Abstract
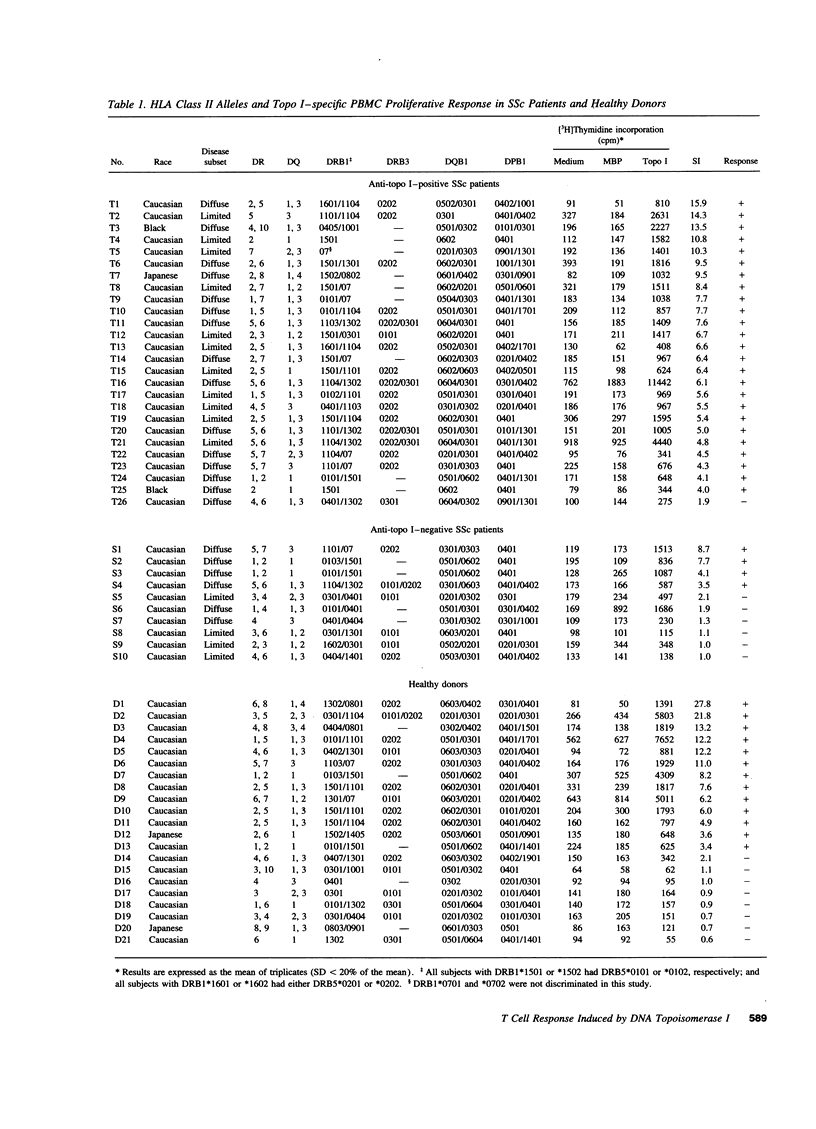
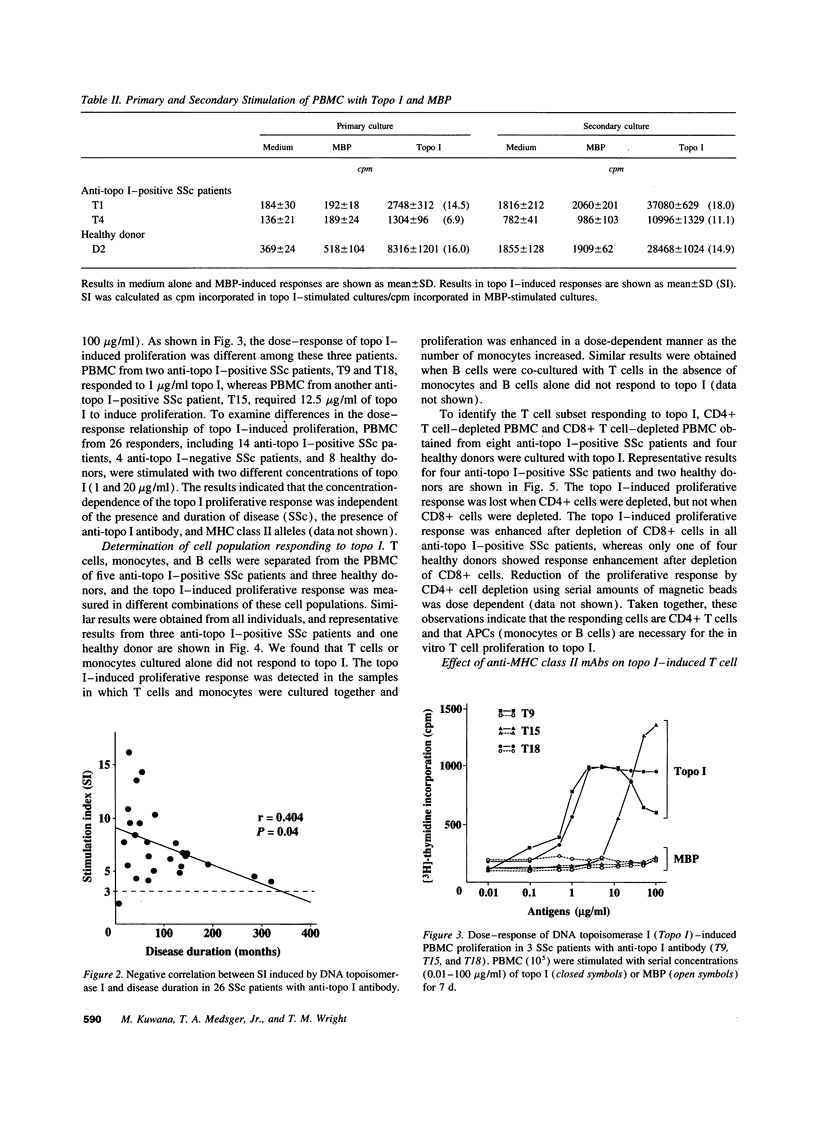
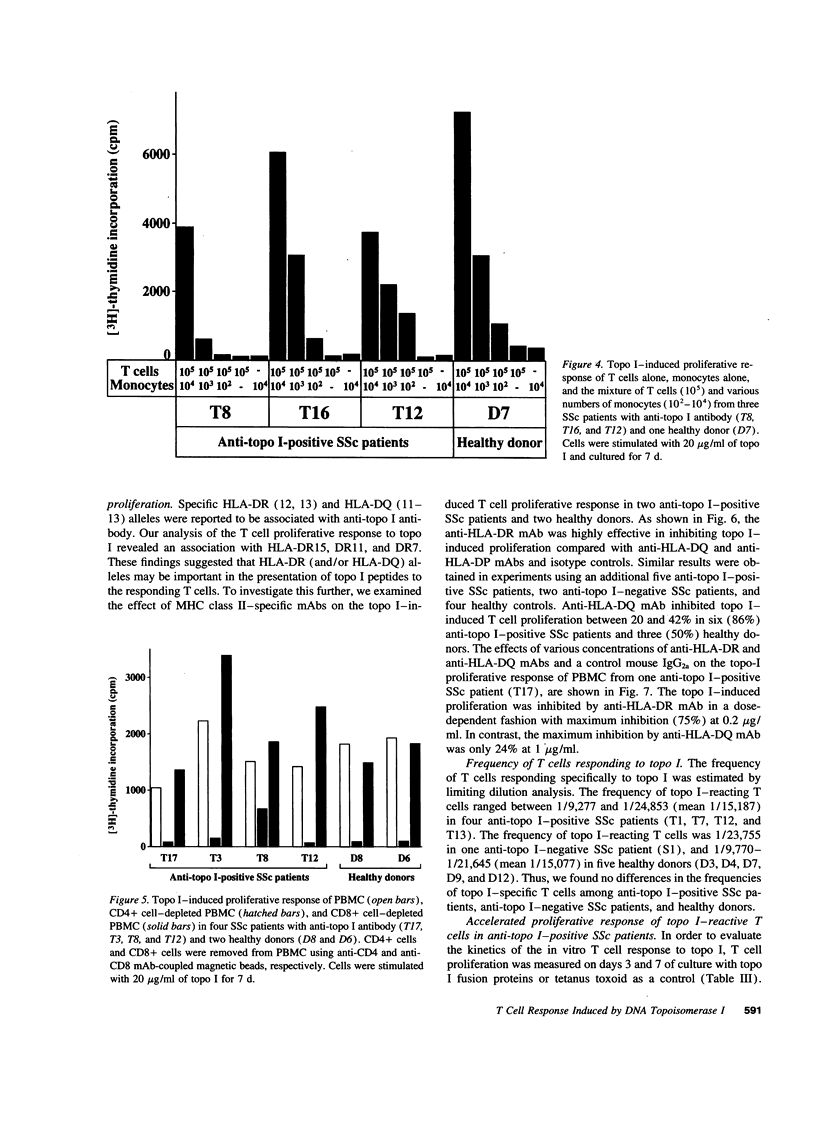
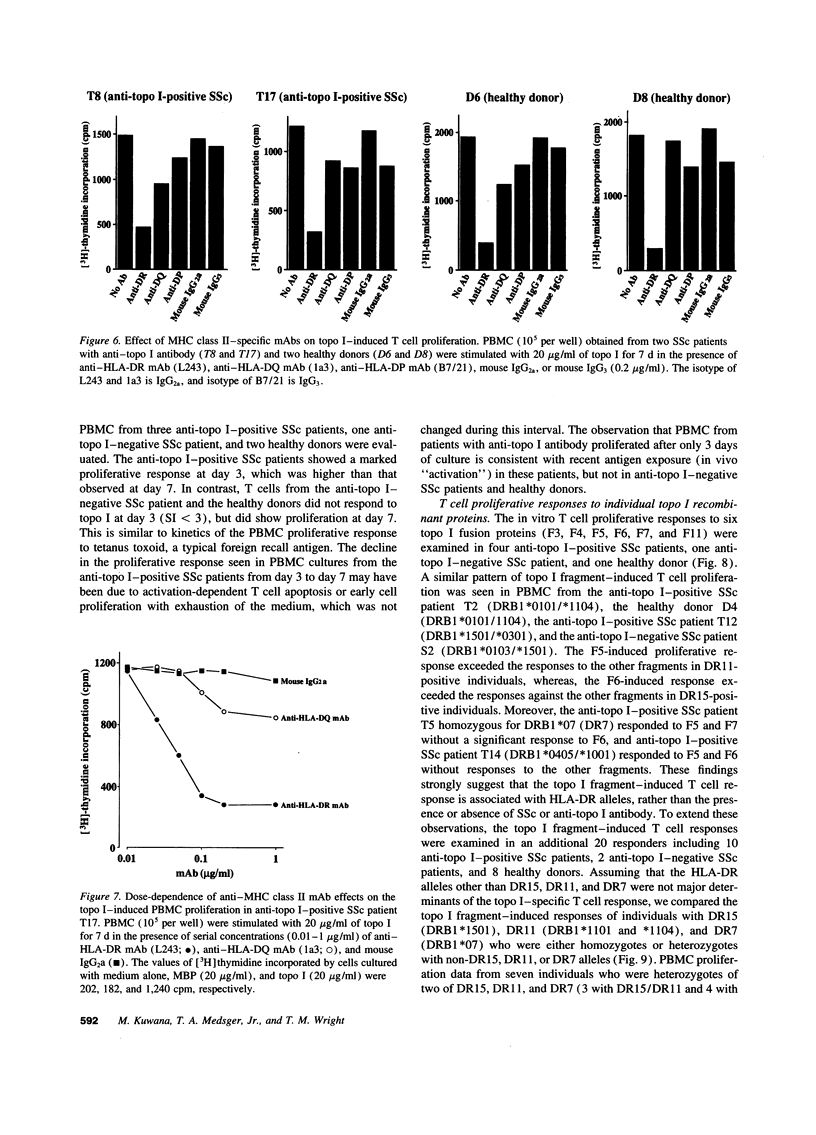
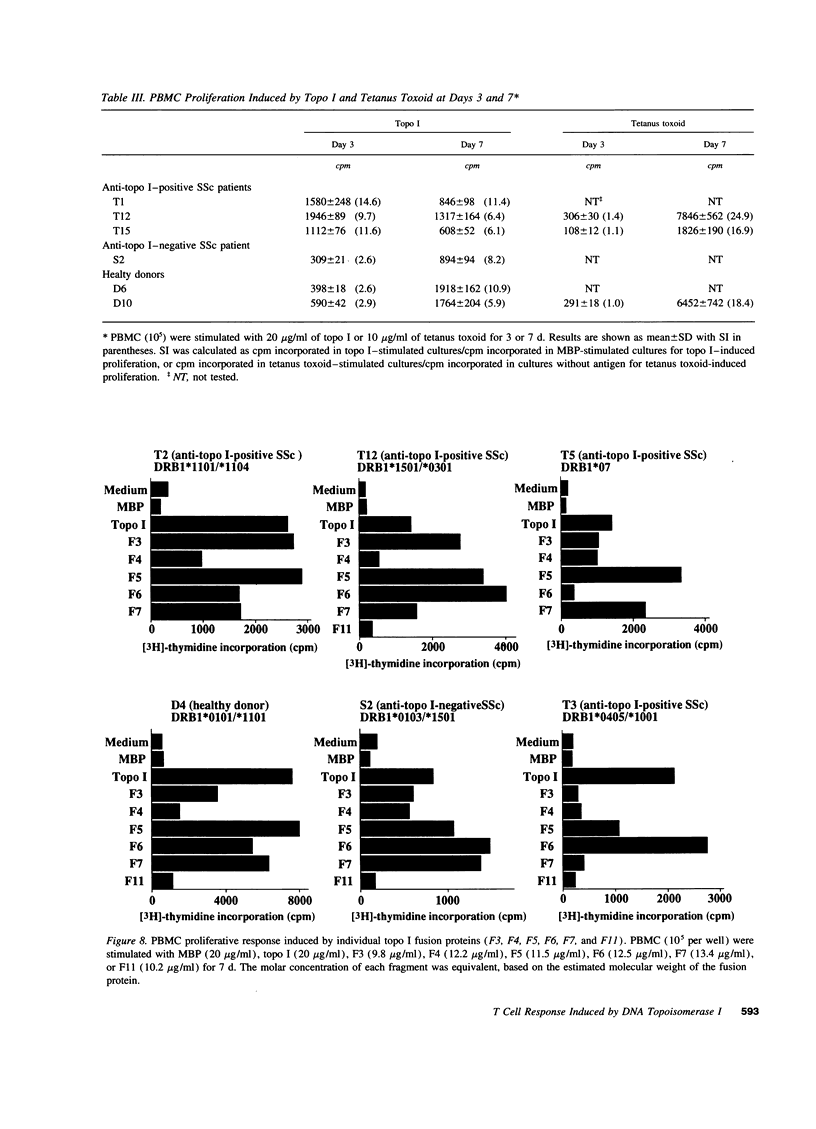
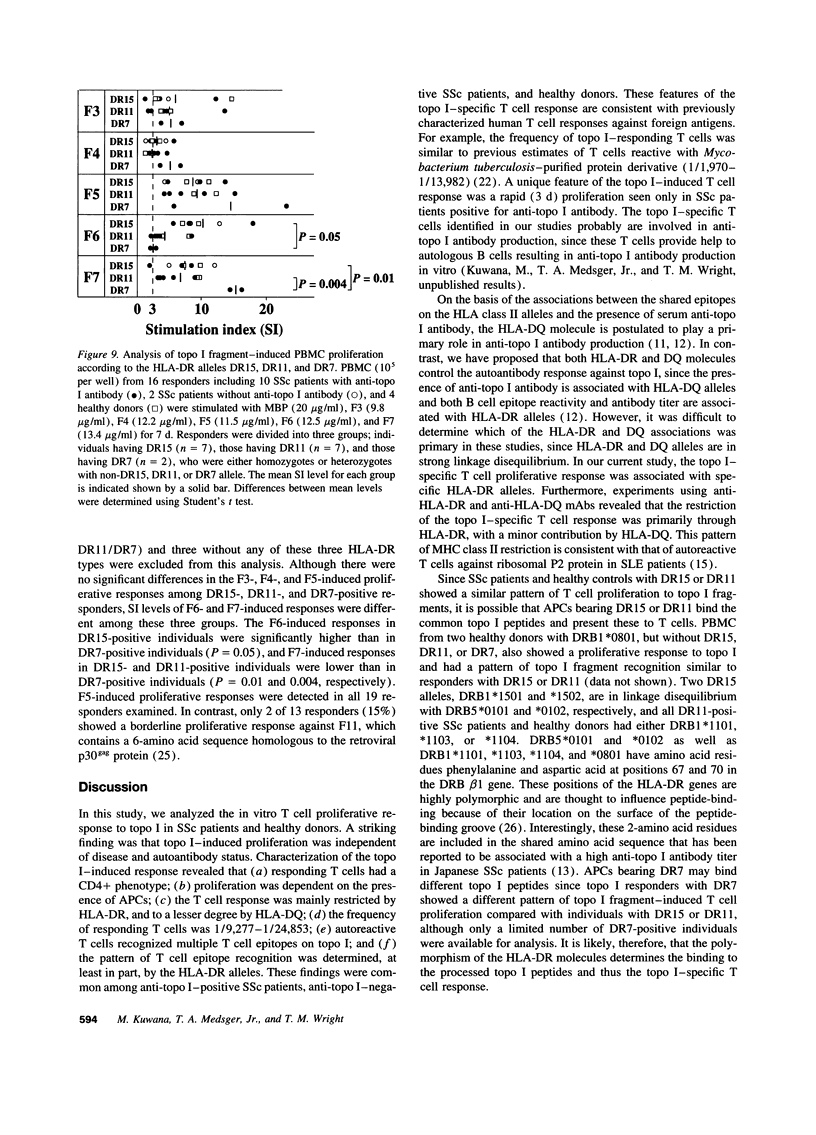
The in vitro T cell proliferative response to DNA topoisomerase I (topo I) was examined in 26 systemic sclerosis (SSc) patients with anti-topo I antibody, 10 SSc patients without anti-topo I antibody, and 21 healthy donors. Using recombinant fusion proteins encompassing the entire human topo I amino acid sequence, a topo I-specific proliferative response was detected in PBMC cultures from 25 (96%) anti-topo I-positive SSc patients, 4 (40%) anti-topo I-negative SSc patients, and 13 (62%) healthy donors. Molecular typing at MHC class II loci revealed that all SSc patients and healthy donors having either DRB1*1501,2 (DR15), DRB1*1101,3,4 (DR11), or DRB1*07 (DR7) were responders. Characterization of the topo I-induced T cell proliferative response showed that (a) the responding cells were CD4+ T cells; (b) antigen-presenting cells were necessary for the response; (c) the response was restricted by HLA-DR, and to a lesser extent by HLA-DQ; and (d) the estimated frequency of the responding T cells determined by limiting dilution analysis was 1/9,277-1/24,853. PBMC cultures from anti-topo I-positive SSc patients showed a high T cell proliferative response after only 3 d of culture with topo I. Anti-topo I-negative SSc patients and healthy donors had no proliferative response after 3 d, but did respond after 7 d of culture. T cell proliferative responses to six truncated topo I fragments tested individually showed different patterns of T cell proliferation that were dependent upon the responder's HLA-DR alleles. These results indicate that T cells reactive with topo I are components of the normal T cell repertoire, and that the topo I-specific T cell proliferative response is not associated with the presence or absence of SSc or anti-topo I antibody, but is restricted by MHC class II alleles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brett S. J., Kingston A. E., Colston M. J. Limiting dilution analysis of the human T cell response to mycobacterial antigens from BCG vaccinated individuals and leprosy patients. Clin Exp Immunol. 1987 Jun;68(3):510–520. [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Burns J., Rosenzweig A., Zweiman B., Lisak R. P. Isolation of myelin basic protein-reactive T-cell lines from normal human blood. Cell Immunol. 1983 Oct 15;81(2):435–440. doi: 10.1016/0008-8749(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Topoisomerase I is preferentially associated with normal SV40 replicative intermediates, but is associated with both replicating and nonreplicating SV40 DNAs which are deficient in histones. Nucleic Acids Res. 1992 Jul 11;20(13):3347–3352. doi: 10.1093/nar/20.13.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. K., DelGiudice-Asch G., Zehetbauer J. B., Lawson J. L., Brot N., Weissbach H., Elkon K. B. Autoantigen-specific T cell proliferation induced by the ribosomal P2 protein in patients with systemic lupus erythematosus. J Clin Invest. 1994 Jul;94(1):345–352. doi: 10.1172/JCI117328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arpa P., Machlin P. S., Ratrie H., 3rd, Rothfield N. F., Cleveland D. W., Earnshaw W. C. cDNA cloning of human DNA topoisomerase I: catalytic activity of a 67.7-kDa carboxyl-terminal fragment. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2543–2547. doi: 10.1073/pnas.85.8.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Dong X., Hamilton K. J., Satoh M., Wang J., Reeves W. H. Initiation of autoimmunity to the p53 tumor suppressor protein by complexes of p53 and SV40 large T antigen. J Exp Med. 1994 Apr 1;179(4):1243–1252. doi: 10.1084/jem.179.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvas A. S., Achten M., Tan E. M. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem. 1979 Oct 25;254(20):10514–10522. [PubMed] [Google Scholar]
- Fatenejad S., Mamula M. J., Craft J. Role of intermolecular/intrastructural B- and T-cell determinants in the diversification of autoantibodies to ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12010–12014. doi: 10.1073/pnas.90.24.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Hoffman R. W., Takeda Y., Sharp G. C., Lee D. R., Hill D. L., Kaneoka H., Caldwell C. W. Human T cell clones reactive against U-small nuclear ribonucleoprotein autoantigens from connective tissue disease patients and healthy individuals. J Immunol. 1993 Dec 1;151(11):6460–6469. [PubMed] [Google Scholar]
- Inoko H., Ando A., Ito M., Tsuji K. Southern hybridization analysis of DNA polymorphism in the HLA-D region. Hum Immunol. 1986 Jul;16(3):304–313. doi: 10.1016/0198-8859(86)90058-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Nieda M., Uchigata Y., Nishimura M., Tokunaga K., Kuwata S., Obata F., Tadokoro K., Hirata Y., Omori Y. Recognition of human insulin in the context of HLA-DRB1*0406 products by T cells of insulin autoimmune syndrome patients and healthy donors. J Immunol. 1993 Nov 15;151(10):5770–5776. [PubMed] [Google Scholar]
- Jardine D., Tachedjian G., Locarnini S., Birch C. Cellular topoisomerase I activity associated with HIV-1. AIDS Res Hum Retroviruses. 1993 Dec;9(12):1245–1250. doi: 10.1089/aid.1993.9.1245. [DOI] [PubMed] [Google Scholar]
- Kawanishi M. Topoisomerase I and II activities are required for Epstein-Barr virus replication. J Gen Virol. 1993 Oct;74(Pt 10):2263–2268. doi: 10.1099/0022-1317-74-10-2263. [DOI] [PubMed] [Google Scholar]
- Koiwai O., Yasui Y., Sakai Y., Watanabe T., Ishii K., Yanagihara S., Andoh T. Cloning of the mouse cDNA encoding DNA topoisomerase I and chromosomal location of the gene. Gene. 1993 Mar 30;125(2):211–216. doi: 10.1016/0378-1119(93)90331-v. [DOI] [PubMed] [Google Scholar]
- Kuwana M., Kaburaki J., Mimori T., Tojo T., Homma M. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest. 1993 Apr;91(4):1399–1404. doi: 10.1172/JCI116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana M., Kaburaki J., Mimori T., Tojo T., Homma M. Autoantigenic epitopes on DNA topoisomerase I. Clinical and immunogenetic associations in systemic sclerosis. Arthritis Rheum. 1993 Oct;36(10):1406–1413. doi: 10.1002/art.1780361013. [DOI] [PubMed] [Google Scholar]
- Kuwana M., Kaburaki J., Okano Y., Inoko H., Tsuji K. The HLA-DR and DQ genes control the autoimmune response to DNA topoisomerase I in systemic sclerosis (scleroderma). J Clin Invest. 1993 Sep;92(3):1296–1301. doi: 10.1172/JCI116703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana M., Kaburaki J., Okano Y., Tojo T., Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994 Jan;37(1):75–83. doi: 10.1002/art.1780370111. [DOI] [PubMed] [Google Scholar]
- Kuwana M., Okano Y., Kaburaki J., Tojo T., Medsger T. A., Jr Racial differences in the distribution of systemic sclerosis-related serum antinuclear antibodies. Arthritis Rheum. 1994 Jun;37(6):902–906. doi: 10.1002/art.1780370619. [DOI] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipham W. J., Redmond T. M., Takahashi H., Berzofsky J. A., Wiggert B., Chader G. J., Gery I. Recognition of peptides that are immunopathogenic but cryptic. Mechanisms that allow lymphocytes sensitized against cryptic peptides to initiate pathogenic autoimmune processes. J Immunol. 1991 Jun 1;146(11):3757–3762. [PubMed] [Google Scholar]
- Maina C. V., Riggs P. D., Grandea A. G., 3rd, Slatko B. E., Moran L. S., Tagliamonte J. A., McReynolds L. A., Guan C. D. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene. 1988 Dec 30;74(2):365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Fatenejad S., Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994 Feb 1;152(3):1453–1461. [PubMed] [Google Scholar]
- Mamula M. J. The inability to process a self-peptide allows autoreactive T cells to escape tolerance. J Exp Med. 1993 Feb 1;177(2):567–571. doi: 10.1084/jem.177.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. Topoisomerase activity is associated with purified SV40 T antigen. Nucleic Acids Res. 1993 Apr 25;21(8):1697–1704. doi: 10.1093/nar/21.8.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Jimenez S. A., Riggs E., Ziemnicka-Kotula D. Determination of an epitope of the diffuse systemic sclerosis marker antigen DNA topoisomerase I: sequence similarity with retroviral p30gag protein suggests a possible cause for autoimmunity in systemic sclerosis. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8492–8496. doi: 10.1073/pnas.86.21.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P. A., Chang H. J., Wilson J. W., Conte C., Saidman S. L., Bray J. D., Tweardy D. J., Medsger T. A., Jr Severe systemic sclerosis with anti-topoisomerase I antibodies is associated with an HLA-DRw11 allele. Hum Immunol. 1994 Jun;40(2):101–110. doi: 10.1016/0198-8859(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Okano Y., Steen V. D., Medsger T. A., Jr Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993 Nov 15;119(10):1005–1013. doi: 10.7326/0003-4819-119-10-199311150-00007. [DOI] [PubMed] [Google Scholar]
- Oki A., Sercarz E. T cell tolerance studied at the level of antigenic determinants. I. Latent reactivity to lysozyme peptides that lack suppressogenic epitopes can be revealed in lysozyme-tolerant mice. J Exp Med. 1985 May 1;161(5):897–911. doi: 10.1084/jem.161.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel E., Showalter S. D., Roberts M., Oroszlan S., Segal S., Aboud M., Blair D. G. Topoisomerase I activity associated with human immunodeficiency virus (HIV) particles and equine infectious anemia virus core. EMBO J. 1990 Dec;9(12):4167–4172. doi: 10.1002/j.1460-2075.1990.tb07640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Zordan T., Tsokos G. C., Datta S. K. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveille J. D., Durban E., MacLeod-St Clair M. J., Goldstein R., Moreda R., Altman R. D., Arnett F. C. Association of amino acid sequences in the HLA-DQB1 first domain with antitopoisomerase I autoantibody response in scleroderma (progressive systemic sclerosis). J Clin Invest. 1992 Sep;90(3):973–980. doi: 10.1172/JCI115974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels D. S., Shimizu N. The predominant form of mammalian DNA topoisomerase I in vivo has a molecular mass of 100 kDa. Mol Biol Rep. 1994 Mar;19(2):99–103. doi: 10.1007/BF00997154. [DOI] [PubMed] [Google Scholar]
- Shero J. H., Bordwell B., Rothfield N. F., Earnshaw W. C. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986 Feb 14;231(4739):737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen V. D., Powell D. L., Medsger T. A., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988 Feb;31(2):196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- Thrash C., Bankier A. T., Barrell B. G., Sternglanz R. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4374–4378. doi: 10.1073/pnas.82.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Opgenorth A., Traktman P., McFadden G. Identification and DNA sequence of the Shope fibroma virus DNA topoisomerase gene. Virology. 1990 Jun;176(2):439–447. doi: 10.1016/0042-6822(90)90013-h. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. L., Hsu M. T. Involvement of topoisomerases in replication, transcription, and packaging of the linear adenovirus genome. J Virol. 1990 Feb;64(2):691–699. doi: 10.1128/jvi.64.2.691-699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Yamamoto N., Maeno K., Nishiyama Y. Role of DNA topoisomerase I in the replication of herpes simplex virus type 2. Arch Virol. 1990;110(1-2):121–127. doi: 10.1007/BF01310708. [DOI] [PubMed] [Google Scholar]



