Abstract
Macroscopic vascular invasion (macroVI) is associated with poor outcomes after liver transplantation (LT) for hepatocellular carcinoma (HCC). Whether microvascular invasion (microVI) is associated with the same adverse prognosis is unclear. One hundred and fifty-five consecutive patients with confirmed HCC after LT from March 1991 to 2004 at our institution were reviewed. Patients had to satisfy Milan criteria to be accepted for LT. They were followed with surveillance images every 3 months while on the waiting list. Disease-free survival (DFS) and overall survival (OS) were evaluated by Kaplan–Meier analysis. Demographic, tumor, and histopathologic characteristics were tested for their prognostic significance. Median follow-up after LT was 30 months. Overall graft survival rates were 87, 74, and 65% at 1, 3, and 5 years, respectively. All recurrences (22/155, 14%) developed within 4 years after LT with an overall 5-year DFS of 79%. Vascular invasion, either microVI or macroVI, was more likely in patients with multicentric HCC (n ≥ 3, p < 0.001) and larger tumor size >4 cm (p = 0.04). Tumor size >5 cm (p = 0.04), advanced pathological TMN stage (p = 0.007), microVI (p = 0.001), and macroVI (p < 0.001) predicted poor tumor-free survival on univariate analysis, but only macroVI was significant in multivariate analysis (hazard ratio 54.2, 95% confidence interval 11, 266). Furthermore, only macroVI was a significant predictor of mortality after LT (p = 0.01). Macrovascular invasion is strongly associated with high rates of recurrence and diminished survival after LT whereas microVI is not an independent risk factor.
Keywords: Hepatocellular carcinoma, Vascular invasion, Liver transplantation, Recurrence, Microvascular invasion
Introduction
In 1996, Mazzaferro et al.1 documented excellent survival results after liver transplantation (LT) in a highly selected group of patients with hepatocellular carcinoma (HCC) with a single tumor <5 cm or as many as three tumors, each smaller than 3 cm. Recent studies have shown that selected patients who do not meet these criteria can still be cured with a transplant; the challenge now is to decide which factors, other than size and number, carry a sufficiently poor prognosis to deny transplantation.2–5
Gross vascular invasion or radiological evidence of tumor invasion in major veins is a known determinant of poor outcome after resection or transplantation for HCC and is an absolute contraindication to LT.4–13 Macrovascular invasion (macroVI) or gross vascular invasion of major portal or hepatic veins evident visually at time of transplant or on pathological evaluation may also be a predictor of recurrence after LT.14 Whether microvascular invasion (microVI), defined as microscopic tumor invasion in smaller intrahepatic vessels identified on pathologic analysis, or macroVI should also be considered a contraindication to LT is controversial. Both types of tumor invasion are difficult to determine pre-LT; therefore, their significance remains uncertain.
Several published studies have found histopathological factors, namely, poorly differentiated grade, microVI, and macroVI, to be independent predictors of poor survival after LT.4,11,15–17 We have previously shown that microVI is an independent predictor of early recurrence after resection for HCC.5 The purpose of this study was to determine if microVI, macroVI, and other pathological factors are associated with tumor recurrence after LT and examine the outcomes thereafter. If these factors are predictors of poor tumor-free survival, then how can we attempt to identify these variables before transplant?
Patients and Methods
One hundred fifty-nine consecutive patients with confirmed HCC on liver explant pathology after LT from October 1991 to October 2004 at our institution were reviewed from a prospective database. The cohort includes 32 patients (20%) with incidental tumors. Patients had to satisfy Milan criteria and have no radiologic evidence of gross vascular invasion to be accepted for LT. Four patients were omitted from analysis for recurrent hepatitis B virus (HBV) before antiviral therapy; after 1991, hepatitis B immune globulin, lamivudine, or combination therapy was used. Patients with known HCC were followed every 3 months while on the waiting list with either an ultrasound or triphasic computed tomography (CT). Patients with HCC were eligible for deceased donor whole organs (n = 149) and split (n = 0) or living donor LT (n = 6).
Patient, tumor, operative, and treatment characteristics were evaluated using a prospective clinical database and review of all pathological explants. The stage, size, and histopathology of the tumor were determined by analysis of the liver explant. Patients were staged based on the explanted specimens using the TNM staging classification of the American Liver Tumor Study Group18. Tumor size was measured as the largest diameter of the major tumor in centimeters. MacroVI was defined as gross vascular invasion into major portal vessels or hepatic veins identified either intraoperatively or on pathologic explant, whereas microVI was determined on pathologic analysis as microscopic vascular invasion of small vessels within the parenchyma of the liver. Pretransplant therapy was used in selective cases; if waiting time was determined to be longer than 6 months, patients commonly underwent radio frequency ablation as a bridge to LT. This was performed in an attempt to keep patients within Milan criteria while on the LT waiting list. Postoperative immunosuppression was similar in all patients and consisted of cyclosporine or tacrolimus and steroids.
After LT, patients were followed with Q3 monthly alpha-fetoprotein (AFP) and CT along with standard post-LT evaluation. Recurrences were defined as new nodules diagnosed by CT with confirmed biopsy in most cases. Overall survival (OS) was death as a result of any cause after LT. Patients were followed until death or study closure (arbitrarily denoted as October 1, 2004). Data was collected until May 1, 2005 to ensure at least 6 months of follow-up for all patients.
Statistical Package for Social Sciences software (SPSS, Inc., Chicago, IL) was used for data analysis. Statistical comparison of categorical and continuous variables was performed using the χ2 test and Mann–Whitney U test, respectively. All data was reported as median with range, mean ± SD or interquartile range (IQR) when appropriate. Analysis of patient OS and disease-free survival (DFS) was performed according to the Kaplan–Meier method. Patient survival in different groups was compared using the log-rank test. All variables with a p value less than 0.1 were then included in a multivariate analysis applying the Cox multiple backward stepwise model.
Results
Patient Characteristics
Of the 1,070 LTs performed during the 13-year period from 1991 to 2004, 159 patients (14%) had HCC. After omission of 4 patients because of recurrent HBV before the antiviral therapy era, 155 patients were included in this analysis (Table 1). The average waiting time for LT was 7 months. There was no perioperative mortality. The median age was 57 years (range 28–70) and the majority of patients were men (79%). The median age of all patients who underwent LT for any cause was 51 years (range 16–71). The most common causes of end-stage liver disease and HCC were hepatitis C virus (HCV) (n = 79; 51%), alcoholic liver disease (n = 34; 22%), and HBV (n = 25; 16%). Selected patients underwent pre-LT therapy, most commonly radio frequency or percutaneous ethanol ablation (ablation, 23%), but the majority of patients received no treatment before LT (72%). Use of ablation did not result in any adverse outcomes in this series of patients who underwent eventual LT.
Table 1.
Pretransplant Demographics of 155 Patients with HCC Who Underwent LT
| Pretransplant Criteria | N = 155 | p Value |
|---|---|---|
| Median age (range) | 57 (28–70) | 0.48 |
| Sex | 0.38 | |
| Male | 123 (79%) | |
| Female | 32 (21%) | |
| Cause of liver disease | 0.10 | |
| HCV | 79 (51%) | |
| HBV | 25 (16%) | |
| Alcohol | 34 (22%) | |
| Cryptogenic | 7 (4.5%) | |
| Alpha-1 antitrypsin | 6 (3.9%) | |
| NASH | 2 (1.3%) | |
| PBC | 2 (1.3%) | |
| Pretransplant therapy | 0.67 | |
| Ablation | 36 (23%) | |
| Resection | 6 (3.9%) | |
| TACE | 1 (0.6%) | |
| EBRT | 1 (0.6%) | |
| None | 111 (72%) |
The p values were determined by log-rank test as predictor of DFS after Kaplan–Meier analysis.
NASH = nonalcoholic steatohepatitis, PBC = primary biliary cirrhosis, EBRT = external beam radiation therapy
Histopathological Analysis
The pathologic features for the 155 explants are shown in Table 2. The median number of tumors was 2 (range 1–20) and 18% were bilobar. The median size of the largest tumor was 2.6 cm and most tumors were graded as well or moderately differentiated (74%). Gross macroVI was evident in 3.9% (6/155) of the explants on pathologic examination, whereas 21% (33/155) of explants had the presence of microVI. Using the pathological TNM classification, 31 patients (20%) had stage I tumors, 69 patients (44%) had stage II tumors, 26 patients had stage III tumors (17%), and 29 patients had stage IV tumors (19%). Patterns of advanced stage were most often because of multifocal HCC or three or greater in number (40%).
Table 2.
Pathologic Characteristics of 155 Liver Explants
| Characteristic | N = 155 | p Value | HR (95% CI) |
|---|---|---|---|
| No. of tumors | |||
| Median (range, cm) | 2 (1–20) | 0.23 | |
| <3 | 126 (81%) | ||
| >3 | 29 (19%) | ||
| Bilobar | 32 (21%) | 0.15 | |
| Size | |||
| Median (range; cm) | 2.6 (.1–16) | 0.04 | 0.47 (0.14,1.61) |
| <5 cm | 145 (94%) | ||
| >5 cm | 15 (10%) | ||
| Stage | |||
| I | 31 (20%) | 0.007 | 1.17 (0.39,3.50) |
| II | 69 (44%) | ||
| III | 26 (17%) | ||
| IV | 29 (19%) | ||
| Positive lymph nodes | 3 (2.0%) | 0.31 | |
| Vascular invasiona | |||
| Microscopic | 33 (21%) | 0.001 | 3.16 (0.92,10.93) |
| Macroscopic | 6 (4%) | <0.001 | 54.2 (11.03,266.4) |
| None | 121 (78%) | b | 1.0c |
| Grade | |||
| Well/mod | 115 (74%) | 0.36 | |
| Poor | 13 (9%) | ||
| N/Ad | 27 (17%) | ||
| Margins | |||
| Positive | 2 (1%) | 0.25 | |
| Negative | 153 (99%) | ||
| Incidental tumor | 34 (22%) | 0.19 |
The p values were determined by chi-square test or log-rank test of variables after Kaplan–Meier analysis (univariate). HR (95% CI) represents multivariate analysis of factors affecting recurrence after resection.
aFive of six patients had characteristics of both microvascular and macrovascular tumor invasion.
bReference category for comparison
cReference category for each categorical variable is assigned HR = 1.0.
dNot available in the analysis
Vascular invasion was associated with both the number and size of tumors. Liver explants, with either microVI or macroVI, had more tumors (3.8 vs 2.0, p < 0.001) and were larger in size (3.6 cm vs 2.8 cm, p = 0.04) compared to those without vascular invasion. Specifically, if microVI or macroVI was present on histopathology, 64 and 100% of the tumors, respectively, were outside of the Milan criteria or were TNM stage III or IV. Histological grade was not associated with either type of vascular invasion; 85% of patients with either type of vascular invasion had favorably differentiated tumors. Ablation did not affect the rate of vascular invasion, either microVI or macroVI, in this series. Eleven of 33 patients (33%) with vascular invasion on explant analysis had undergone ablation of their tumor.
Predictors of Recurrence and Survival
The 5-year disease-specific survival was 79% (Fig. 1a). All recurrences developed within 44 months after LT. The 1-, 3-, and 5-year overall graft OS rates were 87, 74, and 65%, respectively (Fig. 1b). Only 22 patients (14%) developed tumor recurrence after LT with a median follow-up of 30 months (range 6–144 months). Eighty-six percent of patients (122/155) are currently alive and free of cancer. There was no difference in OS in patients with incidental tumors compared to those with known HCC (data not shown), but a significant difference was observed in DFS (5-year OS rate 94 vs 74%, p = 0.02).
Figure 1.

(A) Disease-free survival after LT for HCC of 155 patients. (B) Overall graft survival after LT for HCC of 155 patients.
The median time to recurrence for the 22 patients who developed recurrent HCC was 16.3 months (IQR 8.0–28). Tumor size >5 cm (p = 0.04), pathological TMN stage (p = 0.007), microVI (p = 0.001), and macroVI (p < 0.001) were found to be independent predictors of tumor-free survival. Patients with tumor size of 5 cm or larger had a 5-year DFS of 48 vs 82% for those with tumors smaller than 5 cm. Advanced TMN stage was also associated with poor recurrence-free survival after LT (Fig. 2). Twenty-one of the 22 patients (95%) who developed HCC recurrence were found to have either microVI (n = 15) or macroVI (n = 6) on pathological analysis. This accounts for 68% (15/22) of all patients with microVI and 100% (6/6) of patients with macroVI in the entire study. Patients with macroVI had a median DFS of only 7.1 months compared to a more favorable DFS in patients with microVI or no vascular invasion (median not achieved) (Fig. 3). No significant differences in DFS based on age, sex type, hepatitis status, tumor grade, bilobar disease, tumor number, ablative therapy, or type of transplant were found on univariate analysis. Pre-LT therapy did not result in any improvement in DFS or OS after LT (data not shown).
Figure 2.

The effect of TNM stage on DFS after LT for HCC.
Figure 3.
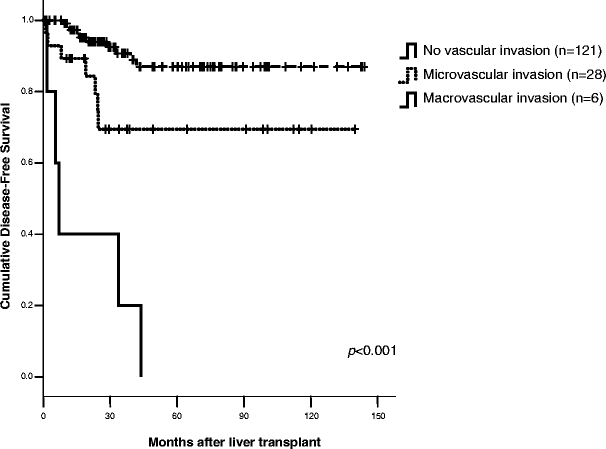
The effect of vascular invasion on recurrence-free survival after LT for HCC.
In a multivariate analysis including factors with an influence on DFS, only macroVI (hazard ratio [HR] 54.2, 95% CI 11.03–266.4) was identified to be predictive (Table 2). This was confirmed with a Cox backward stepwise model of multivariate analysis (data not shown).
Discussion
In this study, we report our experience with LT for HCC with a specific focus on pathological factors affecting long-term outcomes. Overall survival rates of LT for HCC were 87, 74, and 65% at 1, 3, and 5 years, respectively, with a median follow-up time of 30 months. The overall recurrence rate was 14% with 79% 5-year disease-specific survival. Patients with incidental tumors had similar OS rates as those with known tumors consistent with previous reports,8,9,14,15,19 but in this study, a significant difference was found in DFS. Our studies suggest that large tumor size (>4 cm) and multiple tumors (≥3) correlate with an increased incidence of vascular invasion and may provide a surrogate marker for entities that are often difficult to detect before LT.
Recurrence rates after LT may not simply reflect only size and number as suggested in the initial Milan series, but may be a complex interplay of host- and tumor-related factors, which are still largely unknown.20 This report suggests that tumor grade, size, number, and microVI do not influence outcome after LT for HCC; only the presence of macroVI appears to be associated with poor outcomes on multivariate analysis. MacroVI and microVI were more commonly found in multicentric (three or more) and large (>4 cm) HCC. Furthermore, 21 of 22 recurrences had evidence of either microVI or macroVI on pathologic examination. Because we are unable to identify these biologic factors preoperatively, markers of histopathologic or biologic variables that predict poor outcomes are extremely important.14,20–22
MacroVI was shown to be an independent predictor of tumor recurrence after LT in some studies.4,9,10,14 The Pittsburgh group found that microVI and major vascular invasion was associated with increased risk of recurrence by multivariate analysis.4,8 In a report of 344 patients with HCC treated by LT, microVI and macroVI were associated with 4.4- and 15-fold increased risk of recurrence, respectively.8 Shetty et al.14 found that macroVI, but not microVI, was a significant predictor of DSF and OS after LT for HCC. In this study, microVI is associated with higher stage and recurrent tumors, but does not appear to be an independent factor for survival.
The role of microVI on posttransplant recurrence and survival outcomes for HCC still remains unclear. Several published studies have found poorly differentiated histological grade or microVI to be independent predictors of impaired survival after LT.11,15–17 Jonas et al.11 found vascular invasion and histological grade to be the only statistically significant independent predictors of poor survival after LT in 120 patients. In their study, only poorly differentiated tumors larger than 5 cm predicted the presence of microVI. But other studies involving LT and HCC were not able to corroborate poor results regarding microVI.3,14,23,24 The close relationship between histological grade and microVI may explain why microVI is often eliminated in multivariate models for analyzing tumor recurrence prognostic factors that include histological grading and vice versa.4,8,11,15,23,25,26 Multiple tumors, larger tumors, and higher grade of differentiation have all been shown to be associated with microVI after resection for HCC. Esnaola et al.13 reported that tumor size greater than 4 cm and poorly differentiated/undifferentiated histopathologic grade increased the odds of microVI by 3 and 6.3-fold, respectively, but these tumors were primarily in Child class A cirrhotic.
The degree of fibrosis and scarring of the liver may play a significant role in the biological behavior and significance of microVI and macroVI. We have previously shown that microVI was a significant predictor of early recurrence and death after resection for HCC in cirrhotic patients.5 Most recurrences were intrahepatic and away from the staple line, suggesting that liver mobilization and manipulation may cause progression of microVI or a new tumor has developed in the presence of ongoing oncogenic stimulus from cirrhosis. For these reasons, prognostic factors for LT from resection studies should be interpreted with caution and a possible rationale why microVI is so important after resection but not after LT.12,13 Lack of manipulation of the liver and intrahepatic dissection may be a potential explanation for the lack of importance of microVI with LT.
Because of the importance of histological features of HCC, some have advocated pre-LT biopsy to examine grade, vascular invasion, and genetic typing.13,15,20 Complications of needle biopsy such as tumor tract seeding and lack of sensitivity have made routine biopsy unfavorable.27 In our experience, needle biopsy was a poor predictor of microVI or macroVI, and therefore was not considered in our pre-LT work-up for HCC (data not shown).
There are several limitations to this study and therefore some of the results should be interpreted with caution. The need to standardize grading systems for HCC has long been recognized and would allow us to determine if tumor grade is indeed an important prognostic marker for recurrence and survival. Few tumors in this study were graded as poorly differentiated; moreover, in 27 patients, their grade was not determined. Results from histopathological analysis are often met with inherent biases from the pathologist and comprehensive evaluation of the whole liver explant may vary among pathologists and institutions. Finally, with very few tumors containing macroVI, strong conclusions about prognostic characteristics concerning macroVI cannot be made in this report. Whether microVI is a harbinger of macroVI or in some way correlated with a more aggressive form of HCC remains unclear.
The use of pathological and biological features of the tumor may allow us to identify those patients who are at increased risk of recurrence; then these patients should be considered for adjuvant therapy before evidence of a recurrence. Because vascular invasion is more common in multicentric (>3) HCC or large tumors (>4 cm), we propose shortening the interval of pre-LT imaging in patients with these tumors to identify vascular invasion or rapid growth of the tumor before LT. Genetic testing of tumors before LT may be a novel method to predict other prognostic factors affecting recurrence.20 Several studies have reported improved posttransplant survival in HCC patients with transcatheter arterial chemoembolization (TACE) or systemic therapy,28–31 but overall results for adjuvant chemotherapy post-LT were disappointing.32 Because of small numbers, this would be best done in a multicenter-randomized trial. We are currently evaluating the role of tumor size and number in our allocation system in LT for HCC to determine their respective predictive value for prognosis.
In summary, LT for HCC can be performed with acceptable survival outcomes. A single tumor characteristic alone does not appear to determine prognosis or outcome. In the present study, macroVI alone was associated with very poor outcomes after LT. Extending criteria of LT for advanced HCC is possible only with better patient selection using improved pre-LT staging and identification of histopathological biological markers such as macroVI that would preclude LT.
Footnotes
Presented at the 2005 American Transplant Congress, Seattle, WA, May 20–23, 2005.
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699. [DOI] [PubMed]
- 2.Yao FY, Roberts JP. Applying expanded criteria to liver transplantation for hepatocellular carcinoma: Too much too soon, or is now the time? Liver Transpl 2004;10:919–921. [DOI] [PubMed]
- 3.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: Comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765–774. [DOI] [PubMed]
- 4.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer 2000;88:538–543. [DOI] [PubMed]
- 5.Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, Taylor BR, Grant DR, Vollmer CM. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006;202:275–283. [DOI] [PubMed]
- 6.Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: Experience of 480 cases. Hepatogastroenterology 1993;40:328–332. [PubMed]
- 7.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994;106:720–727. [DOI] [PubMed]
- 8.Iwatsuki S, Dvorchik I, Marsh JW, Madariaga JR, Carr B, Fung JJ, Starzl TE. Liver transplantation for hepatocellular carcinoma: A proposal of a prognostic scoring system. J Am Coll Surg 2000;191:389–394. [DOI] [PMC free article] [PubMed]
- 9.Hemming AW, Cattral MS, Reed AI, Van Der Werf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg 2001;233:652–659. [DOI] [PMC free article] [PubMed]
- 10.Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: A retrospective analysis. Ann Surg 1998;227:424–432. [DOI] [PMC free article] [PubMed]
- 11.Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080–1086. [DOI] [PubMed]
- 12.Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, Nagorney DM, Belghiti J, Ng IO, Yamaoka Y, Lauwers GY, Vauthey JN. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg 2005;140;450–457. [DOI] [PubMed]
- 13.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, Ellis LM, Vauthey JN. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg 2002;6:224–232. [DOI] [PubMed]
- 14.Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, Rosen M, Soulen M, Shaked A, Reddy KR, Olthoff KM. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl 2004; 10:911–918. [DOI] [PubMed]
- 15.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: A registry report of the impact of tumor characteristics on outcome. Ann Surg 1998;228:479–490. [DOI] [PMC free article] [PubMed]
- 16.Tamura S, Kato T, Berho M, Misiakos EP, O’Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J, Tzakis AG. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg 2001;136:25–30. [DOI] [PubMed]
- 17.Yokoyama I, Sheahan DG, Carr B, Kakizoe S, Selby R, Tzakis AG, Todo S, Iwatsuki S, Starzl TE. Clinicopathologic factors affecting patient survival and tumor recurrence after orthotopic liver transplantation for hepatocellular carcinoma. Transplant Proc 1991;23:2194–2196. [PubMed]
- 18.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology 2005;128:1752–1764. [DOI] [PubMed]
- 19.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394–1403. [DOI] [PubMed]
- 20.Marsh JW, Finkelstein SD, Schwartz ME, Fiel MI, Dvorchik I. Advancing the diagnosis and treatment of hepatocellular carcinoma. Liver Transpl 2005;11:469–472. [DOI] [PubMed]
- 21.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology 2004;40:667–676. [DOI] [PubMed]
- 22.Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, Subbotin V, Casavilla A, Carr BI, Fung JJ, Iwatsuki S. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: A pilot study. Hepatology 1997;26:444–450. [DOI] [PubMed]
- 23.Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, D'Amico F, Ciarleglio FA, Boccagni P, Brolese A, Zanus G, D'Amico DF. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg 2004;239:150–159. [DOI] [PMC free article] [PubMed]
- 24.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: Analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl 2002;8:873–883. [DOI] [PubMed]
- 25.Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, Burra P, Fagiuoli S, Farinati F, Rugge M, D'Amico DF. The critical issue of hepatocellular carcinoma prognostic classification: Which is the best tool available? J Hepatol 2004;40:124–131. [DOI] [PubMed]
- 26.Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G, Raab R, Ringe B, Manns MP, Pichlmayr R. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol 1999;17:324–331. [DOI] [PubMed]
- 27.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, Sherman M, Grant DR, Greig PD, Gallinger S. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol 2005;16:485–491. [DOI] [PubMed]
- 28.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative c on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997;226:688–701. [DOI] [PMC free article] [PubMed]
- 29.Stone MJ, Klintmalm GB, Polter D, Husberg BS, Mennel RG, Ramsay MA, Flemens ER, Goldstein RM. Neoadjuvant chemotherapy and liver transplantation for hepatocellular carcinoma: A pilot study in 20 patients. Gastroenterology 1993;104:196–202. [DOI] [PubMed]
- 30.Olthoff KM, Rosove MH, Shackleton CR, Imagawa DK, Farmer DG, Northcross P, Pakrasi AL, Martin P, Goldstein LI, Shaked A. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg 1995;221:734–741. [DOI] [PMC free article] [PubMed]
- 31.Carr BI, Selby R, Madariaga J, Iwatsuki S, Starzl TE. Prolonged survival after liver transplantation and cancer chemotherapy for advanced-stage hepatocellular carcinoma. Transplant Proc 1993;25:1128–1129. [PMC free article] [PubMed]
- 32.Pokorny H, Gnant M, Rasoul-Rockenschaub S, Gollackner B, Steiner B, Steger G, Steininger R, Muhlbacher F. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005;5:788–794. [DOI] [PubMed]


