Abstract
Environmental effects on the transmission of many parasitic diseases are well recognized, but the role of specific factors like climate and agricultural practices in modulating transmission is seldom characterized quantitatively. Based on studies of Schistosoma japonicum transmission in irrigated agricultural environments in western China, a mathematical model was used to quantify environmental impacts on transmission intensity. The model was calibrated by using field data from intervention studies in three villages and simulated to predict the effects of alternative control options. Both the results of these interventions and earlier epidemiological findings confirm the central role of environmental factors, particularly those relating to snail habitat and agricultural and sanitation practices. Moreover, the findings indicate the inadequacy of current niclosamide-praziquantel strategies alone to achieve sustainable interruption of transmission in some endemic areas. More generally, the analysis suggests a village-specific index of transmission potential and how this potential is modulated by time-varying factors, including climatological variables, seasonal water-contact patterns, and irrigation practices. These time-variable factors, a village's internal potential, and its connectedness to its neighbors provide a framework for evaluating the likelihood of sustained schistosomiasis transmission and suggest an approach to quantifying the role of environmental factors for other parasitic diseases.
Keywords: disease control, environment
Environmentally mediated parasitic diseases such as malaria, schistosomiasis, hookworm, onchocerciasis, and Chagas disease result in high morbidity and increased mortality, the latter particularly associated with malaria, and affect millions of people living in tropical and subtropical regions (1–5). Various control initiatives are underway to reduce disease burdens caused by these parasitic infections to meet the United Nations' Millennium Development Goals (6–8). However, the benefits of these initiatives are not equally distributed because of regional and country-specific differences in available resources and/or political commitment. For example, the health burden caused by schistosomiasis has been reduced significantly over the past several decades in China (9, 10) and Brazil (11), whereas the disease remains a major public health problem in many parts of Africa, where large-scale control initiatives focused on reducing morbidity through the use of the antihelminthic drug praziquantel have been initiated. More recently, control efforts have been further fueled by funds from international foundations (7, 12).
The importance of seasonal and other environmental factors in the transmission of parasitic diseases, such as malaria, leishmaniasis, and filariasis, has long been recognized (13, 14), but the mechanisms by which environmental factors alter epidemiological parameters and their interaction with public health interventions are seldom well characterized. Understanding these factors is central to the development of comprehensive strategies to supplement drug treatment of affected populations with environmental modifications that may be more sustainable and cost-effective in the long run. In addition, an understanding of these mechanisms can be used to better estimate the long-term impact of impending climate change on environmentally mediated diseases at regional and global scales.
The present study addresses environmental factors in the context of extensive schistosomiasis control efforts underway in China. As they move beyond morbidity control toward the elimination of schistosomiasis transmission, Chinese policy-makers have recognized the need for a more comprehensive approach to control than relying solely on the treatment of humans and animals with praziquantel and the depletion of snail populations using the molluscicide niclosamide (15). This recognition has been motivated by an accumulation of field experience that suggests that the praziquantel/niclosamide strategy may only transiently alter a village's infection risk. For example, recent evidence of the importance of environmental factors to the stability of endemic levels of disease is offered by the reemergence of schistosomiasis transmission in eight counties in Sichuan, where it had formerly been controlled or eliminated mainly through the use of praziquantel and niclosamide (16). Although longitudinal data on reemergence are sparse, three villages in Sichuan Province provide an example of a pattern we suspect may be common. These villages were surveyed in 1987 at the outset of an intensive praziquantel/niclosamide-based control project and followed until the project terminated in 1995 (17). The mean prevalence of infection was 63% in 1987 and 8% at the termination of the intensive control program in 1995 but rebounded to 45% by 2000. Although there had been little control effort before 1987, there was routine county-level control activity, again relying on episodic praziquantel/niclosamide use, in the 1995–2000 interval.
Having recognized the importance of environmental factors in eliminating transmission, the major challenge faced by Chinese public health authorities in tailoring locally effective strategies is in coping with the complex effects of seasonality and heterogeneous local conditions within endemic areas. The importance of local conditions is exemplified by our studies in Sichuan. We have consistently found that, within a climatologically homogeneous region, attributes of village of residence, such as land use and characteristics of the irrigation system, are the most important determinants of human infection intensity and can result in large differences in infection risk occurring in villages separated by as little as 1 km (18). Moreover, village of residence is a sufficiently dominant influence to mask the potential role of individual characteristics, including age, gender, and kinship (18, 19). The central importance of the local environment to levels of human infection in this endemic region in China appears similar to that recently reported by Satayathum et al. for Schistosoma haematobium in Kenya (20).
Our decade-long research on Schistosoma japonicum transmission in irrigated agricultural villages in China has resulted in methods useful for estimating the effect of local environmental factors on transmission intensity. We believe aspects of our findings have relevance for other parasitic diseases. Here we draw on our earlier work and report results aimed at the identification of those environmental variables that can be manipulated locally to contribute to effective and sustainable control. From the outset, we used a mathematical model to guide our fieldwork and to serve as a platform for integrating both general and site-specific information on transmission intensity and control effects. We summarize the model and its use in planning control strategies for three villages below and in more detail in Experimental Procedures. The results of those interventions and forecasts based thereon are presented first and then motivate the model-based definition of three groups of modifiers of disease transmission intensity. These modifiers reflect the relative roles of site-specific factors like the extent of snail habitat and local agricultural practices, the time-variable effects of weather and human water contact activities, and the potential impact of the export and import of the parasite to or from neighboring villages. These modifiers are called internal potential, gating effects, and connectivity, respectively. Although these concepts are developed in a disease-specific context, they have close analogs in the general theory of metapopulation ecology (21). Hence, we feel they offer promise for understanding the role of environmental mediators of other parasitic diseases as well as quantitative approaches to their control.
The use of mathematical models in understanding transmission and control of parasitic diseases has a short but rich history (22). The first model of schistosomiasis transmission dates back to the pioneering work of Macdonald (23), which was later extended to include more biological realism such as worm mating (24), acquired immunity (25, 26), population age structure (22, 27), heterogeneous exposure to infection (28, 29), and multiple disease reservoirs (30). In much of this work, the index of transmission potential and the effect of control measures like chemotherapy upon it are the basic reproductive number, R0, defined for schistosomiasis as the number of female worms reproduced by one female worm during its lifetime in the absence of acquired immunity (25, 27, 31). Environmental factors, including weather, are largely ignored in these models and hence as a factor in R0. Exceptions are studies that have explored weather-driven population dynamics of the intermediate host (32) and the impact of climate change on global distribution of schistosomiasis (33).
The model used in our studies is a direct extension of that of Anderson and May (22), with modifications in its parameterization and the addition of submodels to allow the inclusion of site-specific information. For example, our earlier field studies in the area confirmed that snail populations, cropping patterns, agricultural practices, and the extent of the irrigation system were strong determinants of endemic infection levels (18). Hence, these factors are directly or indirectly represented in the model as detailed in Liang et al. (34) and are summarized here. The model is a nonlinear differential difference-equation model with the additional complexity of including time-variable coefficients and a temperature-dependent developmental delay of the parasite in the snail. The state variables are the mean village infection intensity of the parasite in three human groups: farmers, students, and others. The “others” group is composed of those with low water contact within the village, for example, teachers and administrators. The fourth state variable specifies the population of infected snails in the village. Susceptible snail population dynamics vary according to a temporal pattern established earlier in a longitudinal study in the region (35), but the magnitude is adjusted annually according to the extent of habitat and population density established at one point in time, usually late May or early June. The structure and parameterization of the model are summarized in supporting information (SI) Text.
In 2003, three villages were selected for trials in which environmental modifications were instituted based on model-based analyses of their individual transmission circumstances. Although these are clearly not classical intervention trials, their outcome is relevant here insofar as they test the ability of the current model and parameterization procedures to summarize the key features of the transmission process, including local environmental factors, with reasonable fidelity. The intent is that the model evolves over time as new data become available or new processes are elucidated. The villages are Shian 5 and Xinming 3 of Daxing township and Xinlong 7 of Chuanxing township, all in Xichang County. In 2000, the prevalence of infection in these villages ranged between 44% and 69%, and infection intensity ranged between 10 and 102 e.p.g. (eggs per gram fecal sample by the Kato–Katz test), respectively (18). These villages were selected, because they have irrigation systems typical of the region and are relatively isolated hydrologically from their upstream neighbors. This selection was intended to minimize the effects of conditions and practices external to the villages on their annual transmission experience. The plan was to compare the model-based forecasts of infection intensity in the three human groups and the population of infected snails in each village with field data to be collected at the end of the 2004 infection season.
Results
Simulation Results.
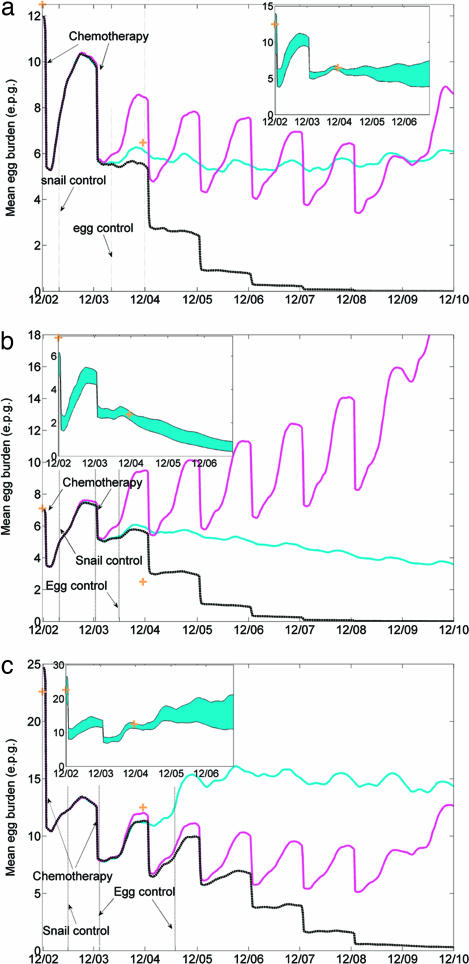
Fig. 1 depicts the median time profile over all Monte Carlo realizations of transmission intensity [shown as e.p.g. (eggs per gram feces)] for three control scenarios for farmers, the largest risk group in the three villages. The scenarios were:
Fig. 1.
Time profiles of median infection intensity for the farmer risk group under the three control scenarios. Simulations were run based on calibrated parameter sets for each village (Scenario I, blue, two chemotherapies in 2002 and 2003, coupled with sustained environmental interventions to 2010; II, cyan, chemotherapies as in scenario I followed by annual chemotherapy at 50% coverage to 2008, no environmental interventions; III, black, a combination of both scenarios I and II). (a) Simulations in village Shian 5; (b) simulations in village Xinming 3; and (c) simulations in village Xinlong 7.
The interventions that actually occurred in the field, i.e., two chemotherapies, administered in 2002 (to those diagnosed as infected) and 2003 (to all residents), coupled with sustained environmental interventions involving snail and egg controls but without further routine chemotherapy.
The 2002–2003 chemotherapies as in scenario I followed by annual chemotherapy at 50% coverage until 2008, which is typical of routine control programs, but without environmental interventions.
A combination of sustained environmental interventions and chemotherapy, i.e., combined scenarios I and II.
Infection intensities were forecast in the at-risk groups by using the model starting in 2002 until the end of 2010 for three scenarios. For all three, it was assumed that the current praziquantel treatment campaign would end in 2008, as current government plans anticipate.
Overall, there is good agreement between the median values of the model realizations and the observations in 2002 and 2004 in Shian 5 and Xinlong 7 but a modest overestimate of infection intensity in 2004 in Xinming 3. This is illustrated Fig. 1 with the blue line representing the Scenario I predictions vs. yellow crosses representing the infection survey results from 2002 and 2004, respectively. The cyan line is the hypothetical praziquantel only scenario, Scenario II, and the black line the combined but still hypothetical Scenario III. The simulation results show that December 2004 was clearly too early to discriminate between the actual and hypothetical control options. The 5-year forecasts, on the other hand, show important differences. In Scenario I, sustained environmental interventions are forecast to suppress transmission to relatively steady low levels (Fig. 1 a and c) or to lead to a decreasing trend (Fig. 1b) even in the absence of further chemotherapy. Sustained annual chemotherapy in the absence of environmental interventions has mixed results with slowly decreasing infection intensity in Shian 5 and Xinlong 7 but increasing intensity in Xinming 3.
Not surprisingly, in all three villages under Scenario II the cessation of chemotherapy at year 8 was followed by an immediate but variable rate of increase in infection intensity. Of note in the context of achieving current governmental goals is the outcome of Scenario III, the combination of chemotherapy until 2008 and sustained environmental interventions, which are forecast to suppress disease transmission to a very low level and even to terminate transmission in most of our simulations by 2008. This outcome underscores the importance of comprehensive controls, that is, environmental modifications in addition to the use of praziquantel and niclosamide.
The blue bands (Fig. 1 Insets) reflect the variation in the Scenario I forecast, depicting the 10% of simulations that come closest to the 2004 field data point shown as the yellow cross in the main panel. This reflects the residual parametric variability remaining after the 2000–2002 calibration and illustrates there were trajectories in all three cases, including Xinming 3, which came quite close to the observed data point. Moreover, with the 2004 data point included, the blue bands depict an updated prediction of infection intensity under Scenario I, sustained environmental control only. In Shian 5 and Xinlong 7, this appears to result in a semistable level of infection intensity in farmers, whereas the analogous level is either zero or has not been reached in Xinming 3. Hence, these simulation studies seem to support the field experience of the existence of semistable levels of infection, but also suggest that they are amenable to modification by environmental interventions. In this context also, the results of Satayathum et al. (20) on S. haematobium prevalence in Kenya indicate a persistent semistable endemic level, in that case under a sustained chemotherapy program. In the next section, the existence of this endemic level is used to identify the factors underlying this behavior with the objective of developing an index to guide environmental changes necessary to terminate transmission.
Internal Potential.
The field data from this region, as well as the simulations, suggest the existence of an endemic level in villages that have not been subjected to large-scale environmental change or episodic disease control activities. Moreover, it appears this is not an equilibrium level in the classical sense of Macdonald (23) but something of a meandering level that may include slow upward or downward trends, as seen in Fig. 1, Scenario I. As recounted earlier, our epidemiological investigations suggest that the endemic level is determined by a set of local factors relating to agricultural practices, the snail population, and the extent and operation of the irrigation system. Together, these factors comprise what we define explicitly below as internal potential. The observed endemic level of infection, however, is a result of internal potential modulated by weather and other factors whose temporal patterns may vary from year to year. To explore this behavior, the full model was simplified by reducing the three human risk groups to one comprised of all villagers. To account for the fact that farmers, students, and others have different water contact patterns and different population sizes, a population-weighted average water contact profile was used (SI Text).
The reduced model has two state variables, w(t) the average village worm burden in the human population and z(t) the average density of infected snails in the village. There are two nonlinearities in the full model, the mating function and the density dependent limitation on worm establishment in the human host. Because we are concerned neither with the initiation of transmission nor the density-limited upper bound that lies well above the semistable levels seen in these and most Sichuan villages, we assume these are not operative over the portion of the endemic range of interest here and set each to unity. The simplified model with new parameter groupings is then:
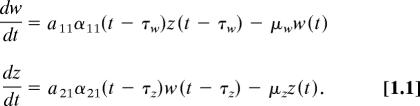 |
The a and α parameters represent the rates of establishment of the parasite in the hosts, whereas the μ parameters, the death rates of worms in vivo and infected snails, respectively. The infection-related parameters are separated into constant, aij, and time variable, αij(t), groups where each time-varying factor has been normalized by its annual maximum value. These maximum values are included within the constant terms. For example, the time-variable water contact parameter in the full model, s(t), is redefined as s(t) = Ssn(t), where 0≤sn(t)≤1, and sn(t) is an element of αij(t), whereas S, the annual maximum, becomes a factor in aij. The time-varying parameters then satisfy the condition that 0 ≤ αi1(t − τ) ≤ 1. The time delays are, as in the full model, that between entry of a parasite and worm development in the human host, τw, and the developmental delay of the parasite in the snail, τz. The parameters of Eq. 1.1 are defined in the SI Text in terms of the original parameters of the full model.
Because of the seasonality now imbedded in the α parameters, these parameters go to zero in the late fall and “turn on” again in the spring. The “on” periods during the transmission season may be further modulated by the rainfall/irrigation effects also contained within the α parameter groups. As a consequence, the α parameters will be termed gating functions. SI Fig. 2 shows the gating functions from a Shian 5 simulation in which α21 decreases abruptly in the fall as a result of the lengthening of the developmental delay, τz, as the environmental temperature undergoes its seasonal decline.
The solutions of Eqs. 1.1 depend on the magnitude of a11, a21, μw, and μz, on the gating functions and on the magnitude of the time lags τw and τz. This complexity precludes a simple analytical specification of the model's qualitative behavior based on parameter values, i.e., a simple algebraic representation of the parametric dependence of the basic reproductive number R0. As an alternative, consider the approximate behavior of the full model shown in the trajectories of Fig. 1 Insets. As noted above, both Xinlong 7 and Shian 5 show trajectories suggesting almost neutral stability behavior in that some trajectories increase slowly, some decrease, and some are essentially flat. We explore this behavior by defining steady state as one in which the time-weighted rate of change of the state variables is approximately zero over the annual cycle. Specifically:
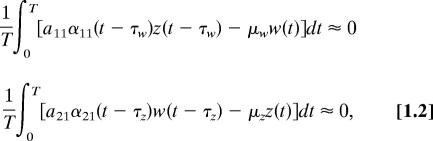 |
where T = 1 year. It is easily shown that, for a nonzero steady state condition to exist,
The condition for zero-state stability, that R0 < 1 for the system 1.1 without delays [and with α11(t) = α21(t) = 1] is the converse of 1.3, that is, μwμz>a11a21.
Hence, to reduce a11 and a21 with respect to μw and μz suggests a first step in developing feasible control strategies. Alternatively, to reduce the transmission by altering the gating functions is the second option, e.g., reducing water-contact duration. It is, of course, something of a truism to say that reducing the rate of infection of people and snails or limiting water contact during the transmission season are sensible strategies.
To link the a–α formulation back to the village environment in terms of the original model parameters, the constant parameter set is divided into two classes, termed site-specific and biological parameters, and denoted by Ps and Pb, respectively. The biological parameters are those that can be expected to be essentially invariant over large geographical areas in contrast to Ps, the village-specific set. Examples of the former are the mortality rate of worms in vivo, μw, the developmental delay of the parasite in the human host, τw, and h, the daily excretion of eggs per worm pair per gram of feces. A site-specific parameter is Ah, the area of snail habitat in the village. The death rate of infected snails, μz, is treated as a site-specific parameter, because it may depend on local conditions. To avoid endemic levels of worm burden in humans or infected snail density >0, the initial objective, then, is to modify the site-specific factors in the village, such that:
We propose that Ps be the intensity-related metric of internal potential, that is, a measure of the time-invariant local determinants of transmission intensity. In terms of the parameters of the full model,
Although each parameter is defined explicitly in SI Text, the environmental factors they represent are easily summarized. The extent of snail habitat is reflected in the area terms Ah and As, snail density and their distribution through Xξ, fertilization practice or sanitation status through βng0, and water contact through Sγ. Hence, all model parameters that can be altered by local environmental intervention are summarized in Ps, which now provides both a quantitative and a qualitative basis for formulating village-specific control strategies. In this context, the interventions described earlier decreased the area of snail habitat, Ah, in Shian 5 and Xinming 3 and decreased βng0 by the biogas installations in all three villages but to differing degrees and on different schedules.
Even postulating that the full model reflects the essential features of the transmission processes, it is necessary to explore the adequacy of environmental interventions aimed at satisfying Eq. 1.4 given the simplifications involved in its derivation. For example, regardless of the value of Ps, the greater the degree of modulation of transmission by the gating functions, the less the effective intensity of transmission. Indeed, there are villages in different areas of Sichuan with very similar agricultural and sanitation characteristics that have experienced quite different endemic levels. Hence, cases may exist where Eq. 1.4 is not satisfied, but infection diminishes over time. Conversely, it can be expected that the effect of the delays will be destabilizing, i.e., there will be increasing levels of infection over time in some cases where Eq. 1.4 is satisfied. We have investigated these possibilities by an analysis of the worm-burden trajectories of the farmer group from 2004 to 2009 for each Monte Carlo realization of Scenario I, sustained environmental control without chemotherapy after 2003, for Shian 5 and Xinming 3. From this analysis, described in SI Text, we conclude that criterion 1.4 is a sensible starting point in specifying the level of environmental modification required to lead to the cessation of disease transmission.
Discussion
The findings reported here most immediately relevant to schistosomiasis control relate to the model-based prediction that a combination of environmental control and chemotherapy will be needed in the three villages to achieve the termination of transmission in the near term (Fig. 1). To the extent these villages are typical of the area, reducing the internal potential of villages will be required, in addition to resetting the initial conditions of the transmission processes with praziquantel, to achieve transmission interruption in the Xichang environment by 2008. Hence, our results support the call for comprehensive control from the Chinese government. However, this implies the need for a new level of local, rather than regional or national, responsibility for selecting control options. This will require local estimates of internal potential to guide this selection. Because all of the factors that determine internal potential are consistent with longstanding qualitative experience of the important determinants of schistosomiasis transmission, we feel the concept points the way toward a practical index of the degree of environmental change necessary to achieve sustainable termination of transmission in some endemic areas. As implicitly recognized in China, this also has important policy implications for schistosomiasis control at provincial and national levels. The need to include environmental elements in a successful control program is not new and, for example, was one of the lessons learned from the successful story of schistosomiasis elimination in Japan in 1979 (36). Although there is, as yet, no evidence of praziquantel resistance by S. japonicum (37), the intensity of its current use in China provides even further motivation to move ahead aggressively with available control technology.
The second schistosomiasis-specific result of practical importance, but with broader implications, concerns the effects of the gating functions on the transmission process and its sensitivity to time-varying environmental factors. The effect of the gating functions was illustrated in SI Fig. 3, which suggests that, if PsPb > 1, the influence of the gating functions determines the existence of a nonzero endemic level and, presumably, the magnitude of that level. An example of the gating effect may be provided by some villages in the Changqiu Mountains of Sichuan, where susceptible snail densities are similar, and disease prevalence in humans has from time to time reached levels similar to that seen in Xichang County, which is >350 kilometers distant. However, both snail-infection rates and sentinel mouse data indicate very low average levels of the parasite in the Changqiu environment, probably because of much more episodic transmission than in Xichang. We hypothesized that this is because of differences in the rainfall/irrigation components of the gating functions that modulate the average daily production and transport of miracidia and cercariae. Recent field studies support this hypothesis (38).
Gating effects have long been associated with vector-borne diseases, generally in their sensitivity to weather fluctuations, for example, western equine encephalitis (39) and, more recently, Barmah Forest virus disease in Australia (40). A second notable example is the model-based malaria map for Africa proposed by Craig et al. (41) that offers a contrast to the schistosomiasis case, in that their analysis focuses on the likelihood that stable transmission can occur, but based on temperature and rainfall gating effects rather than an analog of internal potential. Craig et al. (41) also point out that identifying locales where transmission can occur based on weather conditions is useful but only part of the story, because infection intensity depends also on additional local variables that would come under our definition of internal potential.
It is important to note that all of the foregoing discussion relates to villages uninfluenced by the import or export of parasites from external sources. Few villages in Sichuan are completely isolated, either hydrologically or socioeconomically, the latter, for example, exemplified by exchange of human and animal labor or the purchase of fertilizer from neighboring villages. In previous work, the potential impact of hydrological connectedness was explored by computer simulation, and the results support what practical experience and common sense suggest, that this form of connectedness can be quite important to the patterns of disease transmission and persistence (42). In this context, Clennon et al. (43) have recently reported evidence of the importance of hydrological connectedness in Kenya associated with the transport of Bulinus nastus, the intermediate hose of S. haematobium. D. Gurarie and E.Y.W.S. have further explored the impact of connectivity in irrigated agricultural settings from a theoretical perspective (D. Gurarie and E.Y.W.S., unpublished data). In addition to drawing the distinction between the unidirectional nature of hydrological connectivity and the more diffusive character of socioeconomic connectivity, they show how the transmission determinants at the village level are aggregated into a connected network and, by implication, how this combination of factors is likely to influence the scale of effective control programs.
The concepts of internal potential, its modulation by the gating effects of time-variable environmental factors, and connectivity all have analogs in the transmission of many other infectious diseases (39, 40, 44–46). More generally, however, these ideas also appear quite consistent with current thinking in metapopulation ecology that concerns the distribution and persistence of populations (40). Villages act as connected patches of parasite habitat, which vary in size and quality depending on factors appearing in internal potential, such as the number of snails and human hosts. Manipulating these factors can modify parasite persistence. Reducing the snail population, for example, reduces the likelihood of sustained schistosomiasis transmission, as does reducing the susceptible host population in the control of animal diseases (41).
Although metapopulation analyses often restrict their consideration to habitat area and isolation (42), environmental variables have been shown to be vital in determining occupancy of patches (50). In keeping with the need to include rigorously defined quantitative measures of habitat quality in any analysis of population persistence (51), the internal potential concept incorporates key environmental, agricultural, and social factors known to support the parasite's resource requirement in villages. Likewise, the connectivity concept mentioned above concurs with calls for rigorous quantification of host (and in the case of parasite with free-living stages, parasite) migration between patches in meta population analyses (52). We are optimistic that our approach to identifying environmental factors that can be manipulated to decrease or interrupt schistosomiasis transmission can be usefully extended to other parasitic diseases.
Experimental Procedures
Details of the approaches, methods, and results used to support the analysis presented below have been published in both the Chinese and Western literature. The principal publications in the Western literature relate to epidemiological field methods and results (18, 19, 53), mathematical modeling and model calibration (34, 42), snail distribution and population dynamics (56), and hydrological aspects of parasite transport (38, 57, 58). The new site-specific results reported here relate to the outcome of interventions in three villages in the same region that were the subject of earlier mathematical modeling studies.
The calibration of the model to conditions in each of these villages and the methods and parameter values used in the analyses have been reported (54). The model was calibrated to data collected between the years 2000–2002 for Shian 5 and Xinlong 7, and a third village, Xinming 3, was subsequently added by using identical procedures. Results of model calibration for Shian 5 and Xinlong 7 are detailed elsewhere (54). In summary, 252, 172, and 514 parameter sets (from a Monte Carlo procedure), for Shian 5, Xinlong 7, and Xinming 3, respectively, were identified to lead to simulations consistent with the 2000–2002 field data. Parameter sets meeting the calibration conditions were then used to study the effects of reducing snail populations and interrupting the distribution of parasite eggs back into the environment in fertilizer through treatment of human waste in household biogas digesters used in this region primarily for generating methane gas for cooking. As summarized in SI Tables 1–5, in Shian 5 and Xinming 3, the target was to reduce village snail populations by 25% and 35%, respectively. In this region, irrigation ditches provide the primary snail habitat, although recent studies have shown that highly terraced landscapes offer additional habitat (G.M.D., unpublished results). High-density ditch segments were targeted for intensive niclosamide treatment intended to simulate the concreting of mud-walled ditches, which the project could not afford. Snail populations in these treated areas were monitored and found to be reduced by between 85% and 95%, yielding village population reductions of 21% and 33%. In Xinlong 7, household biogas digesters were installed that are effective at removing and inactivating schistosome eggs from human waste before its use as fertilizer. In three sets of empirical experiments in Xinlong 7, biogas digesters were estimated to result in >2-log reduction in egg concentration by biochemical inactivation, and separately, an additional 1-log reduction by sedimentation. In both Shian 5 and Xinming 3, digesters were also installed in households through the funding from local government. In all three villages, the coverage and timing of installation hinged on availability of funds, as well as on household construction conditions (SI Text).
The field experiments were complicated in 2003 when governmental reaction to the severe acute respiratory syndrome epidemic set in motion a variety of activities for controlling infectious disease in China that extended to schistosomiasis. In particular, residents of all endemic villages in Sichuan, including the three experimental villages, were provided praziquantel treatment late in 2003. To establish the recent history of all control measures taken in the villages as a result of the new campaign, local public health staff and villagers were interviewed. Special attention was focused on determining the actual coverage of praziquantel treatment in each village and niclosamide usage beyond the areas we had stipulated (SI Text). Given these data, the model was used to predict the infection status in each village at the end of 2004 as a result of both our planned interventions as they were actually implemented and the additional interventions carried out locally.
Initial conditions for the computer forecasts of predicted infection intensities after control were established from surveys of human and snail infection status in each village in 2002. At the end of the 2002 infection season, the populations of all three villages were surveyed for infection intensity (18). Simulations were run from 2002 to 2010. The results of the infection surveys, and other site-specific data, such as water contact and weather, were specifically integrated into the model. At the end of the 2004 human infection, surveys were repeated for comparison with the model predictions. Survey results are detailed in SI Text.
Supplementary Material
Acknowledgments
We are indebted to colleagues at the Institute of Parasitic Disease, the Sichuan Center for Disease Control and Prevention, and the Xichang County Anti-Schistosomiasis Station, as well as to Dr. Chen Chunming, who suggested this line of research more than 15 years ago, and to Dr. Bruce Ames, whose early support helped launch our work. We thank Dr. George M. Hornberger and Dr. Thomas McKone for helpful comments on an early version of the manuscript. This work was supported in part by National Institute of Allergy and Infectious Diseases Grant R01 AI 50612.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701878104/DC1.
References
- 1.Michael E, Bundy DAP, Grenfell BT. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 2.Richards FO, Boatin B, Sauerbrey M, Seketeli A. Trends Parasitol. 2001;17:558–563. doi: 10.1016/s1471-4922(01)02112-2. [DOI] [PubMed] [Google Scholar]
- 3.Remme JHF, Blas E, Chitsulo L, Desjeux PMP, Engers HD, Kanyok TP, Kayondo JFK, Kioy DW, Kumaraswami V, Lazdins JK, et al. Trends Parasitol. 2002;18:421–426. doi: 10.1016/s1471-4922(02)02387-5. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The World Health Report 2004–Changing History. Geneva, Switzerland: World Health Organization; 2004. p. 96. [Google Scholar]
- 5.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Sachs SE, Sachs JD. Plos Med. 2006;3:576–584. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molyneux DH. Lancet. 2004;364:380–383. doi: 10.1016/S0140-6736(04)16728-7. [DOI] [PubMed] [Google Scholar]
- 7.Fenwick A, Savioli L, Engels D, Bergquist NR, Todd MH. Trends Parasitol. 2003;19:509–515. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Utzinger J, de Savigny D. Plos Med. 2006;3:585–586. doi: 10.1371/journal.pmed.0030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang QW, Wang LY, Guo JG, Chen MG, Zhou XN, Engels D. Acta Trop. 2002;82:115–125. [Google Scholar]
- 10.Chen M, Feng Z. Parasitol Int. 1999;48:11–19. [Google Scholar]
- 11.Andrade ZA. Memor Inst Oswaldo Cruz. 1998;93:313–316. doi: 10.1590/s0074-02761998000700062. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick A. Trans R Soc Trop Med Hyg. 2006;100:200–207. doi: 10.1016/j.trstmh.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council. Under the Weather: Climate, Ecosystems, and Infectious Disease. Washington, DC: Natl Acad Press; 2001. [PubMed] [Google Scholar]
- 14.Patz JA, Graczyk TK, Geller N, Vittor AY. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 15.State Council of China. Statue of Schistosomiasis Control. China: Beijing; 2006. p. 7. [Google Scholar]
- 16.Liang S, Yang CH, Zhong B, Qiu DC. Bull World Health Org. 2006;84:139–144. doi: 10.2471/blt.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu XG, Zhao WX, Xu FS. Chinese J Schistosomiasis Cont. 1993a;5:82–85. [Google Scholar]
- 18.Spear RC, Seto E, Liang S, Birkner M, Hubbard A, Qiu D, Yang C, Zhong B, Xu F, Gu X, Davis GM. Am J Trop Med Hyg. 2004;70:48–56. [PubMed] [Google Scholar]
- 19.Seto EY, Zhong B, Kouch J, Hubbard A, Spear RC. Am J Trop Med Hyg. 2005;73:1145–1150. [PubMed] [Google Scholar]
- 20.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 21.Hanski I. Metapopulation Ecology. New York: Oxford Univ Press; 1999. [Google Scholar]
- 22.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford Univ Press; 1991. [Google Scholar]
- 23.Macdonald G. Trans R Soc Trop Med Hyg. 1965;59:489–506. doi: 10.1016/0035-9203(65)90152-5. [DOI] [PubMed] [Google Scholar]
- 24.May RM. Math Biosci. 1977;35:301–343. [Google Scholar]
- 25.Anderson RM, May RM. Adv Parasitol. 1985;24:1–101. doi: 10.1016/s0065-308x(08)60561-8. [DOI] [PubMed] [Google Scholar]
- 26.Barbour AD. Am J Trop Med Hyg. 1996;55:135–143. doi: 10.4269/ajtmh.1996.55.135. [DOI] [PubMed] [Google Scholar]
- 27.Chan MS, Guyatt HL, Bundy DAP, Booth M, Fulford AJC, Medley GF. Epidemiol Infect. 1995;115:325–344. doi: 10.1017/s0950268800058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolhouse MEJ. Am J Trop Med Hyg. 1996;55:144–148. doi: 10.4269/ajtmh.1996.55.144. [DOI] [PubMed] [Google Scholar]
- 29.Gurarie D, King CH. Parasitology. 2005;130:49–65. doi: 10.1017/s0031182004006341. [DOI] [PubMed] [Google Scholar]
- 30.Williams GM, Sleigh AC, Li Y, Feng Z, Davis GM, Chen H, Ross AG, Bergquist R, McManus DP. Acta Trop. 2002;82:253–262. doi: 10.1016/s0001-706x(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 31.Woolhouse ME, Hasibeder G, Chandiwana SK. Trop Med Int Health. 1996;1:456–463. doi: 10.1046/j.1365-3156.1996.d01-88.x. [DOI] [PubMed] [Google Scholar]
- 32.Woolhouse ME, Chandiwana SK. Acta Trop. 1990;47:151–160. doi: 10.1016/0001-706x(90)90021-q. [DOI] [PubMed] [Google Scholar]
- 33.Martens P. Health and Climate Change: Modelling the Impacts of Global Warming and Ozone Depletion. London: Earthscan; 1998. [Google Scholar]
- 34.Liang S, Maszle D, Spear RC. Acta Trop. 2002;82:263–277. doi: 10.1016/s0001-706x(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 35.Zhao WX, Gu XG, Xu FS, Li YQ, Zhao LG, Ying HZ, Li XJ, Zhou XF. Sichuan J Zool. 1995;14:119–121. [Google Scholar]
- 36.Tanaka H, Tsuji M. Int J Parasitol. 1997;27:1465–1480. doi: 10.1016/s0020-7519(97)00183-5. [DOI] [PubMed] [Google Scholar]
- 37.Liang YS, Dai JR, Ning A, Yu DB, Xu XJ, Zhu YC, Coles GC. Trop Med Int Health. 2001;6:707–714. doi: 10.1046/j.1365-3156.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 38.Remais J. Berkeley: University of California; 2006. p. 149. PhD dissertation. [Google Scholar]
- 39.Wegbreit J, Reisen WK. J Am Mosq Control Assoc. 2000;16:22–27. [PubMed] [Google Scholar]
- 40.Naish S, Hu W, Nicholls N, Mackenzie JS, McMichael AJ, Dale P, Tong S. Environ Health Perspect. 2006;114:678–683. doi: 10.1289/ehp.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig MH, Snow RW, le Sueur D. Parasitol Today. 1999;15:105–111. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 42.Xu B, Gong P, Seto E, Liang S, Yang CH, Wen S, Gu XG, Spear RC. Ann Am Geogr. 2006;96:31–46. [Google Scholar]
- 43.Clennon JA, King CH, Muchiri EM, Kitron U. Parasitology. 2006:1–11. doi: 10.1017/S0031182006001594. [DOI] [PubMed] [Google Scholar]
- 44.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Ecol Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 45.Earn DJD, Rohani P, Bolker BM, Grenfell BT. Science. 2000;287:667–670. doi: 10.1126/science.287.5453.667. [DOI] [PubMed] [Google Scholar]
- 46.Hoshen MB, Morse AP. Malaria J. 2004;3:32. doi: 10.1186/1475-2875-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanski I. Naturwissenschaften. 2001;88:372–381. doi: 10.1007/s001140100246. [DOI] [PubMed] [Google Scholar]
- 48.Stegeman A, Bouma A, Elbers AR, de Jong MC, Nodelijk G, de Klerk F, Koch G, van Boven M. J Infect Dis. 2004;190:2088–2095. doi: 10.1086/425583. [DOI] [PubMed] [Google Scholar]
- 49.Hanshi I, Gilpin M. Metapopulation Biology: Ecology, Genetics and Evolution. London: Academic; 1997. [Google Scholar]
- 50.Dennis RLH, Eales HT. Biol Conserv. 1999;87:295–301. [Google Scholar]
- 51.Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B. Proc R Soc London Ser B. 2001;268:1791–1796. doi: 10.1098/rspb.2001.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grenfell B, Harwood J. Trends Ecol Evol. 1997;12:395–399. doi: 10.1016/s0169-5347(97)01174-9. [DOI] [PubMed] [Google Scholar]
- 53.Hubbard A, Liang S, Maszle D, Qiu DC, Gu XG, Spear RC. Parasitology. 2002;125:221–231. doi: 10.1017/s003118200200207x. [DOI] [PubMed] [Google Scholar]
- 54.Liang S, Spear RC, Seto E, Hubbard A, Qiu D. Trop Med Int Health. 2005;10:263–278. doi: 10.1111/j.1365-3156.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- 55.Spear RC, Hubbard A, Liang S, Seto E. Environ Health Perspect. 2002;110:907–915. doi: 10.1289/ehp.02110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remais J, Hubbard A, Wu ZS, Spear RC. J Appl Ecol. 2007 in press. [Google Scholar]
- 57.Lowe D, Xi J, Meng X, Wu Z, Qiu D, Spear R. Parasitol Int. 2005;54:83–89. doi: 10.1016/j.parint.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Maszle DR. Berkeley: University of California; 1998. p. 214. PhD dissertation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.