas derived from B lymphocytes each express a unique immunoglobulin (Ig) molecule on the cell surface. Specific antibodies directed against the Ig variable regions can cure lymphomas.1–5 The same Ig variable regions can be made into vaccines to induce a specific immune response by the host against their own tumor.6–10 Such vaccines can be proteins containing the Ig variable regions or DNA encoding these proteins.11–13 In addition, the idiotype protein has been used to pulse dendritic cells for active vaccination.14 Active vaccination induces a polyclonal immune response that should be better than any single monoclonal antibody.15 In addition, active vaccination can induce a T-cell immune response
Previously, we have shown that vaccination of patients with lymphoma can induce an immune response against the Ig molecule on their tumor13,16–18 and that such an immune response correlates with a favorable clinical outcome.18 Prospective, randomized clinical trials are currently under way to determine the efficacy of idiotype vaccination in patients with lymphoma. These vaccines consist of a whole Ig derived from each B-cell tumor and chemically conjugated to a foreign protein, keyhole limpet hemocyanin (KLH), that enhances the immunogenicity of the molecule. Current vaccine manufacturing methods are time consuming and expensive. If personalized therapy with a patient-specific vaccine is to become broadly applicable, a rapid and inexpensive method for vaccine production is needed.
Cell-free protein synthesis (CFPS) technology is a much more rapid and economical vaccine production strategy than traditional protein expression systems that use living cells or other organisms. Cell-free protein synthesis provides high yields19,20 that are not attainable in living hosts because of toxicity21–23 under conditions optimal for protein folding, particularly with regard to proper disulfide bond formation.24,25 Once a patient's lymphoma-specific Ig V genes have been cloned, the proteins can be produced in a cell-free transcription-translation system within hours and can be purified and ready for use as vaccine in a matter of days. Protein produced by stably transfected mammalian or insect cell lines can take months to prepare. These advantages of in vitro expression technology could enable better treatment strategies for patients with lymphoma, because the vaccine could be available for use soon after diagnosis and prior to the use of immunosuppressive chemotherapy.
We developed a cell-free protein expression system that produces high yields of biologically active granulocyte-macrophage colony-stimulating factor (GM-CSF).26 In that study we determined the optimal gene order for combining GM-CSF with an Ig single chain variable fragment (scFv) to maximize protein solubility and biological activity.23 Here, we show that proteins produced in the cell-free system can function as vaccines against a murine B-cell lymphoma. The 2 vaccine candidates we tested were a tumor-derived scFV fused through the bacterial immunity protein, Im9, to GM-CSF and the scFV fragment fused to a 9-amino acid peptide sequence from interleukin-1β (IL-1β9aa).7,27 Both of these fusion proteins induced tumor-specific humoral immune responses rivaling those of the traditional vaccine composed of the whole IgM protein chemically coupled to KLH. Furthermore, the GM-CSF-scFv fusion protein was able to protect mice from tumor challenge with efficacy equal to that of the traditional Ig-KLH vaccine. These results show that the cell-free expression system is a viable option for the production of patient-specific vaccines and confirm that IL-1β9aa and GM-CSF are effective immunostimulatory fusion partners that increase the potency of the cell-free vaccines.
Materials and methods
Mice and cell lines
C3H/Hen female mice, aged 6 to 8 weeks, were obtained from Harlan Sprague-Dawley (San Diego, CA) and were housed at the Laboratory Animal Facility at Stanford University Medical Center (Stanford, CA). All animal experiments were conducted following the Laboratory Animal Facility and National Institute of Health guidelines. The study protocol was approved by the Stanford University institutional animal care and use committee. The carcinogen-induced IgM/κ 38C13 murine B-cell lymphoma cell line28 and the mGM-CSF–dependant NFS-60 cell line have been previously described.29 All cell lines were cultured in RPMI 1640 + L-glutamine (Gibco from Invitrogen, Carlsbad, CA), penicillin-streptomycin (pen-strep; Invitrogen), 10% FCS (Omega Scientific, Tarzana, CA), and 2-mercaptoethanol (2ME; Invitrogen). NFS-60 cells were grown in this media, with the addition of either yeast-derived mGM-CSF (donated by Immunex, now Amgen, Thousand Oaks, CA) or bacterial-derived mGM-CSF (PeproTech, Rocky Hill, NJ).
Construction of scFv and fusion plasmids for Id vaccines
The construction of pK7.38C13scFv (38C13 VH-VL) was reported previously.23,26 For pK7.38C13.IL-1β9aa, a DNA fragment (5′-GTGCAGGGTGAAGAGAGCAACGA TAAA-3′) that encodes a 9-amino acid peptide VQGEESNDK from IL-1β was added onto the VL-VH DNA fragment by polymerase chain reaction (PCR) primer extension of the 3′ region of pK7.VH-VL vector with an opposing T7 forward primer. The 3′ extension was made from the 38C13 VL gene to include the first 15 base pairs of the E coli protein chloramphenicol acetyltransferase (CAT),23,26 38C13 VH-VL, and the IL-1β sequence followed by a GGGGS linker and a C-terminal His-tag. The fragment was then restricted with Nde1 and Sal1 and ligated into the pK7 vector. Another construct was made with an N-terminal IL-1β9aa peptide (IL1β9aa-VL-VH). This construct was made by PCR primer extension of pK7.VH-VL, amplified with a T7 termination reverse primer and a forward primer encoding the first 15 base pairs of CAT, followed by the IL1β9aa sequence (listed above), a sequence encoding a GGGGS linker and overlap with the 38C13 VH. The entire forward primer sequence was 5′-CATATGGAGAAAAAAATCGTTCAGGGTGAAGAAAGTAACGATAAATGCCAGGTGAAGCTGCA-GGAG-3′. The fragment was cloned into the pK7 vector after restriction digest of the Nde1 and Sal1 sites.
To construct pK7.GM-Im9-VL-VH, the Im9 linker was used to replace the GGGGS linker between GM-CSF and VL in the pK7.GM-VL-VH construct23 yielding a new fusion of mGM-Im9-VL-VH. The DNA sequence encoding the Im9 protein was obtained by using codons favored for protein expression in the Escherichia coli system. The resulting sequence of Im9 was 5′-GAACTGAAACATAGCATCTCCGACTATACCGAAGCGGAG-TTTTTACAGCTGGTGACCACGATTTGCAACGCCGATACCAGCT-CGGAAGAAGAGCTGGTGAAATTAGTGACGCATTTTGAAGAGA-TGACCGAGCATCCGAGCGGTTCCGATCTGATTTACTATCCGAAA-GAGGGCGATGACGATAGCCCGAGTGGGATTGTTAACACCGTT-AAACAGTGGCGTGCGGCCAATGGTAAAAGCGGGTTTAAACAA-GGG-3′. The PCR-amplified Im9 fragment was ligated to mouse GM-CSF through overlapping PCR. mGM-CSF was amplified with primers 5′-ATATACATATGGAGAAAAAAATCGCACC-3′ and 5′-GTCGG AGATGCTATGTTTCAGTTCAGAGCCACCTCCTCCTTTTTG-3′ and pK7. GM-CSF26 as the template. The PCR-amplified mGM-CSF was mixed with the Im9 fragment and amplified using 10 rounds of annealing and extension, followed by PCR with 5′-ATATACATATGGAGAAAA-AAATCGCACC-3′ and 5′-AGACTGGGTGAGCTC AATGTC-3′. Finally, the PCR-amplified GM-Im9 fragment was digested by NdeI and SacI and ligated into NdeI/SacI-digested pK7.mGM-VL-VH, yielding pK7.mGM-Im9-VL-VH.
The pk7.hGM-Im9-VL-VH plasmid was constructed from the mGM-Im9-VL-VH template by exchanging human GM-CSF for mouse GM-CSF. The hGM-CSF gene was amplified by PCR from a construct previously used6 and inserted into the same location as mGM-CSF by overlapping PCR of the 3′ region of hGM-CSF with the 5′ region of the GGGGS linker/Im9 region of mGM-Im9-38VL-VH. A CAT 15-base pair sequence, 6X His-tag, and GGGGS linker was also added to the 5′ region of hGM-CSF by primer extension. The resulting fragment was then restricted with Nde1 and Sal1 and ligated into the pK7 vector.
In vitro GM-CSF bioassays
The biological activity of cell-free–expressed GM-CSF and GM-CSF fusion proteins was assayed using a murine GM-CSF–dependent cell line, NFS-60.29 GM-CSF–dependent NFS-60 cells were grown in RPMI media (Invitrogen) with 10% FCS in the presence of E coli–derived GM-CSF (PeproTech). Once in log-phase growth, cells were centrifuged and washed 3 times with RPMI/10%FCS to remove residual GM-CSF. Cells were plated at a concentration of 5000 cells/well in a flat-bottom 96-well tissue culture plate (Falcon Microtest 96; Fisher Scientific, Rockville, MD), and dilutions of standard GM-CSF (E coli–derived GM-CSF from R&D Systems, Minneapolis, MN) or cell-free expressed GM-CSF/fusion proteins were added to the wells in serial dilutions. Cultures containing each dilution were performed in triplicate. After 20 hours, 50 μL [3H]-thymidine (Amersham/GE Healthcare, Piscataway, NJ) was added to each well to a final concentration of 6.7 μCi/mL (0.248 MBq). Seven hours later, the cells were harvested onto glass fiber filter mats, and [3H]-thymidine incorporation was measured using a Wallach 1450 MicroBeta scintillation counter (PerkinElmer Life Sciences, Wellesley, MA).
Generation of mAbs for Id-KLH vaccines and immunoassays
The 38C13 immunoglobulin was produced from a hybridoma (38C13/A1-2) that secretes soluble 38C13 IgM.30 To produce a vaccine, this protein was conjugated to KLH using glutaraldehyde. The anti-38C13 idiotype Ab preparation used as a standard in the immunoassays was an equimolar cocktail of different isotypes produced from the hybridomas S1C5, S5A8, S3H5, and S4C8, which have been previously described.30
Expression and purification of fusion proteins produced in the cell-free system
Cell-free reaction conditions are described in our previous report.23 For production of protein for the current experiments, the in vitro reactions were scaled up to 5 mL. The reaction mixture was placed as a large 5-mL drop in a Petri dish and incubated at 30°C for 4 hours.31 After 4 hours, the soluble protein was separated from the insoluble fraction of the reaction by centrifugation at 15 000g for 15 minutes. The soluble fraction was loaded on a 5-mL Ni-NTA column (Qiagen, Valencia, CA), pre-equilibrated with 50 mM phosphate buffer (pH 8.0) containing 300 mM NaCl and 10 mM imidazole. The column was washed with 30 mL 25 mM imidazole in the same buffer and eluted with 250 mM imidazole. The purified products were then concentrated by filtration in an Amicon (Bedford, MA) 15-mL ultrascale centrifugal device (5000 MWCO) and dialyzed against PBS buffer. Finally, the vaccine protein was brought to a concentration of 0.1% Tween-20 and sterilized by filtration through a 0.2-μM filter (Nalgene, Rochester, NY). The vaccines were stored at 4°C for up to 5 weeks before use.
Vaccine protein quantification
Following protein purification, vaccine proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (NuPage 10% Bis-Tris; Invitrogen) under reducing conditions. Purified protein (8 μL) or BSA (Pierce, Rockford, IL) of varying concentration was loaded with 1 mM dithiothreitol and NuPage sample buffer (Invitrogen) in a final volume of 20 μL. Protein concentration was determined by staining with Coomassie blue and comparing the relative intensity of purified vaccine products with that of the BSA standards of known concentration. Further analysis of the stored products by nondenaturing gel electrophoresis revealed that both the GM-CSF fusion proteins were in the form of monomers, and both of the proteins lacking GM-CSF were in the form of dimers.
Immunization with in vitro fusion proteins and Id-KLH and tumor challenge
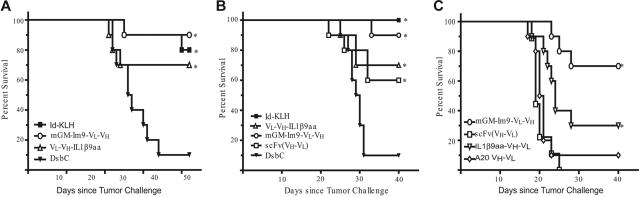
For immunization, C3H/Hen mice were vaccinated intraperitoneally with 50 μg idiotype Ig conjugated to KLH, 15 μg of the appropriate scFv, the molar equivalent of scFv fusion proteins, or cell-free–produced DsbC (irrelevant control) diluted into 200 to 500 μL PBS. Mice were vaccinated on days 0, 14, and 28, and tail-bled 10 days following the second and third vaccinations to assess immune responses. Two weeks following the third vaccination, mice were challenged with 38C13 tumor cells intraperitoneally in 200 μL PBS (400 cells in Figure 5A–B, 1200 cells in Figure 5C). Before tumor challenge, cells were thawed, washed 3 times in RPMI/10% FCS/pen-strep/L-glutamine/2ME, and grown in culture for 3 days. Before tumor challenge, cells were washed 3 times in PBS then diluted to the correct concentration in PBS for injection. After tumor challenge, mice were followed for survival. Survival analysis was performed using Prism software (GraphPad, San Diego, CA), and P values were calculated using the log-rank statistical test.
Figure 5.
Vaccine produced in the cell-free system protected mice from tumor challenge comparably to the Id-KLH vaccine. In 3 independent experiments mice were immunized biweekly with 3 vaccinations of the indicated vaccine and were challenged with 400 38C13 tumor cells (or 1200 tumor cells in panel C) 2 weeks following the third vaccination. (A) First tumor challenge experiment (n = 10 mice per group). (B) Second tumor challenge experiment (n = 10 mice per group). For both groups, *statistical significance (P < .05 compared with DsbC control) determined by comparing survival curves using the log-rank test. (C) Third tumor challenge experiment (n = 10 except n = 9 for scFv and Im9-38ScFv and n = 7 for hGM-Im9-scFv). *significance (P < .05) by the log-rank test compared with both 38C13 scFv (VH-VL) and A20 scFv (VH-VL). Actual P values for all experiments are listed in the text.
Immunoassays for measurement of antitumor immune responses
To assess the humoral immune response in the 38C13 tumor model, we used an enzyme-linked immunosorbent assay (ELISA) to quantify 38C13-specific IgG antibodies and fluorescence-activated cell sorting (FACS) staining to assess the ability of the vaccine-induced Ab response to recognize the native target on the 38C13 tumor. For the ELISA, 96-well flat-bottom Nunc Maxisorp Immunoplates (Nalgene) were coated with 5 μg/mL 38C13 Ab in carbonate buffer overnight. The following day, the plates were blocked with 5% nonfat milk in PBS. Serum (from C3H/Hen mice 10 days after second or third vaccination) or standard (anti-38C13 Id cocktail of 4 different isotypes) was diluted in 1%BSA/PBS and applied for 1 hour, and bound IgG from serum was detected using a goat anti–mouse IgG-specific HRP-conjugated Ab (Southern Biotech, Birmingham, AL). Plates were washed 4 times between steps with ELISA wash buffer (PBS/NaCl/Triton X), and detection was performed with ABTS chromogenic substrate (Roche, Basel, Switzerland) and observed by measuring optical density at 405 to 490 nm with a Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA).
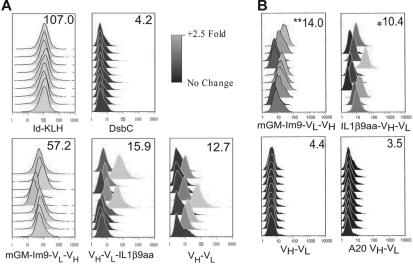
Serum of immunized mice was tested for the ability of the antibodies to recognize the native 38C13 protein on the surface of the tumor cells by flow cytometry. Cells were grown in RPMI, washed in PBS with 1% BSA and 0.05% sodium azide, and resuspended in the same buffer to be divided into aliquots at 500 000 cells/tube. Cells were stained with serum for 30 minutes at 1:20 (Figure 4A) or 1:800 dilutions (Figure 4B), washed twice in FACS buffer, then stained with FITC-conjugated goat anti–mouse IgG. The cells were analyzed on a FACScalibur flow cytometry instrument (Becton Dickinson, San Diego, CA).
Figure 4.
Vaccines prepared in a cell-free system are capable of inducing an immune response that recognizes the native 38C13 protein on the cell surface. Flow cytometry was used to analyze 38C13 tumor cells incubated with mouse sera obtained after 3 vaccinations with the indicated proteins. Each histogram represents results from one mouse. The scale shows fold change over secondary antibody alone on a log2 scale. The numbers in the upper right corner of each box indicate mean median fluorescence for that group. (A) Flow cytometry of cells incubated with sera at a 1:20 dilution. (B) Analysis of an independent experiment by flow cytometry. Cells were incubated with sera at a 1:800 dilution to determine the relative efficacy of vaccines in induction of a specific Ab response. **Significance (P < .05) by Student 2-tailed t test compared with both 38C13 scFv (VH-VL) and A20 scFv (VH-VL) and *statistical significance (P < .05) compared with A20 scFv (VH-VL).
Results
Expression and purification of proteins
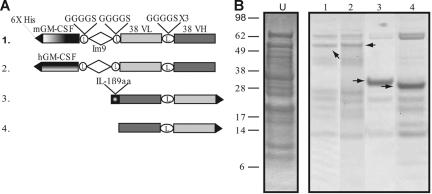
The constructs used to generate the cell-free proteins are variations on the scFv derived from the mouse B-cell tumor 38C13 and are diagrammed in Figure 1A. The fusion proteins were histidine tagged to facilitate purification. The first construct, GM-Im9-VL-VH, is a fusion of mouse GM-CSF to the 38C13 scFv via a linker sequence derived from the E coli immunity protein Im9.32 Im9 is a 4-helix, antiparallel bundle protein that folds rapidly during biosynthesis, and we reasoned that the Im9 domain would separate the GM-CSF and the scFv components and allow each of them to fold correctly. We also constructed 2 different fusions of the 38C13 scFv with a 9-amino acid epitope from IL-1β (IL1β9aa), one with the IL1β9aa peptide at the C terminus (VH-VL-IL1β9aa) and one with the scFv component at the C terminus (IL1β9aa-VH-VL, only one is shown in Figure 1A), because we knew from previous work23 that the order of the individual components could affect protein folding. A fusion protein made with human GM-CSF, which is not biologically active in murine cells, served as a test of the requirement for GM-CSF bioactivity. In addition, a construct with the scFv from the unrelated A20 B-cell lymphoma (A20 VH-VL) served as a control for idiotype specificity. The cell-free reaction was optimized for redox potential and disulfide bond formation as previously described.23 Protein synthesis was allowed to proceed for 4 hours in 5-mL batches using the thin film format,31 with a total of approximately 30 mL cell-free reaction volume produced per construct. The His6-tagged proteins were purified immediately from the soluble fraction of the cell-free reaction mixture using a nickel affinity column. A Coomassie-stained gel of the purified fusion proteins is shown in Figure 1B. The purity varied for the different constructs and ranged between 50% and 90%, based on calibrated estimates of gel band intensities. The 30-mL reaction volume usually produced approximately 1 mg protein after purification, but yields ranged between 0.5 and 3 mg (data not shown).
Figure 1.
Constructs and purified protein products from cell-free reactions. (A) Diagram of 38C13 idiotype constructs produced in the cell-free expression system. Construct 1 is an scFv linked to mouse GM-CSF via an Im9 linker. Construct 2 links human GM-CSF to the scFv fragment. The third construct is a fusion of IL-1β9aa to the 38C13 scFv. Construct 4 is the 38C13 scFv alone. Constructs not shown are the C-terminal IL-1β9aa fusion to the 38C13 scFv, the A20 scFv, and the E coli disulfide bond isomerase (DsbC). (B) After 4 hours of protein synthesis, products were enriched from the cell-free reaction supernatant using a nickel affinity column. The partially purified products of constructs were analyzed by SDS-PAGE and stained with Coomassie blue. The arrows indicate the band that corresponds to the desired product. For comparison, unpurified cell-free product is shown (lane U).
Biological activity of GM-CSF fusion proteins
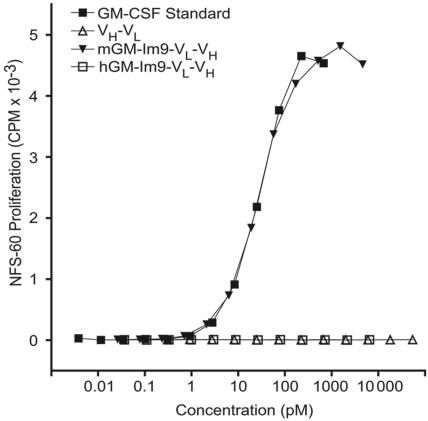
Our previous report on immunoglobulin–GM-CSF fusion proteins6 demonstrated that their efficacy as vaccines depended on the biological activity of the GM-CSF. Therefore, we tested the GM-CSF bioactivity of the fusion proteins using a murine GM-CSF–dependant cell line, NFS-60 (Figure 2). We found that our GM-Im9-VL-VH fusion protein supported the growth of NFS-60 cells with equal efficacy to that of the commercially available murine GM-CSF, produced in bacteria. In contrast, and as expected, neither 38C13 scFv nor the scFv fused to human GM-CSF were able to support the proliferation of these murine GM-CSF–dependent cells.
Figure 2.
mGM-CSF fusion proteins are bioactive as measured by growth of GM-CSF–dependent NFS-60 cells. NSF-60 cells were grown in the presence of varying concentrations of 38C13 idiotype fusion proteins prepared in the cell-free expression system or commercial murine GM-CSF (mGM-CSF) standard. All dilutions were performed in triplicate. Neither the human fusion (hGM-Im9-scFv) nor scFv alone induced cell growth, but the fusion protein containing mGM-CSF fused to scFv promoted the growth of NFS-60 cells with the same potency as a commercial standard.
Immunogenicity of scFv fusion proteins
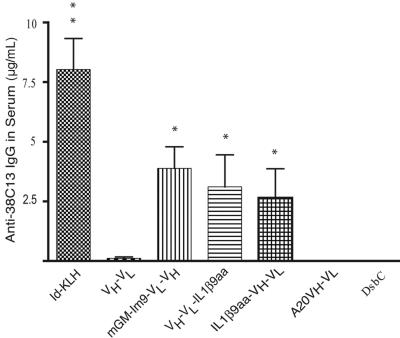
We determined the relative ability of 38C13 scFv fusion proteins to induce antibodies that recognize the native 38C13 protein, both as a free molecule and as a cell-surface protein. Such recognition is a minimal requirement of the desired immune response and a good test for proper folding of the cell-free vaccines. After 3 immunizations, all groups of mice vaccinated with either idiotype Ig conjugated to KLH (Id-KLH) or with a cell-free produced 38C13 scFv proteins had an antibody response detectable by ELISA (Figure 3). As negative controls, we used 2 different irrelevant protein products produced using the cell-free method: the scFv derived from the BALB/c A20 tumor (A20 VH-VL) and the E coli disulfide bond isomerase (DsbC) protein, also produced by CFPS. As expected, neither of the nonspecific controls elicited an anti-antibody response against the 38C13 tumor Ig protein. Mice immunized with Id-KLH produced in mammalian cells had the highest antibody titer of all the groups. The scFv fusion proteins produced in the cell-free system, GM-Im9-VL-VH, VH-VL-IL1β9aa, and IL1β9aa-VH-VL, all induced higher titers of anti-38C13 antibodies than did vaccination with scFv alone (P < .001, P = .035, and P = .007, respectively). The different scFv fusions elicited similar responses, although GM-Im9-VL-VH had a slight advantage over IL1β9aa-VH-VL (P = .473).
Figure 3.
Cell-free vaccines induced humoral immune responses; anti-38C13 Ab levels were comparable to that of the Id-KLH standard. The concentration of anti-38C13 IgG antibodies in mouse serum 10 days after the third vaccination was determined by ELISA. Data plotted is combined from 3 independent experiments (n = 29 mice for mGM-Im9-VL-VH, 20 mice for VL-VH-IL1β9 and DsbC, 19 mice for Id-KLH and scFv (VH-VL), and 10 mice for A20 VH-VL and IL1β9aa-VH-VL). Variations in group sizes are due to groups being tested in 1, 2, or 3 experiments and to the loss of serum samples in a few cases. Data shown are means with SEM; *P < .05 compared with scFv (VH-VL); **P < .05 compared with mGM-Im9-VL-VH and VH-VL-IL1β9aa using a 2-tailed Student t test. Exact P values are stated in the text.
To further assess the efficacy of the vaccines, we used flow cytometry to test whether the elicited antibodies recognized the idiotypic Ig on the surface of the 38C13 tumor cells (Figure 4). Sera from individual mice immunized with Id-KLH or with mGM-Im9-VL-VH gave strong staining of the tumor cells (Figure 4A). The scFv fragment alone and VH-VL-IL1β9aa also induced antitumor antibodies, but staining was much weaker and not every mouse responded (Figure 4A). These antibodies were specific, because they did not bind to the cells of an unrelated mouse B-cell tumor (data not shown).
In an independent experiment we tested a construct with the IL1β9aa peptide fused to the N-terminus of the scFv component because in our previous studies23 a GM-CSF fusion protein worked well with this gene order. We also tested a lower concentration of the immune serum so as to better compare the relative efficacies of the vaccines (Figure 4B). At this lower concentration, the serum from the scFv-immunized mice showed no staining of the 38C13 tumor. In contrast, both fusion proteins tested, GM-Im9-VL-VH and IL1β9aa-VH-VL, induced detectable anti-idiotype antibodies. However, when compared with the response to the scFv fragment alone, only the GM-Im9-VL-VH fusion protein had a statistical advantage (P < .001), and only in this group did every mouse respond (Figure 4B).
Tumor protection
We tested the ability of the vaccines to protect mice from tumor challenge in 3 separate experiments (Figure 5). As shown in Figure 5A–B, mice were challenged with 400 38C13 tumor cells after 3 vaccinations with the indicated proteins. All of the vaccines tested provided significant protection as compared with mice immunized with an irrelevant control protein (DsbC, also produced in the cell-free system). Both experiments showed that mGM-Im9-VL-VH was as effective as the conventional protein product (a full-length, mammalian-produced Ig coupled to KLH). The GM-Im9-VL-VH vaccine offered the best protection of the fusion proteins tested when all 3 experiments were taken into account, although it provided no statistical advantage over the other cell-free fusion vaccines within a particular experiment. There was no statistical difference between the efficacy of the IL-1β9aa fusion, which consistently protected 70% of the mice, and the 38scFv vaccine, which protected 60% (Figure 5B). Therefore, we found no advantage when an IL-1β9aa molecule was fused to the C terminus of the scFv relative to the scFv fragment alone. On the basis of the results of these 2 tumor challenge experiments, GM-Im9-VL-VH appeared to be as effective a vaccine as Id-KLH and performed better than the other vaccines tested (although efficacy was not statistically different from that of VH-VL-IL1β9aa).
We then challenged immunized mice (immune response shown in Figure 4B) with 3 times the number of tumor cells used in the first 2 in vivo experiments to more stringently assess the relative efficacies of the different vaccines (Figure 5C). This experiment included IL1β9aa-VH-VL and the negative control vaccine, A20 VH-VL. In this experiment, the 38C13 scFv had no advantage over the control A20 scFv. Immunization with either GM-Im9-VL-VH or IL1β9aa-VH-VL had a protective advantage over the A20 scFv (P < .001 and P = .038, respectively) and over the 38C13 scFv (P < .001 and P = .009). With the increased number of tumor cells, GM-Im9-VL-VH was superior to IL1β9aa-VH-VL (P = .047).
Discussion
Several clinical trials are under way to test tumor-derived Ig as a therapeutic vaccine for patients with B-cell lymphoma. Patients are treated with whole Ig molecules, prepared using mammalian or insect cell expression systems, conjugated chemically to KLH, and are coadministered with GM-CSF as an immune stimulant. To make customized immune therapy feasible and potentially available soon after diagnosis, prior to the use of immunosuppressive chemotherapy, more streamlined production methods are needed.
Here, we report that idiotype fusion proteins produced in an E coli–based cell-free expression system serve as effective vaccines for eliciting tumor protection. In addition, we have identified 2 potential proteins that, when fused to 38C13 scFv, increased its immunogenicity and ability to protect mice from a tumor challenge. One advantage of the in vitro expression platform in comparison to mammalian or insect cell systems is that much higher soluble yields can be obtained. Using the model protein E coli chloramphenicol acetyltransferase (CAT), batch reactions can produce up to 800 μg/mL,31 and in one semicontinuous system, up to 6 mg/mL.19
A second advantage of the cell-free system is that the reaction milieu can be altered to facilitate correct protein folding, particularly by changing the sulfhydral redox potential, a parameter important for proteins with disulfide bonds. In previous publications, several measures have been used to encourage oxidative protein folding: (1) using a sulfhydral buffer composed of oxidized and reduced glutathione,24,25,33 (2) pretreating the E coli extract with iodoacetamide to inactivate endogenous sulfhydral-dependant oxidoreductases,24,33 and (3) adding exogenous bacterial disulfide isomerase DsbC.24,33 Combining these approaches, we were able to increase the yield of soluble, biologically active murine GM-CSF26 as well as proteins containing biologically active mGM-CSF fused to the scFv of the mouse 38C13 B-cell lymphoma.23
In this report, we showed that idiotype vaccines produced in a cell-free system can be as effective as full-length tumor Ig fused to KLH produced in a mammalian cell system. Our experiments showed that the scFv portions of the fusion proteins produced using CFPS technology were sufficiently well folded to induce an antitumor humoral response. In addition, the vaccine formulations, GM-Im9-VL-VH and IL1β9aa-VH-VL, induced substantial antibody responses against the soluble native 38C13 protein and specifically bound the idiotype expressed on the tumor cell surface. The most effective construct tested was the fusion of GM-CSF to the single-chain Fv (GM-Im9-VL-VH); this fusion consistently protected mice from tumor challenge with efficacy comparable to conventional Id-KLH.
We also showed that the IL1β9aa-VH-VL vaccine (the smallest scFv-fusion protein tested) protected mice from tumor challenge. Because the IL-1β 9 amino acid peptide retains the immunostimulatory activity of the full-length IL-1β molecule,27 it is thought to act through the IL-1β receptor. However, this remains to be proven. It was clear from our experiments that the ILβ9aa-VH-VL vaccine was superior to VH-VL alone, but only when IL-1β9aa was placed on the N-terminus of the scFv fragment.
The speed and relative simplicity of the cell-free system allowed us to produce and evaluate a variety of vaccine candidates for this study. The same advantages will be extremely important for the rapid and economical production of patient-specific human lymphoma vaccines. We are currently optimizing the necessary technology to express the vaccine proteins directly from PCR products obtained from Ig V regions, eliminating the time needed for transforming and culturing bacterial cells and recovering the plasmid DNA. We believe that the cell-free protein expression system will ultimately yield the most practical approach to customized vaccine therapy. Because the patient's own Ig V regions can be rapidly cloned and expressed using the CFPS technology, vaccines may be available for patients shortly after a diagnosis of lymphoma. This may make it possible to use individualized therapy as frontline treatment, before the use of chemotherapy, radiation therapy, or both.
Acknowledgments
We thank Debbie Czerwinski for advice on immunoassays and flow cytometry and Jonathan Irish for help with flow cytometry data analysis.
This work was supported by the United States Public Health Services (grants CA34233 and CA33399) and by the Leukemia and Lymphoma Society (Specialized Center of Research [SCOR] 7017-06 grant). R.L. is an American Cancer Society Clinical Research Professor.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.K. performed the research, analyzed the data, and wrote the paper; J.Y. and A.V. performed the research and analyzed data; S.L., J.R.S., and R.L. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: J.R.S. is the inventor on patents describing cell-free protein technology that have been assigned to Stanford University.
Correspondence: Ronald Levy, Division of Oncology, 269 Campus Dr, CCSR 1105, Stanford University Medical Center, Stanford, CA 94305-5151; e-mail: levy@stanford.edu.
References
- 1.Brown SL, Miller RA, Horning SJ, et al. Treatment of B-cell lymphomas with anti-idiotype antibodies alone and in combination with alpha interferon. Blood. 1989;73:651–661. [PubMed] [Google Scholar]
- 2.Levy R, Miller RA. Tumor therapy with monoclonal antibodies. Fed Proc. 1983;42:2650–2656. [PubMed] [Google Scholar]
- 3.Maloney DG, Brown S, Czerwinski DK, et al. Monoclonal anti-idiotype antibody therapy of B-cell lymphoma: the addition of a short course of chemotherapy does not interfere with the antitumor effect nor prevent the emergence of idiotype-negative variant cells. Blood. 1992;80:1502–1510. [PubMed] [Google Scholar]
- 4.Meeker TC, Lowder J, Maloney DG, et al. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985;65:1349–1363. [PubMed] [Google Scholar]
- 5.Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 6.Tao MH, Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature. 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 7.Hakim I, Levy S, Levy R. A nine-amino acid peptide from IL-1beta augments antitumor immune responses induced by protein and DNA vaccines. J Immunol. 1996;157:5503–5511. [PubMed] [Google Scholar]
- 8.Lambert SL, Okada CY, Levy R. TCR vaccines against a murine T cell lymphoma: a primary role for antibodies of the IgG2c class in tumor protection. J Immunol. 2004;172:929–936. doi: 10.4049/jimmunol.172.2.929. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MJ, Esserman L, Byars NE, Allison AC, Levy R. Idiotype vaccination against murine B cell lymphoma. Humoral and cellular requirements for the full expression of antitumor immunity. J Immunol. 1990;145:1029–1036. [PubMed] [Google Scholar]
- 10.Chen TT, Tao MH, Levy R. Idiotype-cytokine fusion proteins as cancer vaccines. Relative efficacy of IL-2, IL-4, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1994;153:4775–4787. [PubMed] [Google Scholar]
- 11.King CA, Spellerberg MB, Zhu D, et al. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- 12.Rice J, Buchan S, Dewchand H, Simpson E, Stevenson FK. DNA fusion vaccines induce targeted epitope-specific CTLs against minor histocompatibility antigens from a normal or tolerized repertoire. J Immunol. 2004;173:4492–4499. doi: 10.4049/jimmunol.173.7.4492. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman JM, Singh G, Hermanson G, et al. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 2002;62:5845–5852. [PubMed] [Google Scholar]
- 14.Timmerman JM, Levy R. Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J Immunol. 2000;164:4797–4803. doi: 10.4049/jimmunol.164.9.4797. [DOI] [PubMed] [Google Scholar]
- 15.Caspar CB, Levy S, Levy R. Idiotype vaccines for non-Hodgkin's lymphoma induce polyclonal immune responses that cover mutated tumor idiotypes: comparison of different vaccine formulations. Blood. 1997;90:3699–3706. [PubMed] [Google Scholar]
- 16.Timmerman JM, Czerwinski DK, Davis TA, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 17.Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 18.Hsu FJ, Caspar CB, Czerwinski D, et al. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma—long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 19.Kigawa T, Yabuki T, Yoshida Y, et al. Cell-free production and stable-isotope labeling of milligram quantities of proteins. FEBS Lett. 1999;442:15–19. doi: 10.1016/s0014-5793(98)01620-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim DM, Swartz JR. Regeneration of adenosine triphosphate from glycolytic intermediates for cell-free protein synthesis. Biotechnol Bioeng. 2001;74:309–316. [PubMed] [Google Scholar]
- 21.Koga Y, Kato K, Nakano H, Yamane T. Inverting enantioselectivity of Burkholderia cepacia KWI-56 lipase by combinatorial mutation and high-throughput screening using single-molecule PCR and in vitro expression. J Mol Biol. 2003;331:585–592. doi: 10.1016/s0022-2836(03)00782-4. [DOI] [PubMed] [Google Scholar]
- 22.Rungpragayphan S, Kawarasaki Y, Imaeda T, Kohda K, Nakano H, Yamane T. High-throughput, cloning-independent protein library construction by combining single-molecule DNA amplification with in vitro expression. J Mol Biol. 2002;318:395–405. doi: 10.1016/S0022-2836(02)00094-3. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Kanter G, Voloshin A, et al. Rapid expression of vaccine proteins for B-cell lymphoma in a cell-free system. Biotechnol Bioeng. 2005;89:503–511. doi: 10.1002/bit.20283. [DOI] [PubMed] [Google Scholar]
- 24.Kim DM, Swartz JR. Efficient production of a bioactive, multiple disulfide-bonded protein using modified extracts of Escherichia coli. Biotechnol Bioeng. 2004;85:122–129. doi: 10.1002/bit.10865. [DOI] [PubMed] [Google Scholar]
- 25.Ryabova LA, Desplancq D, Spirin AS, Pluckthun A. Functional antibody production using cell-free translation: effects of protein disulfide isomerase and chaperones. Nat Biotechnol. 1997;15:79–84. doi: 10.1038/nbt0197-79. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Kanter G, Voloshin A, Levy R, Swartz JR. Expression of active murine granulocyte-macrophage colony-stimulating factor in an Escherichia coli cell-free system. Biotechnol Prog. 2004;20:1689–1696. doi: 10.1021/bp034350b. [DOI] [PubMed] [Google Scholar]
- 27.Antoni G, Presentini R, Perin F, et al. A short synthetic peptide fragment of human interleukin 1 with immunostimulatory but not inflammatory activity. J Immunol. 1986;137:3201–3204. [PubMed] [Google Scholar]
- 28.Bergman Y, Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977;7:413–417. doi: 10.1002/eji.1830070702. [DOI] [PubMed] [Google Scholar]
- 29.Hara K, Suda T, Suda J, et al. Bipotential murine hemopoietic cell line (NFS-60) that is responsive to IL-3, GM-CSF, G-CSF, and erythropoietin. Exp Hematol. 1988;16:256–261. [PubMed] [Google Scholar]
- 30.Maloney DG, Kaminski MS, Burowski D, Haimo-vich J, Levy R. Monoclonal anti-idiotype antibodies against the murine B cell lymphoma 38C13: characterization and use as probes for the biology of the tumor in vivo and in vitro. Hybridoma. 1985;4:191–209. doi: 10.1089/hyb.1985.4.191. [DOI] [PubMed] [Google Scholar]
- 31.Voloshin AM, Swartz JR. Efficient and scalable method for scaling up cell free protein synthesis in batch mode. Biotechnol Bioeng. 2005;91:516–521. doi: 10.1002/bit.20528. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson N, Capaldi AP, James R, Kleanthous C, Radford SE. Rapid folding with and without populated intermediates in the homologous four-helix proteins Im7 and Im9. J Mol Biol. 1999;286:1597–1608. doi: 10.1006/jmbi.1998.2548. [DOI] [PubMed] [Google Scholar]
- 33.Yin G, Swartz JR. Enhancing multiple disulfide bonded protein folding in a cell-free system. Biotechnol Bioeng. 2004;86:188–195. doi: 10.1002/bit.10827. [DOI] [PubMed] [Google Scholar]