Abstract
Large and rapid changes in light scattering accompany secretion from nerve terminals of the mammalian neurohypophysis (posterior pituitary). In the mouse, these intrinsic optical signals are intimately related to the arrival of the action potential E-wave and the release of arginine vasopressin and oxytocin (S-wave). Here we have used a high bandwidth atomic force microscope to demonstrate that these light-scattering signals are associated with changes in terminal volume that are detected as nanometer-scale movements of a cantilever positioned on top of the neurohypophysis. The most rapid mechanical response (“spike”), having a duration shorter than the action potential but comparable to that of the E-wave, represents a transient increase in terminal volume due to water movement associated with Na+-influx. The slower mechanical event (“dip”), on the other hand, depends upon Ca2+-entry as well as on intraterminal Ca2+-transients and, analogously to the S-wave, seems to monitor events associated with secretion.
INTRODUCTION
The mammalian neurohypophysis, a neuroendocrine organ that consists primarily of the nerve terminals of hypothalamic magnocellular neurons, has long provided an important model system for studying the rapid release of neuropeptides (1–5). Magnocellular neurons from the supraoptic and paraventricular nuclei project their axons as bundles of fibers through the median eminence and infundibular stalk to arborize extensively and terminate in the neurohypophysis, where the neuropeptides vasopressin and oxytocin are released into the circulation by a Ca2+-dependent mechanism. In the rat, some 20,000 neurons give rise to ∼40,000,000 neurosecretory terminals and swellings (6) whose plasmalemma accounts for nearly 99% of the excitable membrane in the neurohypophysis (6). Indeed, the dense packing of these terminals and their collective very large surface/volume ratio account for the unusually robust extrinsic (impermeant voltage-sensitive dye) (7,8) and intrinsic (light-scattering) (9–11) optical signals that we have recorded from this tissue. Whereas the extrinsic signals are a direct measure of fast voltage changes associated with electrical stimuli invading the terminal arbor, the light-scattering signals that we observe represent changes in the optical transparency of the tissue that range in duration from milliseconds to seconds. Some of these components correspond to distinct events leading to neuropeptide release (9). Because our earlier experiments suggested that some features of the light-scattering changes might reflect rapid volume changes in the neurosecretory terminals and/or in the dense core granules they contain, and in light of early reports of mechanical events associated with the action potential in nerve (12,13), we decided to monitor, directly, changes in tissue thickness using high bandwidth atomic force microscopy (HBAFM) (14–23). The work presented here focuses exclusively on events that occur during, or immediately after, the arrival of the action potential into the terminals. Preliminary reports of our findings have appeared in abstract form (24,25).
METHODS
Preparation
The neurointermediate lobe of the pituitary, which comprises pars nervosa (neurohypophysis) and pars intermedia, was obtained, as previously described (9), from female CD-1 mice that had been anesthetized by CO2 inhalation and decapitated in accordance with institutional guidelines. Briefly, the mouse head was pinned to the bottom of a Sylgard-lined dissection dish, and, after removal of the skin, the skull was opened along the dorsal midline and the bone removed bilaterally. Cutting the optic and olfactory nerves allowed caudal reflexion of the brain and rupture of the infundibular stalk, leaving the infundibular stump together with the entire pituitary gland attached to the base of the skull. The gland, gently removed using fine forceps and iridectomy scissors, was transferred to a dish containing oxygenated mouse Ringer's solution (in mM: 154 NaCl, 5.6 KCl, 1.0 MgCl2, 2.2 CaCl2, 10 glucose, 20 HEPES, adjusted to pH 7.4 with NaOH) where the anterior pituitary (pars anterior) was separated from the neurointermediate lobe and discarded. The pars intermedia, which consists of a delicate lacework of cells supporting the neurohypophysis, provided a convenient border through which the specimen could be pinned to the bottom of the experimental chamber while preserving the integrity of the neurohypophysis.
Stimulation was achieved using a pair of Pt-Ir (90%–10%) electrodes clasping the infundibular stump and consisted of brief (100–500 μs) shocks delivered through a stimulus isolator. The experimental chamber was mounted on the stage of either an upright (Zeiss UEM) or a modified inverted (Zeiss IM-35) microscope (Zeiss, Jena, Germany).
Detection of intrinsic and extrinsic optical changes in nerve terminals
Light-scattering changes were recorded (9) as compound changes in transparency of the neurohypophysis using a large area PIN silicon photodiode (PV-444, Perkin Elmer Optoelectonics, Vaudreuil, Canada) and a current-to-voltage converter having a 1-MΩ feedback resistor together with a 500× second stage amplifier. Illumination was provided by either a high power 740-nm light-emitting diode (Roithner LaserTechnik, Vienna, Austria) (26,27) or a 250-W tungsten-halogen lamp with a 675 ± 25 nm (Chroma Technology, Brattleboro, VT) filter.
Extrinsic fluorescence changes (−ΔF) proportional to ΔVm were recorded after staining the neurohypophysis for 40 min in a Ringer's solution containing 50 μg/ml of the naphthyl-styrylpyridinium potentiometric probe di-4-ANEPPDHQ (JPW5029) (28) in 0.25% ethanol. The current-to-voltage converter for these measurements was a single stage device (DLPCA-200, Femto Messtechnik, Berlin, Germany) having either a 10-MΩ or a 100-MΩ feedback resistor, and the light source was, once again, the 250-W tungsten halogen lamp with a 530 ± 45 nm excitation filter, 585-nm dichroic mirror, and 620 ± 20 nm emission filter (Chroma Technology) in an epi-illumination configuration. KG-1 heat filters (Schott, Duryea, PA) were incorporated into all illumination paths, and all optical records were either low-pass filtered at 1 kHz with an 8-pole Bessel filter and sampled at 2 kHz or low-pass filtered at 7 kHz with a 1-pole Butterworth (equivalent to an RC) filter and sampled at 20 kHz. All optical records were digitized at 16-bit resolution.
Atomic force microscopy
The atomic force microscope (AFM) was an ESPM 3D (Novascan, Ames, IA), with the following modifications: a) the X-Y scanning was disabled and the Z axis feedback was turned off, providing a high bandwidth measurement of the Z-position of the cantilever, which was sampled at 10 or 20 kHz; b) the cantilever (MikroMasch CSC12/tipless/no Al, MikroMasch, Portland, OR) was uncoated silicon (width 35 μm, length 350 μm, thickness ∼1 μm) without any tip. The resonant frequency of this cantilever is ∼10 kHz in air; however, in Ringer's solution and in contact with the tissue, it would have been reduced to DC to a few hertz. The force constant of the cantilever is 0.03 N/m. The AFM system's frequency response is >20 kHz and, thus, able to follow the nerve terminal action potential with very high fidelity. The HBAFM laser wavelength was 670 nm. ΔZ, recorded using the HBAFM, was filtered at 7 kHz with a 1-pole Butterworth (RC) filter and digitized at 16-bit resolution.
During an experiment, the preparation was submerged in Ringer's solution. Next, the AFM head was tilted down and positioned over the center of the preparation using the X-Y stage micrometer. The AFM probe was brought nearly into contact with the center of the top of the neurohypophysis using the picomotor screws of the ESPM 3D. The 670-nm laser spot of the AFM was aligned to the center of the distal end of the cantilever where it was about to contact the neurohypophysis, and the quadrant detector position was adjusted for optimal laser signal. The AFM was then instructed (in software written for the ESPM 3D) to “Auto Engage”, after which the feedback was turned off. Then, with a completely passive AFM cantilever in stable contact with the neurohypophysis, measurement of ΔZ in response to electrical stimulation of action potentials could proceed.
RESULTS
To determine whether volume changes accompany light-scattering changes in the mouse neurohypophysis, we used a modified HBAFM to monitor rapid (millisecond) mechanical events during and immediately after an electrically evoked action potential. By employing a tipless cantilever (width 35 μm, length 350 μm, thickness ∼1 μm), disabling the X-Y scanning, and sampling the Z axis position of the cantilever without feedback at up to 20 kHz, we could record mechanical events in a single sweep, and these records had a S/N > 25:1. This apparatus permitted us to detect dynamic changes in the height of a column of neurohypophyseal terminals.
Clearly, the linear movement of the cantilever along the Z axis is an indirect measure of nerve terminal volume change. (Since the cantilever is seated atop the tissue, changes in its position reflect changes in the thickness of the intact neurohypophysis.) However, this experimental approach afforded us a real time account of a parameter that is covariant with terminal volume while preserving the anatomical and functional integrity of the neurohypophysis. Using this high bandwidth apparatus, we demonstrated that mammalian neurosecretory terminals exhibit interesting mechanical changes during excitation-secretion coupling. To validate these observations, we also configured the HBAFM with additional lenses and detectors that permitted optical measurements of light-scattering changes and/or ΔVm simultaneously with the mechanical recordings (see Figs. 2–4).
FIGURE 2.
Comparison of the action potential and the associated mechanical events triggered by a single electrical stimulus (500 μs) delivered to the infundibular stalk. Simultaneous recordings of the action potential (−ΔF, green trace) and the mechanical spike and dip (ΔZ, red trace) evoked in the same preparation. The thin black traces represent 10-point binomial smoothing of the data. Both traces were sampled at 20 kHz and low-pass filtered at 7 kHz.
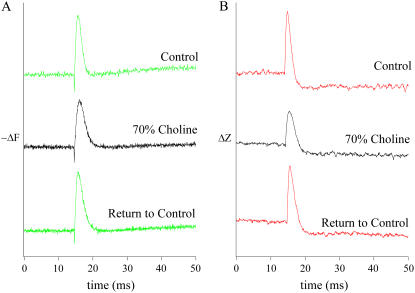
FIGURE 3.
The effect of replacing sodium with choline in the Ringer's solution bathing the neurohypophysis. (A) Optical recordings (−ΔF) of a single action potential in normal mouse Ringer's solution (Control, top trace), in Ringer's solution in which 70% of the Na+ was replaced by choline (middle trace), and the recovery in Control (bottom trace). (B) HBAFM measurements of the mechanical spike and dip recorded simultaneously with the corresponding action potentials in panel (A). All traces were sampled at 20 kHz and low-pass filtered at 7 kHz.
FIGURE 4.
The effect of tonicity (and turgor) on the amplitude of the mechanical spike. (A) The action potential recorded optically (−ΔF) was virtually unaffected (middle-black trace) by a 17-min exposure to a Ringer's solution made 25% hypertonic by the addition of sucrose. (B) Under the same conditions, the mechanical spike was reduced in amplitude (middle-black trace). The corresponding action potentials and mechanical spikes were recorded simultaneously. All traces were sampled at 20 kHz and low-pass filtered at 7 kHz.
Fig. 1 illustrates optical and mechanical signals recorded in single trials from neurosecretory terminals of the intact CD-1 mouse neurohypophysis stimulated seven times at 15 Hz. Fig. 1 A shows the action potentials themselves, recorded as extrinsic fluorescence changes, after staining the preparation with the potentiometric probe di-4-ANEPPDHQ (28). Fig. 1 B illustrates the light-scattering changes (ΔI), during an identical stimulus train, recorded in an unstained preparation. The early upward deflection (E-wave), a decrease in transmission and an increase in large angle light scattering (9), reflects the arrival of the action potential and/or action currents at the neurohypophyseal terminals. The sustained downward deflection of the trace (S-wave), an increase in transmitted light intensity, is intimately related to secretion of the neuropeptides arginine vasopressin and oxytocin (9). Fig. 1 C illustrates the HBAFM recording (cantilever movement along the Z axis) of the mechanical spikes that accompany a train of seven stimuli identical to those applied in A and B. This panel reveals components of the HBAFM signals that require further analysis. Noteworthy is the downward drift of the baseline, which probably reflects the subsidence of the tissue under the weight of the cantilever. (This explanation, although plausible, has not been tested experimentally.) Other significant features of the HBAFM signal are highlighted in the inset, which shows the first mechanical spike of this train on an expanded time base. Notice that the spike is followed by a downward deflection of the cantilever (“dip”). The accumulation of the dips during a train results in the pronounced steepening in slope exhibited by the envelope of the signal during stimulation.
FIGURE 1.
Optical and mechanical signals recorded independently from the neurosecretory terminals of three mouse neurohypophyses in response to trains of stimuli delivered at 15 Hz. (A) Action potentials recorded optically (−ΔF) using the fluorescent potentiometric dye di-4-ANEPPDHQ. (B) Light-scattering changes recorded as changes in transmission (−ΔI) in response to an identical stimulus in an unstained preparation. (Increase in transmitted light intensity is plotted downward.) (C) Mechanical events triggered by action potentials, monitored with a modified HBAFM. The inset shows the first mechanical spike and dip from this train on an expanded time base. All traces were low-pass filtered at 1 kHz.
Fig. 2 illustrates the action potential recorded optically (upper trace) and the simultaneously recorded mechanical spike (ΔZ) (lower trace) evoked by a single 500-μs stimulus. The data in this experiment were low-pass filtered at 7 kHz and sampled at 20 kHz. The thin black traces represent 10-point binomial smoothing of the data. The mechanical spike had duration (fullwidth at half-maximum) of ∼1.6 ms, comparable to that of the action potential. Both the action potential and the mechanical spike were eliminated in the presence of 1 μM tetrodotoxin (TTX) (data not shown.).
Because the mechanical spike reflects a transient increase in the volume of the electrically stimulated nerve terminals, it is reasonable to suppose that it results from water entry along with, or as part of, the inward Na+-current during the action potential. Substitution of an impermeant cation for Na+ in the Ringer's solution is expected to reduce the total number of Na+-ions entering the nerve terminals during the action potential. Thus, by titrating the Na+-replacement it should be possible to preserve most of the voltage excursion during the spike, compromising only its rate of rise. Fig. 3 illustrates the effect on the nerve terminal action potential (A) and on the mechanical spike (B) of replacing 70% of the Na+ in the Ringer's solution with choline. Fig. 3 A shows optical recordings (−ΔF) of a single action potential in normal mouse Ringer's solution (upper trace) in a Ringer's solution in which 70% of the Na+ was replaced by choline (middle trace) and upon recovery in normal Ringer's solution (lower trace). Note the broadening of the action potential without a dramatic loss of amplitude. The records in Fig. 3 B illustrate HBAFM measurements of the mechanical spikes (ΔZ) recorded simultaneously with the corresponding action potentials depicted in Fig. 3 A. The disproportionate decrease in amplitude of the mechanical spike and the broadening of the signal in the reduced-Na+ Ringer's solution are evident.
If the mechanical spike represents a transient increase in the volume of the terminals due to water entry, one might expect this signal to vary with the membrane tension in the terminals. To test this hypothesis we modified turgor pressure by varying the tonicity of the extracellular solution. A typical experiment is illustrated in Fig. 4. Panel A shows the optical recordings (−ΔF) of a single action potential in normal mouse Ringer's solution (upper trace), after 17 min in a Ringer's solution rendered 25% hypertonic with sucrose (middle trace), and upon recovery in normal Ringer's solution (lower trace). Increasing tonicity should have the effect of shrinking the terminals (and corrugating the membrane). The records shown in Fig. 4 A demonstrate that hypertonicity has no effect on the size or the time-course of the nerve terminal action potential. By contrast, the records in Fig. 4 B, which represent the volume changes (ΔZ) triggered by the same action potentials shown in Fig. 4 A, reveal a dramatic effect of hypertonicity (and, consequently, terminal shrinkage) on the amplitude of the mechanical spike.
The S-wave of the light-scattering change is exquisitely sensitive to [Ca2+]o (9,10). Since the time course of the light-scattering change is similar (albeit not identical) to that of the mechanical signal (Fig. 1, B and C), it was of interest to examine whether or not the dip in the mechanical signal also depended on Ca2+-entry. Fig. 5 A shows the light-scattering signal (−ΔI) in response to a train of seven stimuli delivered at 15 Hz in normal Ringer's solution (blue trace) and in a Ringer's solution containing 1 mM CdCl2 (black trace). Fig. 5 B illustrates the mechanical response to a train of stimuli identical to that in Fig. 5 A, recorded in normal Ringer's solution (red trace) and in a Ringer's solution containing 1 mM CdCl2 (black trace). The slope change induced by the train of mechanical spikes, resulting from the summation of dips, was eliminated by Cd2+. This effect mimics the Cd2+-induced loss of the S-wave illustrated in Fig. 5 A (black trace) (9).
FIGURE 5.
The effect of Cd2+ on the light-scattering change and on the mechanical signal in response to a train of electrical stimuli. (A) The light-scattering change (−ΔI) recorded from secretory terminals of a mouse neurohypophysis in response to seven stimuli delivered at 15 Hz in normal mouse Ringer's solution (Control, blue trace) and after 15 min in a Ringer's solution containing 1 mM CdCl2 (black trace). (B) The mechanical response (ΔZ), recorded from a different preparation, to an identical train of stimuli. Red trace, Control Ringer's solution; black trace, 15 min in a Ringer's solution containing 1 mM CdCl2. Note that both the S-wave of the light-scattering change and the summated dips (slope change) of the train of mechanical spikes are eliminated when Ca2+-entry into the terminals is blocked by Cd2+. All traces were low-pass filtered at 1 kHz.
In light of the results from Fig. 5, it was important to determine whether or not the dip of the HBFAM signals was also dependent on Δ[Ca2+]i resulting from intraterminal Ca2+-release. Fig. 6 illustrates the effect of caffeine on both the light-scattering signals (Fig. 6 A) and the volume changes (Fig. 6 B) triggered simultaneously by a train of stimuli. Note that caffeine affects the two signals differently. Whereas caffeine enhances the cumulative sizes of the S-waves and the dips, its effect on the individual responses within each train is remarkably different.
FIGURE 6.
The effect of caffeine on the light-scattering change and on the mechanical signal in response to a train of electrical stimuli. (A) The light-scattering change (−ΔI) recorded from secretory terminals of the mouse neurohypophysis in normal mouse Ringer's solution (Control, blue trace) and after 15 min in a Ringer's solution containing 10 mM caffeine (black trace). (B) The mechanical response (ΔZ) to an identical train of stimuli recorded from a different preparation by the HBAFM. Red trace, Control Ringer's solution; black trace, 15 min in a Ringer's solution containing 10 mM caffeine. All traces were low-pass filtered at 1 kHz.
DISCUSSION
The action potential in the neurosecretory terminals of the mouse neurohypophysis is accompanied by light-scattering changes (9–11) and a rapid, transient volume change (mechanical spike) having duration comparable to or shorter than that of the electrical event itself (Figs. 2–4). This mechanical spike, recorded as an upward deflection of the HBAFM cantilever, represents an increase in thickness of the intact neurohypophysis on the order of 5–10 nm. We have deliberately omitted ordinate scales in the figures as the records are population recordings and, although the HBAFM is calibrated, the precise amplitude of ΔZ does not translate directly to a measure of the diameter change of a single terminal. Since the tissue probably owes its thickness to a stack of ∼100 terminals, this deflection may reflect an increase in diameter of as little as 0.05 nm (0.5 Å) in a single terminal. When 70% of the Na+ was replaced by an impermeant cation (choline), the action potential was somewhat affected (Fig. 3 A); the mechanical spike was dramatically reduced in amplitude (Fig. 3 B), however, suggesting that this signal may monitor the water entry that, presumably, accompanies the inward Na+-current in the terminals. Indeed, action potential simulations using a 70% reduction in [Na+]o yield similar results, with a disproportionate share of the effect on the integrated Na+-current and little effect on the amplitude of the action potential. If the mechanical spike reflects water movement, one would expect it to be sensitive to osmotic changes. Indeed, rendering the terminals shrunken or corrugated using a hypertonic Ringer's solution decreased the amplitude of the mechanical spike (Fig. 4 B) without affecting significantly the amplitude of the action potential (Fig. 4 A). Although these data strongly suggest that the rapid volume changes result from water moving across the terminal membrane, we have, as yet, no basis for concluding whether this water moves through voltage-gated Na+-channels or through nearby aquaporins (29–31).
It is instructive to estimate the number of water molecules that might enter a nerve terminal during the action potential and whether this number could account for the observed volume changes. Such a calculation is clearly approximate, as it depends upon several uncertain assumptions. Nonetheless, an order of magnitude value for the anticipated volume increase in a single neurohypophyseal terminal during the action potential can begin to corroborate our hypothesis. If we assume a 5-μm spherical terminal, its volume will be 65.4 femtoliters and its capacitance (assuming 1 μF/cm2) will be 0.785 pF. During a 100-mV action potential at least 4.9 × 107 Na+-ions will enter the terminal and, if we assume that N water molecules enter along with the Na+, this amounts to the addition of 1.48 × 10−18 N liters of water to the terminal. Here, two critical assumptions are required. First, we assume that the entire interior of the terminal is accessible to the water even though it is tightly packed with dense core granules. Second, we assume that N ≈ 2. This seems a reasonable assumption based on the streaming potential measurements of Benos and colleagues (32) in epithelial amiloride-sensitive Na+ channels from which they concluded that between two and three water molecules are translocated together with a single Na+-ion. On the assumption that a pair of water molecules accompanies each Na+-ion, 2.96 × 10−18 liters of water will enter each terminal during the action potential and this will increase the diameter of a 5-μm terminal by ∼0.0015% or 0.8 Å. This is entirely consistent with the estimate of 0.5 Å per terminal derived from our HBAFM measurement of ΔZ.
The very brief duration of the mechanical spike (fullwidth at half-maximum <2 ms at room temperature) requires some further comment, especially considering that it is shorter than that of the action potential (Fig. 2). The difference may have a trivial explanation inasmuch as the recordings of membrane potential and volume are both population recordings, and the apparatus is necessarily configured so that different populations are sampled. The optical record of membrane potential is obtained from a larger population of terminals than is the HBAFM record, and there is probably some temporal dispersion in both signals, depending upon the number of terminals included. The cantilever samples the slab of terminals directly underneath it, whereas the fluorescence measurement of membrane voltage is derived from a larger circular cylinder of terminals defined by a diaphragm in the image plane of the microscope.
Sachs and colleagues (20,23) used an AFM to measure voltage-induced membrane displacements in voltage-clamped HEK293 cells. They confirmed thermodynamic predictions (Lippmann model (33)) that voltage modulates membrane tension and that this will cause movement. Indeed, the volume increase that we measure agrees with the sign of the displacement that they reported. They also speculated (23) that this electromechanical coupling might be physiologically significant in highly curved membranes, including, presumably, nerve terminals. However, the experiment illustrated in Fig. 3, in which reduction in [Na+]o had a profound effect on ΔZ, with a much smaller effect on ΔV, suggests that the mechanical spike that we observe is not a purely electromechanical effect (i.e., direct coupling of transmembrane voltage to membrane tension). Very significant differences between the two preparations preclude any direct comparison of the results. These considerations notwithstanding, we cannot completely exclude the possibility that the mechanical spike has its origin in the membrane voltage. Indeed, Cohen et al. (34,35) measured both current- and potential-dependent components of light-scattering signals in squid giant axons, and only the potential-dependent light-scattering signals had time courses comparable to those of the action potential. However, since the surface/volume ratio in 5-μm diameter nerve terminals is at least 150 times greater than in the squid giant axon, much faster current-dependent responses might be observed in the neurohypophysis. (Note also that the amplitude of each of the two potential-dependent components of the light-scattering signal in squid axon was proportional, not to the voltage, but to its square.)
Whereas the mechanical spike exhibits similarities with the E-wave of the light-scattering signal, including its short duration and its insensitivity to Cd2+, the dip that follows not only is sensitive to Ca2+-levels, but also shares additional features with the S-wave (e.g., both signals are enhanced in high [Ca2+]o and reduced in low [Ca2+]o or when D2O is substituted for H2O (9); data not shown) and both exhibit facilitation and depression during a train of stimuli. By implication, this downward deflection of the cantilever (dip) seems to be related to neuropeptide release or to a prerelease modification of the secretory granules. Since secretion produces transient increases in terminal capacitance, and hence surface area, it may seem paradoxical that it would also be associated with a decrease in volume. However, exocytotic expulsion of dense core granules provides a plausible mechanism for the observed volume decrease.
Parpura and Fernandez studied the mechanical properties of submicrometer-sized secretory granules isolated from rat mast cells using an AFM (21) and reported that these secretory granules contain an insoluble matrix that reversibly shrinks and swells in response to ion exchange with different cations. In particular, these authors demonstrated that this matrix has the mechanical properties of an ion exchange gel and that rapid swelling of the matrix occurred when the interior of the granule was exposed to normal Ringer's solution. A similar mechanism could account for the dip in the HBAFM signal that follows the action potential, although the direction of the change is opposite to that described in the work on mast cell granules. The gel matrices that form the dense cores of different secretory cells must differ, so it is possible that the Ca2+-dependent volume decrease (dip) that we observe could be related to an ion exchange mechanism.
The experiment in Fig. 5 compares the dependence of the S-wave of the light-scattering change and the dip of the mechanical signal upon Ca2+-entry into the terminals. This figure illustrates the stimulation-induced changes in slope of both ΔI (Fig. 5 A, blue trace) and ΔZ (Fig. 5 B, red trace) observed in normal [Ca2+]o (2.2 mM), resulting from the summation of the S-waves and the dips, respectively, during the train of stimuli. A careful comparison of these traces, however, reveals that the detailed patterns of facilitation and depression for both experimental modalities are not identical. Instead, the data suggest that ΔI and ΔZ may monitor distinct but closely related processes. The addition of 1 mM Cd2+ to the bathing solution reduces or eliminates both the S-waves and the dips (Fig. 5, A and B, black traces), thereby largely abolishing the change in slope during the train of stimuli. (It should be noted, however, that for technical reasons, the ΔZ and ΔI traces were not recorded from the same preparation.)
In contrast to Fig. 5, which demonstrates the dependence of these signals on Ca2+-entry, Fig. 6 illustrates the effect of enhancing Δ[Ca2+]i during a train of stimuli using caffeine (36). Caffeine is known to mobilize Ca2+ from intracellular stores, including those found in mammalian nerve terminals (37–45). In addition, our laboratory has reported large effects of caffeine on the S-wave of the light-scattering signal (46) and on Δ[Ca2+]i as monitored with low affinity fluorescent Ca2+-indicator dyes (36) (but see Muschol et al. (47)). The effects of caffeine on the light-scattering change and the mechanical signals illustrated in Fig. 6 suggest once again that the S-wave and the dip are indicators of quasisynchronous but independent events and that the two experimental modalities provide complementary descriptions of events that contribute to excitation-secretion coupling.
Although there have been earlier observations of mechanical events in nerve, none of these reports identified mechanical phenomena in nerve terminals and none of them could demonstrate signals without extensive averaging. These nanometer scale mechanical phenomena associated with nerve terminal excitation and secretion, studied together with the light-scattering changes, may provide new insight into the relation between terminal volume, intraterminal Ca2+-stores, and excitation-secretion coupling.
Acknowledgments
We are grateful to Dr. Paul DeWeer for his critical reading of the manuscript and to Drs. Clay Armstrong, Raj Lartius, and Zhe Lu for helpful discussions.
This work was supported by U.S. Public Health Service grant NS40966 (B.M.S.).
References
- 1.Douglas, W. W. 1963. A possible mechanism of neurosecretion release of vasopressin by depolarization and its dependence on calcium. Nature (Lond.). 197:81–82.13988418 [Google Scholar]
- 2.Douglas, W. W., and A. M. Poisner. 1964. Stimulus-secretion coupling in a neurosecretory organ: the role of calcium in the release of vasopressin from the neurohypophysis. J. Physiol. 172:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann, J. J. 1983. Stimulus-secretion coupling. Prog. Brain Res. 60:281–304. [DOI] [PubMed] [Google Scholar]
- 4.Salzberg, B. M., and A. L. Obaid. 1988. Optical studies of the secretory event at vertebrate nerve terminals. J. Exp. Biol. 139:195–231. [DOI] [PubMed] [Google Scholar]
- 5.Stuenkel, E. L., and J. J. Nordmann. 1993. Intracellular calcium and vasopressin release of rat isolated neurohypophysial nerve endings. J. Physiol. 468:335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordmann, J. J. 1977. Ultrastructural morphometry of the rat neurohypophysis. J. Anat. 123:213–218. [PMC free article] [PubMed] [Google Scholar]
- 7.Muschol, M., P. Kosterin, M. Ichikawa, and B. M. Salzberg. 2003. Activity-dependent depression of excitability and calcium transients in the neurohypophysis suggests a model of “stuttering conduction”. J. Neurosci. 23:11352–11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainer, H., S. A. Wolfe Jr., A. L. Obaid, and B. M. Salzberg. 1986. Action potentials and frequency-dependent secretion in the mouse neurohypophysis. Neuroendocrinology. 43:557–563. [DOI] [PubMed] [Google Scholar]
- 9.Salzberg, B. M., A. L. Obaid, and H. Gainer. 1985. Large and rapid changes in light scattering accompany secretion by nerve terminals in the mammalian neurohypophysis. J. Gen. Physiol. 86:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obaid, A. L., R. Flores, and B. M. Salzberg. 1989. Calcium channels that are required for secretion from intact nerve terminals of vertebrates are sensitive to omega-conotoxin and relatively insensitive to dihydropyridines. Optical studies with and without voltage-sensitive dyes. J. Gen. Physiol. 93:715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons, T. D., A. L. Obaid, and B. M. Salzberg. 1992. Aminoglycoside antibiotics block voltage-dependent calcium channels in intact vertebrate nerve terminals. J. Gen. Physiol. 99:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasa, K., I. Tasaki, and R. Gibbons. 1980. Swelling of nerve fibers associated with action potentials. Science. 210:338–339. [DOI] [PubMed] [Google Scholar]
- 13.Lettvin, J., W. Pitts, and O. Sten-Knudsen. 1962. Mechanical response in nerve. Quart. Report of the Research Lab for Electronics, M.I.T. 64:291–292.
- 14.Bustamante, C., C. Rivetti, and D. J. Keller. 1997. Scanning force microscopy under aqueous solutions. Curr. Opin. Struct. Biol. 7:709–716. [DOI] [PubMed] [Google Scholar]
- 15.Fisher, T. E., P. E. Marszalek, A. F. Oberhauser, M. Carrion-Vazqeuz, and J. M. Fernandez. 1999. The micro-mechanics of single molecules studied with atomic force microscopy. J. Physiol. 520:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadegaard, N. 2006. Atomic force microscopy in biology: technology and techniques. Biotech. Histochem. 81:87–97. [DOI] [PubMed] [Google Scholar]
- 17.Giocondi, M. C., P. E. Milhiet, E. Lesniewska, and C. Le Grimellec. 2003. Med. Sci. (Paris). 19:92–99 [Atomic force microscopy: from cellular imaging to molecular manipulation]. [DOI] [PubMed] [Google Scholar]
- 18.Hansma, H. G., and J. H. Hoh. 1994. Biomolecular imaging with the atomic force microscope. Annu. Rev. Biophys. Biomol. Struct. 23:115–139. [DOI] [PubMed] [Google Scholar]
- 19.Hansma, H. G., and L. Pietrasanta. 1998. Atomic force microscopy and other scanning probe microscopies. Curr. Opin. Chem. Biol. 2:579–584. [DOI] [PubMed] [Google Scholar]
- 20.Mosbacher, J., M. Langer, J. K. Horber, and F. Sachs. 1998. Voltage-dependent membrane displacements measured by atomic force microscopy. J. Gen. Physiol. 111:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parpura, V., and J. M. Fernandez. 1996. Atomic force microscopy study of the secretory granule lumen. Biophys. J. 71:2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar, A., R. B. Robertson, and J. M. Fernandez. 2004. Simultaneous atomic force microscope and fluorescence measurements of protein unfolding using a calibrated evanescent wave. Proc. Natl. Acad. Sci. USA. 101:12882–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, P. C., A. M. Keleshian, and F. Sachs. 2001. Voltage-induced membrane movement. Nature. 413:428–432. [DOI] [PubMed] [Google Scholar]
- 24.Kim, G. H., P. Kosterin, R. Lartius, A. L. Obaid, and B. M. Salzberg. 2007. High bandwidth atomic force microscopy reveals a mechanical spike during the action potential in mammalian nerve terminals. Biophys. J. 92:86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, G. H., P. Kosterin, R. Lartius, A. L. Obaid, and B. M. Salzberg. 2006. A mechanical spike during the action potential in mammalian nerve terminals monitored with an atomic force microscope. J. Gen. Physiol. 128:15a.16801381 [Google Scholar]
- 26.Rumyantsev, S. L., M. S. Shur, Yu Bilenko, P. V. Kosterin, and B. M. Salzberg. 2004. Low frequency noise and long-term stability of noncoherent light sources. J. Appl. Phys. 96:966–969. [Google Scholar]
- 27.Salzberg, B. M., P. V. Kosterin, M. Muschol, A. L. Obaid, S. L. Rumyantsev, Y. Bilenko, and M. S. Shur. 2005. An ultra-stable non-coherent light source for optical measurements in neuroscience and cell physiology. J. Neurosci. Methods. 141:165–169. [DOI] [PubMed] [Google Scholar]
- 28.Obaid, A. L., L. M. Loew, J. P. Wuskell, and B. M. Salzberg. 2004. Novel naphthylstyryl-pyridinium potentiometric dyes offer advantages for neural network analysis. J. Neurosci. Methods. 134:179–190. [DOI] [PubMed] [Google Scholar]
- 29.Agre, P. 2004. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 43:4278–4290. [DOI] [PubMed] [Google Scholar]
- 30.Agre, P., G. M. Preston, B. L. Smith, J. S. Jung, S. Raina, C. Moon, W. B. Gugino, and S. Nielsen. 1993. Aquaporin CHIP: the archetypal molecular water channel. Am. J. Physiol. 265:F463–F476. [DOI] [PubMed] [Google Scholar]
- 31.Preston, G. M., and P. Agre. 1991. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc. Natl. Acad. Sci. USA. 88:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ismailov, I. I., V. G. Shlyonsky, and D. J. Benos. 1997. Streaming potential measurements in alphabetagamma-rat epithelial Na+ channel in planar lipid bilayers. Proc. Natl. Acad. Sci. USA. 94:7651–7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bockris, J., and A. Reddy. 1973. Modern Electrochemistry: An Introduction to an Interdisciplinary Area, Vol. 2. Plenum, New York.
- 34.Cohen, L. B., R. D. Keynes, and D. Landowne. 1972. Changes in axon light scattering that accompany the action potential: current-dependent components. J. Physiol. 224:727–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen, L. B., R. D. Keynes, and D. Landowne. 1972. Changes in light scattering that accompany the action potential in squid giant axons: potential-dependent components. J. Physiol. 224:701–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muschol, M., B. R. Dasgupta, and B. M. Salzberg. 1999. Calcium and barium kinetics in mammalian nerve terminals during exocytosis. Biophys. J. 76:A400.
- 37.Brorson, J. R., D. Bleakman, S. J. Gibbons, and R. F. Miller. 1991. The properties of intracellular calcium stores in cultured rat cerebellar neurons. J. Neurosci. 11:4024–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llano, I., J. Gonzalez, C. Caputo, F. A. Lai, L. M. Blayney, Y. P. Tan, and A. Marty. 2000. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 3:1256–1265. [DOI] [PubMed] [Google Scholar]
- 39.Neering, I. R., and R. N. McBurney. 1984. Role for microsomal Ca storage in mammalian neurones? Nature. 309:158–160. [DOI] [PubMed] [Google Scholar]
- 40.Pessah, I. N., R. A. Stambuk, and J. E. Casida. 1987. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol. Pharmacol. 31:232–238. [PubMed] [Google Scholar]
- 41.Sharma, G., and S. Vijayaraghavan. 2003. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 38:929–939. [DOI] [PubMed] [Google Scholar]
- 42.Shmigol, A., P. Kostyuk, and A. Verkhratsky. 1995. Dual action of thapsigargin on calcium mobilization in sensory neurons: inhibition of Ca2+ uptake by caffeine-sensitive pools and blockade of plasmalemmal Ca2+ channels. Neuroscience. 65:1109–1118. [DOI] [PubMed] [Google Scholar]
- 43.Thayer, S. A., L. D. Hirning, and R. J. Miller. 1988. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol. Pharmacol. 34:664–673. [PubMed] [Google Scholar]
- 44.Usachev, Y., A. Shmigol, N. Pronchuk, P. Kostyuk, and A. Verkhratsky. 1993. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience. 57:845–859. [DOI] [PubMed] [Google Scholar]
- 45.Weber, A. 1969. The mechanism of action of caffeine on sarcoplasmic reticulum. J. Gen. Physiol. 52:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salzberg, B. M., S. D. Kraner, M. Muschol and A. L. Obaid. 1997. Calcium release from intraterminal stores plays a direct role in release from peptidergic nerve terminals in mammals: evidence from light scattering in mouse neurohypophysis. J. Gen. Physiol. 110:16A. [Google Scholar]
- 47.Muschol, M., B. R. Dasgupta, and B. M. Salzberg. 1999. Caffeine interaction with fluorescent calcium indicator dyes. Biophys. J. 77:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]