Abstract
Node-positive esophageal cancer is associated with a dismal prognosis. The impact of a solitary involved node, however, is unclear, and this study examined the implications of a solitary node compared with greater nodal involvement and node-negative disease. The clinical and pathologic details of 604 patients were entered prospectively into a database from1993 and 2005. Four pathologic groups were analyzed: node-negative, one lymph node positive, two or three lymph nodes positive, and greater than three lymph nodes positive. Three hundred and fifteen patients (52%) were node-positive and 289 were node-negative. The median survival was 26 months in the node-negative group. Patients (n = 84) who had one node positive had a median survival of 16 months (p = 0.03 vs node-negative). Eighty-four patients who had two or three nodes positive had a median survival of 11 months compared with a median survival of 8 months in the 146 patients who had greater than three nodes positive (p = 0.01). The survival of patients with one node positive [number of nodes (N) = 1] was also significantly greater than the survival of patients with 2–3 nodes positive (N = 2–3) (p = 0.049) and greater than three nodes positive (p < 0001). The presence of a solitary involved lymph node has a negative impact on survival compared with node-negative disease, but it is associated with significantly improved overall survival compared with all other nodal groups.
Keywords: Lymph node, Esophagectomy, Lymphadenectomy, Survival
Introduction
Carcinoma of the esophagus carries a dismal prognosis, and for patients presenting with localized resectable disease, multivariate analysis has established that the presence or absence of involved lymph nodes confers the greatest prognostic significance.1 In surgical management, the extent and type of lymphadenectomy undertaken varies from no formal lymphadenectomy to two and three field dissection.2–5 The presence and extent of lymph node involvement is important as selective approaches may be considered depending on the nodal stage at presentation. In early tumors, for instance, the sentinel node concept initially developed in melanoma and breast cancer was explored to help identify patients who may not require lymph node dissection.6–8 The advent of minimally invasive esophagectomy may also highlight the need to subselect patients for lymphadenectomy.9
In the observations of the senior author (JVR), patients with solitary involved lymph nodes may achieve good outcomes, and this hypothesis was evaluated in this analysis of a large prospective database. We report herein that the cohort with a solitary node involved had cancer outcomes closer to node-negative disease than other node-positive subgroups, and suggest that this represents a distinct prognostic subgroup.
Patients and Methods
The study population consisted of all patients with tumors of the esophagus and esophagogastric junction who underwent surgical resection, either alone or preceded by neoadjuvant chemoradiation, between 1993 and 2005. Patients receiving multimodal therapy received cisplatin, 5-fluorouracil, and external beam radiotherapy (40–44 Gy, 2–2.67 Gy/fraction) as previously described.10 Data concerning the clinical and pathologic parameters for all patients was obtained from a detailed prospective database maintained by a full-time data manager. Pathologic parameters analyzed included the location of the tumor, tumor morphology, i.e., adenocarcinoma or squamous cell carcinoma, histological differentiation (grade), TNM staging, number and site of involved lymph nodes, and R classification after surgical resection. Staging of tumors was performed according to the American Joint Committee on Cancer TNM system.11
A subtotal esophagectomy was performed with a sutured anastomosis either in the right thorax (two-stage) or neck (three-stage). All cases underwent a formal abdominal lymphadenectomy and mediastinal lymph node dissection up to and including the subcarinal nodes. Thoracic nodes were submitted separately to abdominal nodes.
Statistical Analysis
Data are presented as frequencies, means, and percentages. ANOVA was used for comparison of the four demographic groups. Survival probability was estimated using the Kaplan–Meier method. Survival was calculated from the date of clinical diagnosis to date of death or date last seen. In the multivariate analysis, independent prognostic factors for survival were determined by using a Cox regression hazard model. Two analyses were performed, one for all patients and the other exclusive to node-positive patients. All statistical analyses were performed using Stata software (version 9.1 for Windows, Statcorp, TX). A p value <0.05 was considered statistically significant.12
Results
Patients/histology
Six hundred and four patients underwent surgery for localized malignancy of the esophagus or esophagogastric junction. The mean age was 62 ± 10.4 (median = 64, range 56 to 70). Four hundred and twelve (68%) patients were men. The mean number of lymph nodes examined per specimen was 12 ± 6 (median = 10, range = 6 to 55). Two hundred and eighty-nine patients (48%) had node-negative disease [number of nodes (N) = 0], 84 (14%) had one node positive (N = 1), 84 had two or three nodes positive, and 147 (24%) had greater than three nodes positive (N > 3). In patients with one involved node, in all cases the node was adjacent to the tumor, mediastinal for esophageal tumors, and periesophageal or along the left gastric artery for junctional tumors (Tables 1 and 2).
Table 1.
Demographics of Nodal Subgroups
| Histologic Data | N = 0 (n = 289) | N = 1 (n = 84) | N = 2–3 (n = 84) | N > 3 (n = 147) |
|---|---|---|---|---|
| Tumor site (%) | ||||
| Lower esophagus | 138 (47) | 39 (46) | 37 (44) | 57 (39) |
| EG junction | 80 (28) | 35 (42) | 33 (39) | 75 (51) |
| Middle esophagus | 55 (19) | 10 (12) | 12 (14) | 11 (7) |
| Upper esophagus | 16 (6) | 0 | 2 (3) | 4 (3) |
| Morphology (%) | ||||
| Adenocarcinoma | 140 (48) | 51 (61) | 57 (68) | 113 (77) |
| Squamous cell carcinoma | 140 (48) | 29 (35) | 25 (30) | 32 (22) |
| Others | 9 (4) | 4 (5) | 2 (1) | 2 (1) |
| Treatment (%) | ||||
| Multimodal therapy | 129 (44) | 28 (33) | 24 (29) | 21 (14) |
| Surgery alone | 161 (56) | 56 (76) | 60 (71) | 125 (86) |
| Residual tumor (%) | ||||
| R0: no residual tumor | 250 (86) | 71 (85) | 64 (76) | 108 (73) |
| R1: residual tumor found | 39 (13) | 13 (15) | 19 (23) | 39 (27) |
| Rx: unknown | 1 (1) | – | 1 (1) | – |
| Pathological stage (%) | ||||
| Stage 0 | 53 (18) | – | – | – |
| Stage I | 59 (20) | 1 (1) | – | – |
| Stage II | 170 (59) | 21 (25) | 25 (30) | 16 (11) |
| Stage III | 5 (2) | 58 (29) | 53 (63) | 110 (76) |
| Stage IV | 1 (1) | 4 (5) | 6 (7) | 20 (13) |
| pT stage (%) | ||||
| Tx | 3 (1) | 0 | 2 (3) | 1 (0.5) |
| Tis | 12 (4) | 0 | 0 | 0 |
| T0 | 40 (14) | 1 (1) | 2 (3) | 2 (1) |
| T1 | 56 (19) | 5 (6) | 4 (5) | 3 (2) |
| T2 | 35 (12) | 16 (19) | 18 (21) | 12 (8) |
| T3 | 138 (48) | 60 (71) | 54 (64) | 120 (82) |
| T4 | 6 (2) | 2 (3) | 4 (5) | 8 (5) |
EG = esophagogastric
Table 2.
Histology of Nodal Subgroups
| Histologic Data | N = 0 | N = 1 | N = 2–3 | N > 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adeno | SCC | Adeno | SCC | Adeno | SCC | Adeno | SCC | |||||||||
| n = 140 | N = 140 | n = 51 | n = 29 | n = 57 | n = 25 | n = 113 | n = 32 | |||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | No | % | No | % | |
| Tumor site | ||||||||||||||||
| Lower Esophagus | 64 | (46) | 66 | (47) | 19 | (52) | 15 | (52) | 23 | (40) | 13 | (52) | 39 | (35) | 17 | (53) |
| EG Junction | 73 | (52) | 6 | (4) | 31 | (10) | 3 | (10) | 33 | (58) | 0 | 0 | 74 | (65) | 1 | (3) |
| Middle Esophagus | 3 | (2) | 52 | (37) | 1 | (28) | 8 | (28) | 1 | (2) | 10 | (40) | 0 | 0 | 10 | (31) |
| Upper Esophagus | 0 | 0 | 16 | (11) | 0 | (10) | 3 | (10) | 0 | 0 | 2 | (8) | 0 | 0 | 4 | (13) |
| Treatment | ||||||||||||||||
| Multimodal | 80 | (57) | 46 | (34) | 23 | (45) | 5 | (17) | 20 | (35) | 4 | (16) | 19 | (13) | 3 | (10) |
| Surgery alone | 60 | (43) | 93 | (66) | 28 | (55) | 24 | (83) | 37 | (65) | 21 | (84) | 94 | (87) | 28 | (90) |
| Path stage | ||||||||||||||||
| Stage 0 | 29 | (21) | 18 | (13) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stage 1 | 42 | (30) | 15 | (10) | 1 | (2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stage 2 | 66 | (47) | 102 | (73) | 15 | (29) | 4 | (14) | 19 | (33) | 5 | (20) | 15 | (13) | 1 | (3) |
| Stage 3 | 2 | (1) | 4 | (3) | 32 | (63) | 24 | (83) | 35 | (61) | 17 | (68) | 82 | (73) | 27 | (84) |
| Stage 4 | 0 | 0 | 1 | (1) | 3 | (6) | 1 | (3) | 3 | (6) | 3 | (12) | 16 | 914) | 3 | (10) |
| Unknown | 1 | (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | (3) |
| pT stage | ||||||||||||||||
| Tx | 2 | (1) | 1 | (1) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | (8) | 0 | 0 | 1 | (3) |
| Tis | 9 | (6) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | (4) | 0 | 0 | 0 | 0 | 0 | 0 |
| T0 | 19 | (14) | 17 | (12) | 1 | (2) | 0 | 0 | 3 | (5) | 0 | 0 | 2 | (2) | 0 | 0 |
| T1 | 39 | (29) | 15 | (11) | 4 | (8) | 0 | 0 | 14 | (24) | 0 | 0 | 3 | (3) | 0 | 0 |
| T2 | 16 | (11) | 19 | (14) | 12 | (23) | 3 | (10) | 36 | (63) | 4 | (16) | 11 | (10) | 1 | (3) |
| T3 | 53 | (38) | 84 | (60) | 33 | (65) | 26 | (90) | 2 | (4) | 17 | (68) | 91 | (80) | 28 | (88) |
| T4 | 2 | (1) | 4 | (2) | 1 | (2) | 0 | 0 | 0 | 0 | 2 | (8) | 6 | (5) | 2 | (6) |
Adeno = adenocarcinoma, SCC = small cell carcinoma, EG = esophagogastric
Two hundred and two patients (33%) had multimodal therapy and 402 patients (67%) had surgery alone. Of the multimodal cohort, 129 (64%) were ypN0 on histopathologic assessment, 28 (14%) had one node positive, 24 (12%) had two to three positive nodes, and 21 (10%) had greater than three positive nodes. The attainment of an R0 resection was significantly greater in patients with none or one node involved compared with both other groups (p < 0.05). The majority of patients in all groups had pT3 tumors, 48% in the pN0 group compared with 71, 64, and 82% in the N = 1, N = 2–3, and N > 3 groups, respectively (p < 0.05). One hundred and forty (62%) of the squamous cell carcinoma cohort were node-negative (N = 0) compared with 140 (39%) of cases with adenocarcinoma (39%) (p < 0.05).
Survival
The median survival for all patients was 20 months at a median follow-up of 19 months (3–167). Patients who were node-negative (N = 0) had a median survival of 26 months (Table 3), compared with 16 months when one node was positive (p = 0.03). Patients who had two to three nodes positive had a median survival of 11 months, and 8 months in patients who had greater than three nodes positive (p = 0.01; N = 2–3 vs N > 3). The survival of patients with one node positive (N = 1) was significantly greater than the survival of patients with 2–3 nodes positive (p = 0.04) and the cohort with greater than three involved nodes (p < 0.0001).
Table 3.
Univariate and Multivariate Analysis: All Patients
| Variables | No. of Patients | Median Survival (moths) | p Valuea (Univariate) | HR | 95% CIa | p valueb (Multivariate) | HR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| Surgery only | 401 | 13 | 0.077 | 1 | – | – | – | |
| Multimodal | 203 | 19 | 0.84 | 0.69–1.02 | ||||
| Tumor site | ||||||||
| Upper esophagus | 25 | 16 | 0.371 | 1 | – | – | – | |
| Middle esophagus | 87 | 14 | 0.946 | 0.98 | 0.58–1.66 | |||
| Lower esophagus | 268 | 14 | 0.658 | 1.16 | 0.69–1.81 | |||
| EG junction | 224 | 14 | 0.624 | 1.13 | 0.69–1.84 | |||
| Depth of invasion | ||||||||
| T0 | 57 | 55 | <0.001 | 1 | 0.652 | 1 | ||
| T1 | 68 | 26 | 0.537 | 1.16 | 0.73–1.83 | 0.472 | 0.71 | 0.21–2.3 |
| T2 | 81 | 26 | 0.419 | 1.20 | 0.77–1.85 | 0.573 | 1.11 | 0.31–3.94 |
| T3 | 373 | 11 | <0.001 | 2.28 | 1.60–3.26 | 0.871 | 1.40 | 0.79–2.41 |
| T4 | 19 | 7 | <0.001 | 4.34 | 2.46–7.68 | 0.649 | 2.59 | 1.42–4.08 |
| No. of nodes | ||||||||
| 0 | 289 | 26 | <0.001 | 1 | <0.001 | 1 | 0.63–1.87 | |
| 1 | 84 | 16 | 0.038 | 1.36 | 1.02–1.82 | 0.774 | 1.08 | 0.83–2.43 |
| 2–3 | 84 | 11 | <0.001 | 1.91 | 1.45–2.52 | 0.202 | 1.42 | 1.07–3.18 |
| >3 | 147 | 8 | <0.001 | 2.61 | 2.08–3.29 | 0.027 | 1.84 | |
| Histology | ||||||||
| Squamous | 361 | 14 | 0.916 | 1 | ||||
| Adenocarcinoma | 224 | 13 | 0.596 | 1.05 | 0.87–1.28 | – | – | – |
| Other | 19 | 26 | 0.483 | 0.80 | 0.44–1.48 | |||
| Stage | ||||||||
| 0 | 53 | 55 | <0.001 | 1 | 0.118 | 1 | ||
| I | 63 | 55 | 0.747 | 0.92 | 0.56–1.51 | 0.576 | 0.68 | 0.18–2.59 |
| II | 230 | 20 | 0.037 | 1.49 | 1.02–2.17 | 0.508 | 1.55 | 0.42–4.69 |
| III | 225 | 10 | <0.001 | 2.71 | 1.86–3.95 | 0.527 | 1.68 | 0.34–5.58 |
| IV | 31 | 6 | <0.001 | 6.16 | 3.72–10.2 | 0.182 | 3.14 | 1.14–7.76 |
| Residual tumor | ||||||||
| R0 | 492 | 17 | <0.001 | 1 | 0.052 | 1 | ||
| R1 | 110 | 8 | 1.70 | 1.37–2.12 | 1.25 | 0.99–1.58 | ||
aχ2
bCox regression
HR = hazard ratio, CI = 95% confidence intervals, EG = esophagogastric
The 1-, 3-, and 5-year survival of the pN0 group was 78, 51, and 44%, respectively (Fig. 1). Where one node was involved, survival was 67, 41, and 35%, respectively. Where two to three nodes were involved, the 1-, 3-, and 5-year survival was 57, 25, and 13%, respectively, and where greater than three nodes were involved, this was 40, 14, and 8%, respectively.
Figure 1.
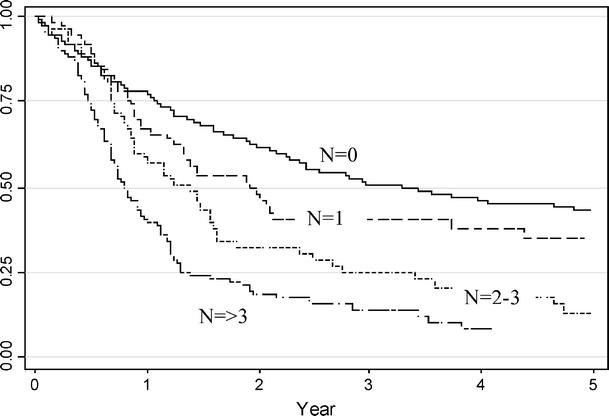
Overall survival by number of nodes positive.
Univariate analysis (Table 3) revealed nodal status, pT stage, pathologic stage, and R status as predictors of survival. Multivariate analysis revealed nodal status alone to significantly (p < 0.0001) impact on survival. By this analysis the hazards ratio increased from 1.08 for one involved node to 1.42 for two to three involved nodes, and 1.84 for greater than three nodes.
Excluding node-negative patients, univariate analysis (Table 4) revealed pT stage, pathologic stage, R status, and number of nodes as predictive of survival. By multivariate analysis (Table 5), pathologic stage (p = 0.010) and number of nodes were significant determinants of survival. Compared with the cohort with one involved node, the hazard ratio for two to three nodes was 1.56 (p = 0.049) and 2.06 (p = 0.007) for greater than three nodes.
Table 4.
Univariate Analysis: Node-positive Alone
| Variables | No. of Patients | Median Survival (moths) | p valuea (Univariate) | HR | 95% CI |
|---|---|---|---|---|---|
| Treatment | |||||
| Surgery only | 241 | 11 | 0.234 | 1 | 0.63–1.11 |
| Multimodal | 74 | 11 | 0.84 | ||
| Tumor site | |||||
| Upper esophagus | 9 | 18 | 0.650 | 1 | |
| Middle esophagus | 32 | 10 | 0.556 | 1.31 | 0.54–3.18 |
| Lower esophagus | 130 | 10 | 0.183 | 1.75 | 0.77–3.98 |
| OG junction | 144 | 12 | 0.350 | 1.48 | 0.65–3.36 |
| Depth of invasion | |||||
| T0 | 5 | 11 | 0.001 | 1 | |
| T1 | 12 | 8 | 0.917 | 1.06 | 0.33–3.41 |
| T2 | 46 | 24 | 0.176 | 1.12 | 0.43–1.78 |
| T3 | 235 | 11 | 0.757 | 1.43 | 0.74–2.14 |
| T4 | 14 | 5 | 0.157 | 2.23 | 0.74–6.78 |
| Histology | |||||
| Squamous | 86 | 11 | 0.638 | 1 | |
| Adenocarcinoma | 221 | 11 | 0.638 | 1.07 | 0.81–1.40 |
| Other | 8 | 3 | 0.848 | 1.07 | 0.49–2.35 |
| Stage | |||||
| 1–II | 63 | 19 | <0.001 | 1 | |
| III–IV | 251 | 10 | 2.01 | 1.43–2.83 | |
| Residual tumor | |||||
| R0 | 259 | 12 | 0.035 | 1 | |
| R1 | 61 | 9 | 1.33 | 1.02–1.73 | |
| No. of nodes | |||||
| 1 | 84 | 17 | <0.001 | 1 | |
| 2–3 | 84 | 13 | 0.021 | 1.67 | 1.06–2.29 |
| >3 | 147 | 9 | <0.001 | 2.53 | 1.50–3.62 |
aχ2
HR = hazard ratio, CI = 95% confidence intervals
Table 5.
Mutivariate Analysis: Node-positive Only
| Variables | p valuea (Multivariate) | HR | 95% CI |
|---|---|---|---|
| Depth of invasion | |||
| T0 | 1 | ||
| T1 | 0.544 | 0.82 | 0.31–1.75 |
| T2 | 0.679 | 1.23 | 0.74–1.81 |
| T3 | 0.313 | 1.49 | 0.99–2.21 |
| T4 | 0.202 | 1.83 | 1.39–3.24 |
| Stage | |||
| I–II | 0.010 | 1 | |
| III–IV | 1.59 | 0.82–3.06 | |
| No. of nodes | |||
| 1 | 1 | ||
| 2–3 | 0.049 | 1.56 | 1.21–2.35 |
| >3 | 0.007 | 2.06 | 1.51–2.82 |
| Residual tumor | |||
| R0 | 0.283 | 1 | |
| R1 | 1.22 | 0.80–1.79 | |
aCox regression
HR = hazard ratio, CI = 95% confidence intervals
Discussion
Cancers of the esophagus and esophagogastric junction are aggressive tumors, which are typically diagnosed at an advanced stage of disease progression.13 This large retrospective review of a tertiary center’s experiences over 12 years highlights the importance of lymph node involvement in the prognosis of these tumors. The study shows that the presence of a solitary node, although a significantly negative factor compared with pN0 disease, is associated with significantly improved median and 1-, 3-, and 5-year survival compared with cohorts of patients with greater nodal involvement. The 5-year survival, for instance, was 35% compared with 13 and 8%, respectively, for cohorts with two to three positive nodes and greater than three positive nodes.
There is no uniform consensus on the number of lymph nodes that must be sampled. In a study by Ito et al.,3 the median number of lymph nodes examined per specimen was 6 (range 0 to 35) and only 20% of patients had at least 15 lymph nodes examined. In this study, the median number of lymph nodes examined per specimen was 12 (range 6 to 55), and 24% of the patients had at least 15 lymph nodes examined. These results appear consistent with practice in the United States where an analysis of the National Cancer Database indicated that only 18% of patients undergoing surgery for gastric cancer have more than 15 lymph nodes analyzed.14 In this Unit, lymph node clearance involves a D2 dissection of abdominal nodes, and wide mediastinal clearance to the carina and paratracheal node dissection if they appear involved. No cervical dissection is performed, consistent with recommendations from another group.15 It is acknowledged that variation in lymph node yield may mask stage migration, particularly in a retrospective analysis, but the standardization of lymphadenectomy is likely to minimize the impact of this potential bias.
The association between extent of nodal involvement and outcome is well described.16–18 No study to our knowledge has previously focused on the impact of one positive node on outcome in esophageal cancer. The observation, however, of the unique prognostic significance of a solitary involved node was recently reported.19 In a study of 187 patients with esophageal adenocarcinoma treated with neoadjuvant chemoradiotherapy, Gu et al.19 at the MD Anderson observed from their analysis that patients with a solitary involved node had better overall and relapse-free survival compared with other nodal groups. Moreover, the 5-year survival outcomes and 2-year relapse-free survival was not significantly different from the node-negative cohort. Although in our series survival figures were better for node-negative patients than patients with a solitary involved node, the overall pattern of outcome data in our series is consistent with the report from the Anderson group, with prognosis in this cohort closer to node-negative than other node-positive subgroups.
The clinical implication of this finding is not clear at this time, but it should, at minimum, encourage a more optimistic view of patients who have a solitary lymph node identified after adequate lymphadenectomy, as approximately 35% of patients with this pathologic stage may be cured. In the future, it is possible that advances in endoscopic US staging, fluorodeoxyglucose PET, and sentinel node assessment may improve pre- and intraoperative assessment of nodal involvement, defining node-negative, solitary involved node and micrometastatic-involved subgroups, and selective lymphadenectomy and minimally invasive approaches may be evaluated in these situations. This demands prospective evaluation, but it may be noteworthy that all involved nodes in the solitary involved node cohort were close to the primary site and may possibly have been identified as sentinel nodes.
In conclusion, this study shows that in a large cohort of patients, lymph node status and the number of lymph nodes positive at the time of surgical resection is directly linked to survival. Extensive nodal involvement is confirmed as carrying a dismal prognosis, but greater optimism is justified where a solitary involved lymph gland defines the pN stage after an adequate lymphadenectomy.
References
- 1.Roder JD, Busch R, Stein HJ, et al. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the esophagus. Br J Surg. 1994;81:410–413. [DOI] [PubMed]
- 2.HulscherJB, Van Sandick JW, De Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed]
- 3.Ito H, Clancy TE, Osteen RT, et al. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg. 2004;199: 880–886. [DOI] [PubMed]
- 4.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581–587. [DOI] [PMC free article] [PubMed]
- 5.Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: Analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520–531. [DOI] [PMC free article] [PubMed]
- 6.Cox CE, Pendas S, Cox JM, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227:645–651. [DOI] [PMC free article] [PubMed]
- 7.Lamb PJ, Griffin SM, Burt AD, et al. Sentinel node biopsy to evaluate the metastatic dissemination of oesophageal adenocarcinoma. Br J Surg. 2005;92:60–67. [DOI] [PubMed]
- 8.Burian M, Stein HJ, Sendler A, et al. Sentinel node detection in Barrett’s and cardia cancer. Ann Surg Oncol. 2004;11:255S–258S. [DOI] [PubMed]
- 9.Luketich JD, Alvero-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: Outcomes in 222 patients. Ann Surg. 2003;238:486–494. [DOI] [PMC free article] [PubMed]
- 10.Walsh TN, Noonan N, Hollywood D, Kelly A, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. [DOI] [PubMed]
- 11.Digestive system: The esophagus. In: AJCC Cancer Staging Manual, 5th ed. Philadelphia: Lippincott, Williams and Wilkins, 1997, pp. 65–67.
- 12.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:197–219.
- 13.Siewert JR, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: Results of surgical therapy based on anatomical/topographical classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. [DOI] [PMC free article] [PubMed]
- 14.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [DOI] [PubMed]
- 15.Dresner SM, Wayman J, Shenfine J, et al. Pattern of recurrence following subtotal oesophagectomy with two field lymphadenectomy. Br J Surg. 2000;87:362–373. [DOI] [PubMed]
- 16.Akiyama H, Tsurumara M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364–372. [DOI] [PMC free article] [PubMed]
- 17.Eloubeidi MA, Desmond R. Prognostic factors for the survival of patients with esophageal cancer in the US. Cancer. 2002;95:1434–1443. [DOI] [PubMed]
- 18.Hsu CP, Chen CY, Hsia JY, Shai SE. Prediction of prognosis by the extent of lymph node involvement in squamus cell carcinoma of the thoracic esophagus. Eur J Cardio-thorac Surg. 2001;19:10–13. [DOI] [PubMed]
- 19.Gu Y, Swisher SG, Ajani JA, Correa AM. et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer. 2006;106:1017–1025. [DOI] [PubMed]


