Abstract
Background
Cold ischemia time and the presence of postoperative hepatic arterial thrombosis have been associated with biliary complications (BC) after liver transplantation. An ABO-incompatible blood group has also been suggested as a factor for predisposal towards BC. However, the influence of Rh nonidentity has not been studied previously.
Materials
Three hundred fifty six liver transplants were performed from 1995 to 2000 at our hospital. BC incidence and risk factors were studied in 345 patients.
Results
Seventy patients (20%) presented BC after liver transplantation. Bile leakage (24/45%) and stenotic anastomosis (21/30%) were the most frequent complications. Presence of BC in Rh-nonidentical graft–host cases (23/76, 30%) was higher than in Rh-identical grafts (47/269, 17%) (P = 0.01). BC was also more frequent in grafts with arterial thrombosis (9/25, 36% vs 60/319, 19%; P = 0.03) and grafts with cold ischemia time longer than 430 min (26/174, 15% vs 44/171, 26%; P = 0.01). Multivariate logistic regression confirmed that Rh graft–host nonidentical blood groups [RR = 2(1.1–3.6); P = 0.02], arterial thrombosis [RR = 2.6(1.1–6.4); P = 0.02] and cold ischemia time longer than 430 min [RR = 1.8(1–3.2); P = 0.02] were risk factors for presenting BC.
Conclusion
Liver transplantation using Rh graft–host nonidentical blood groups leads to a greater incidence of BC.
Keywords: Liver transplantation, Biliary complications, Rh nonidentity
Introduction
Biliary complications (BC) after liver transplantation (LT) are still a significant cause of morbidity and mortality.1,2 Hepatic arterial thrombosis, long ischemia time, inadequate exposure of the biliary epithelium to the preservation solution, and chronic rejection have been associated with BC.3–5 However, complications may also occur in the absence of these pathogenic factors. The expression of donor ABH antigen in the vascular and biliary epithelium of hepatic allografts has been linked to higher risk of biliary and arterial complications, especially in ABO-incompatible grafts.6 However, the influence of Rh nonidentity in LT has not been analyzed until now.
The aim of this study is to analyze the incidence of BC after LT, to define their etiological risk factors, and to study the influence of Rh nonidentity on presentation of BC after LT.
Materials
Three hundred and fifty-six (n = 356) liver transplants were performed at our hospital between January 1995 and November 2000. Eleven were excluded from the study because important data were missing (n = 345). The study was closed on May 2001 with a 6-month-minimum graft follow-up. The procurement procedure was based on the rapid flush technique.7 After reperfusion of the allograft, cholecystectomy was performed, the donor bile duct being divided just above the cystic duct junction in most cases. Biliary anastomosis was performed with interrupted stitches of 6-0 Polydioxanone suture Ethicon® (Johnson & Johnson, Brussels, Belgium). The biliary reconstruction technique was end-to-end anastomosis in the majority of LTs (315 patients, 91%). T-tubes were used in four patients due to diameter discrepancy between the biliary ducts of the donor and the recipient. If the primary disease affected the biliary tract, or if technical factors made end-to-end anastomosis difficult, Roux-en-Y-hepaticojejunostomy was performed (25 out of 345, 7%). The outcome was assessed in terms of biliary and arterial complications and patient status (alive, retransplanted, or dead). BCs were studied routinely with abdominal ultrasonography at day 1 post LT, weekly before discharge from hospital and monthly thereafter or when there was clinical or biochemical suspicion of BC, and this was confirmed with magnetic resonance cholangiography, percutaneous transhepatic cholangiography, or endoscopic retrograde cholangio-pancreatography when necessary. The immunosuppressive regimen was based on quadruple sequential therapy with antilymphocyte globulin, Sandimmune Neoral® (Novartis, Basel, Switzerland), steroids, and azathioprine in most patients, as reported elsewhere.8 Cellular rejection was diagnosed according to histological criteria.9
Statistical analysis Chi-squared analysis was used to compare dichotomous variables and the presence of BC. Variables that were statistically significant in the univariate analysis were introduced in a multivariate logistic regression analysis. Other known risk factors for developing BC were introduced into the model as covariates: donor age, acute rejection, and chronic rejection. Variables with P > 0.05 were excluded from the final equation. Kaplan–Meier estimates of the rate of BC for both groups and the results were compared with a log-rank test.
Results
Perioperative data and surgical details The preservation solutions used were the University of Wisconsin solution in 332 (97%), Celsior solution in 5 (1%), and histidine-tryptophan-ketoglutarate solution in 8 (2%). Twenty grafts (6%) were shipped by another team. The study of donor risk factors demonstrated that there was no hemodynamic instability (systolic pressure lower than 60 mmHg for more than 1 h) in 265 cases (77%), and that the main cause of death was traumatic head injury (129/36%). Donor Rh blood group was positive in 301 (87%) and negative in 44 cases (13%). Recipient Rh blood group was positive in 284 (82%) patients, and negative in 61 (18%). Rh blood groups of donor and recipient were positive to positive in 255 cases (74%), positive to negative in 46 (13%), negative to positive in 30 (9%), and negative to negative in 14 (4%). Donors and recipients had identical Rh in 269 cases (78%) and nonidentical in 76 (22%). The donor ABO blood group was A in 157 (46%), B in 29 (8%), AB in 19 (5%), and O in 140 (41%), while recipients were A in 161 cases (46%), B in 34 (10%), AB in 20 (6%) and O in 130 (38%). The ABO groups of transplanted grafts and hosts were identical in 97% of cases (335 patients) and compatible in 10 cases. There were no cases of incompatible grafts.During postoperative development, 25 LTs presented arterial thrombosis (7%). Eight grafts presented primary nonfunction and were retransplantated (2.2%), and 43 presented initial poor function that recovered spontaneously (12%).3 Biopsy-proven acute rejection was diagnosed in 66 grafts (19%) after transplantation, with development to chronic ductopenic rejection in 8 (2%).
BCs and Rh mismatch BCs appeared in 70 of the 345 patients (20%). Cross-tabs were built to analyze differences between the grafts that suffered BCs and the rest. Both groups were similar in terms of donor preoperative evaluation and support, recipient descriptive data, and surgical terms. The incidence of BC in nonidentical Rh graft–host cases (23/76; 30%) was significantly higher than in cases of identical Rh graft–host (47/269; 17%, P = 0.01). Cases of arterial thrombosis after LTs and ischemia time longer than 430 min were also associated with a higher incidence of BC (Table 1).
Table 1.
Demographics and Major Complications Occurring in Both Groups Studied, Chi-square Test
| BCs (n = 70) | No BCs (n = 275) | Chi-square | |
|---|---|---|---|
| Donor data | |||
| ABO blood group | 0.2 | ||
| A | 25/157 (16%) | 132/157 (84%) | |
| B | 6/29 (21%) | 23/29(79%) | |
| AB | 6/19 (32%) | 13/19(68%) | |
| O | 33/140 (24%) | 107/140 (76%) | |
| Rh blood group | 0.4 | ||
| Positive | 59/301 (19%) | 242/301 (81%) | |
| Negative | 11/44 (25%) | 33/44 (75%) | |
| Sex | 0.4 | ||
| Male | 51/233 (22%) | 182/233 (75%) | |
| Female | 19/110 (17%) | 91/110 (83%) | |
| Donor age | 0.4 | ||
| ≤70 years | 66/320 (20%) | 254/320 (80%) | |
| >70 years | 4/25 (16%) | 21/25 (84%) | |
| Recipient data | |||
| Rh blood group | 0.2 | ||
| Positive | 54/284 (19%) | 230/284 (81%) | |
| Negative | 16/61 (26%) | 45/61 (74%) | |
| Rh D-R crossing | 0.1 | ||
| Positive–positive | 45/255 (18%) | 210/255 (82%) | |
| Positive–negative | 14/46 (30%) | 32/46 (70%) | |
| Negative–positive | 9/30 (30%) | 21/30 (70%) | |
| Negative–negative | 2/14 (14%) | 12/14 (86%) | |
| Rh D-R identity | 0.01 | ||
| Identical | 47/269 (17%) | 222/269 (83%) | |
| Nonidentical | 23/76 (30%) | 53/76 (70%) | |
| ABO blood group | 0.4 | ||
| A | 27/161 (17%) | 134/161 (83%) | |
| B | 7/34 (20%) | 27/34(80%) | |
| O | 30/130 (23%) | 100/130(77%) | |
| AB | 6/20 (30%) | 14/20 (70%) | |
| ABO D-R identity | 0.4 | ||
| Identical | 67/335 (20%) | 268/335 (80%) | |
| Nonidentical | 3/10 (30%) | 7/10 (70%) | |
| Sex of recipient | 0.4 | ||
| Male | 48/214(22%) | 166/214 (78%) | |
| Female | 22/130(17%) | 108/130(83%) | |
| Recipient age | 0.2 | ||
| <60 years | 41/221 (18%) | 180/221 (82%) | |
| ≥60 years | 29/124 (24%) | 95/124 (76%) | |
| Diagnosis | 0.3 | ||
| Choleostasis | 2/14(15%) | 12/14(85%) | |
| Cirrhosis | 32/162 (20%) | 130/162(80%) | |
| Hepatocarcinoma | 27/103(26%) | 76/103(74%) | |
| Other etiology | 1/18(5%) | 17/18(95%) | |
| Re-OLT | 7/39(18%) | 32/39 (89%) | |
| Other tumors | 1/9(11%) | 8/9 (89%) | |
| Surgical data | |||
| Cold ischemic time | 0.01 | ||
| ≤430 min | 26/178 (15%) | 148/178 (85%) | |
| >430 min | 44/171 (26%) | 127/171 (74%) | |
| Type of anastomosis | 0.6 | ||
| Termino-terminal | 64/315(20%) | 251/315(80%) | |
| Graft evolution data | |||
| Arterial thrombosis | 0.03 | ||
| Yes | 9/25 (36%) | 16/25(64%) | |
| No | 60/319 (19%) | 259/319(81%) | |
| Initial poor function | 0.6 | ||
| Yes | 10/43 (23%) | 33/43 (77%) | |
| No | 60/302 (20%) | 242/302 (80%) | |
| Primary nonfunction | 0.1 | ||
| Yes | 0 | 8/8 (100%) | |
| No | 70/336 (20%) | 266/336 (80%) | |
| Acute rejection | 0.4 | ||
| Yes | 11/66(17%) | 55/66(83%) | |
| No | 59/279(21%) | 220/279(79%) | |
| Chronic rejection | 0.5 | ||
| Yes | 1/8(12%) | 7/8(88%) | |
| No | 69/337 (20%) | 268/337(80%) | |
OLT orthotopic liver transplantation
Multivariate Analysis of Risk Factors for BCs
In multivariate analysis, arterial thrombosis presented an adjusted relative risk (aRR) = 2.6, CI 95% = (1.1–6.4) (P = 0.02), cold ischemia time aRR = 1.8 (CI 95% = 1–3.2) (P = 0.02), and Rh graft–host nonidentity aRR = 2 (CI 95% = 1.1–3) (P = 0.02) were confirmed as independent risk factors for BC. Other variables included in the initial analysis were nonsignificant (Table 2). Kaplan–Meier estimator and log-rank test confirmed these findings (P = 0.01, Fig. 1). To discard the possible association between the Rh match and the two other risk factors, we demonstrated that arterial thrombosis had similar incidence in Rh-nonidentical grafts (3/75, 4%) and in Rh-identical grafts (22/269, 8%; P = 0.1). Moreover, grafts with long ischemia times (>430 min) had similar incidence in the Rh-nonidentical (42/76, 55%) group and in Rh-identical patients (129/269, 48%; P = 0.1).
Table 2.
Biliary Complications
| Univariate logistic regression | Multivariate logistic regression | |
|---|---|---|
| Donor age >70 years | 0.5 | 0.7 |
| Rh D-R identity identical nonidentical | 0.01; 2(1.1–3.6) | 0.02; 2 (1.1–3.6) |
| Cold ischemic time >430 min | 0.01; 1.9(1.1–3.3) | 0.02; 1.8(1–3.2) |
| Arterial thrombosis (yes) | 0.04; 2.4(1–5.7) | 0.02; 2.6 (1.1–6.4) |
| Acute rejection (yes) | 0.4 | 0.4 |
| Chronic rejection (yes) | 0.5 | 0.5 |
Univariate and multivariate logistic regression.
Figure 1.
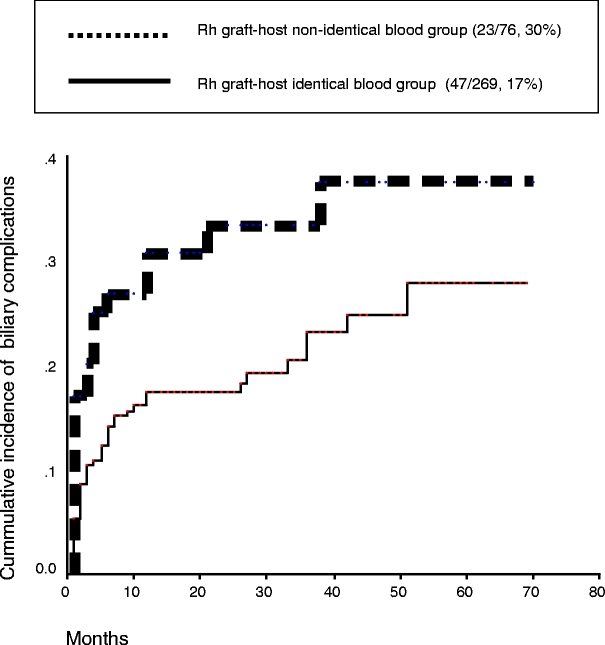
Kaplan–Meier estimates for the onset of BCs for nonidentical Rh graft–host (P = 0.01).
Type and Management of BCs
BCs were diagnosed in 70 patients. Biliary duct anastomosis stricture was the main complication, presented clinically, with (7/10%) or without postoperative leak (21/30%). Solitary leaks (17/24%), ischemic-type BCs (ITBC) with arterial thrombosis (6/9%), ITBC without arterial thrombosis (10/14%), and lithiasis (9/13%) were also related complications. The therapeutic approach was surgical in 23 patients (33%), endoscopic in 20 (28%), retransplantation in 11 (16%), and conservative treatment in 16 (23%).
Chi-square test was performed to analyze differences between the type of BC and the three risk factors found. As would be expected, arterial thrombosis was identified in all the cases of ITBC with thrombosis, in a higher percentage than the other BCs (P < 0.001). However, the different types of BCs were not associated with long ischemia time (P = 0.2) or Rh-identity (P = 0.4).
Finally, chi-square test was also performed to analyze differences among the three BC risk factors and medical management. The therapeutic approach regarding Rh-mismatch was similar in both groups (P = 0.3). Retransplantation was a frequent approach in arterial thrombosis management (4/9, 40%; P = 0.04). Interestingly, when the BC was presented in grafts with short ischemic time (<430 min), management by surgery (10/26, 38%) or an endoscopic approach (10/26, 38%) were sufficient, and it was not necessary to retransplant. However, when the BC arose in a graft with a long ischemic time (>430 min) the management was more aggressive, with 29% of patients (13/44) needing surgery and 25% (11/44) needing retransplantation.
Discussion
Etiopathogenesis of BC
Currently, orthotopic liver transplants are performed with good results at several centers without taking the donor–recipient Rh relationship into account. In fact, no prior work has shown greater morbidity or mortality after the usage of Rh-incompatible liver grafts. However, it was in studying the causes of BC in our grafts in prior studies that we began to suspect the existence of a possible relationship between BC and Rh incompatibility. First, we observed a greater rate of BC in the presence of preservation lesions in postreperfusion biopsies.10 Thus, we found that the biliary epithelium is very sensitive to changes during preservation. Second, the description of lesions like ITBC,11 to which surgical technique does not contribute as a primary cause, led us to suspect possible immunological pathogenesis of the BC. Lastly, it is paradoxical to find that, while surgical technique is improving and satisfactory results are achieved in liver transplants, BCs are still a problem, leading us to suspect that there are unidentified factors that cause them. The arterial thrombosis and long cold ischemia time were independent risk factors for developing BC, as was expected and reported by others.11,12 In our study we have demonstrated that even though BC in grafts with short ischemia can be resolved with surgery or endoscopy, the prognosis of BC was worse in cases of long ischemic grafts requiring retransplantation.
Relationship Between ABO and Rh in Liver Transplants
As for the donor–recipient ABO relationship, the usage of ABO-incompatible grafts has been discouraged due to the high rate of BC and poor graft survival.6,13,14 In the Sanchez-Urdazpal study,6 82% of the 18 ABO-incompatible grafts presented BC in comparison with 6% of the control group, while Farges,14 published slightly better results several years later with BC of 54%. Therefore, these grafts are used in extremely urgent cases when there is no other possible alternative. Immunological phenomena, such as rejection,3 may also lead to biliary strictures. In the same way, the ABO system was shown to cause more BC and worse graft survival in LT.6,13–15 However, Rh nonidentity seems to have better tolerance and is not a cause of graft refusal when a donor appears. Surprisingly, our study demonstrated a higher incidence of BC in the Rh-nonidentical group.
Some authors16,17 reported low rates of alloimmunization in Rh-negative recipients of Rh-incompatible transfusion after LT. It was suspected that immunosuppressant drugs modified the immunosuppressive response.16 However, debate still exists as to whether the D barrier can be crossed in LT. Previous studies of ABO barrier and BC suggested the hypothesis of an immunological injury to the bile duct epithelium, and the expression of ABH antigens in the donor 150 days after transplantation.6 However, the D antigen is only expressed in erythrocytes.
The nonidentical Rh group has two mismatch possibilities: positive donor to a negative recipient or negative donor to positive recipient. In the first case (positive to negative) the immunologic mechanism is easy to understand because the humoral anti-D (Rh) response may be responsible for the graft injury.
The other subgroup (negative to positive) may have a different pathogenic explanation. Bryan et al.17 hypothesized that two mechanisms could be involved in the same process in kidney transplantation: other Rh antigenic loci (C and E) and histocompatibility antigenic crossings. A negative liver graft in a positive recipient with lymphatic cells and tissues predisposes to cellular response against it. Finally, the biliary tract can probably suffer immunological damage, and thus further BC. Therefore, while the results of the study lead to suspicion of an immunological pathogenesis, the mechanism is still unclear.
In conclusion, Rh-nonidentical LT involves a higher rate of BCs. Future studies should examine the influence of Rh donor and blood group on graft development. Finally, our results suggest that there is a summation effect of BC risk factors. In our opinion, Rh-nonidentical liver grafts should not undergo a very long ischemia time.
References
- 1.Greif, F., Bronsther, O. L., Van Thiel, D. H., Casavilla, A., Iwatzuki, S., Tzakis, A., et al. (1994). The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Annals of Surgery, 219, 40–45. [DOI] [PMC free article] [PubMed]
- 2.Colonna, J. O. (1996). Technical problems: biliary. In R. W. Busutil & G. B. Klintmalmm (Eds.), Transplantation of the liver, 1st edn (pp. 617–625). Philadelphia: Saunders.
- 3.Oguma, S., Belles, S., Starzl, T. E., & Demetris, A. J. (1989). A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology, 9, 204–209. [DOI] [PMC free article] [PubMed]
- 4.Li, S., Stratta, R. J., Langnas, A. N., Wood, P., Marujo, W., Shaw, B. W. (1992). Diffuse biliary tract injury after orthotopic liver transplantation. American Journal of Surgery, 164, 536–540. [DOI] [PubMed]
- 5.Belzer, F. O., D’Alessandro, A., Hoffman, R., Kalayoglu, M., & Sollinger, H. (1992). Management of common bile duct in extended preservation of the liver. Transplantation, 53, 1166–1167. [DOI] [PubMed]
- 6.Sanchez-Urdazpal, L., Batts, K. P., Gores, G. J., Moore, S. B., Sterioff S., Wiesner, R. H., et al. (1993). Increased bile duct complications in liver transplantations across ABO barrier. Annals of Surgery, 218, 152–158. [DOI] [PMC free article] [PubMed]
- 7.Starzl, T. E., Hakala, T. R., Shaw, B. W. Jr., Hardesty, R. L., Rosenthal, T. J., Griffith, B. P., et al. (1984). A flexible procedure for multiple cadaveric organ procurement. Surgery, Gynecology & Obstetrics, 158, 223–230. [PMC free article] [PubMed]
- 8.Fabregat, J., Fradera, R., Figueras, J., Rafecas, A., Torras, J., Casanovas, T. et al. (1994). Hepatic allograft rejection under quadruple immunosuppressive regimen with cyclosporine A in liver transplantation: incidence of viral and fungal infection. Transplantation Proceedings, 26, 2697–2699. [PubMed]
- 9.Ludwig, J. (1989). Classification and terminology of hepatic allograft rejection: whither bound? Mayo Clinic Proceedings, 64, 676–679. [DOI] [PubMed]
- 10.Busquets, J., Figueras, J., Serrano, T., Torras, J., Ramos. E., Fabregat, J., et al. (2001). Postreperfusion biopsies are useful in predicting complications after liver transplantation. Liver Transplant, 5, 432–435. [DOI] [PubMed]
- 11.Sanchez-Urdazapal, L., Gores, G. J., Ward, E. M., Maus, T. P., Wahlstrom, H. E., Moore, S. B., et al. (1992). Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology, 16, 42–53. [DOI] [PubMed]
- 12.Langas, A. N., Marujo, W., Stratta, R. J., Wood, R. P., Li, S. J., & Shaw, B. W. (1991). Hepatic allograft rescue following arterial thrombosis: role of urgent revascularization. Transplantation, 51, 86–90. [DOI] [PubMed]
- 13.Guggenheim, J., Samuel, D., Reynes, M., & Bismuth, H. (1990). Liver transplantation across ABO blood group barriers. Lancet, 336, 519–523. [DOI] [PubMed]
- 14.Farges, O., Kalil, A. N., Samuel, D., Saliba, F., Arulnaden, J. L., Debat, P., et al. (1995). The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplantation, 59(8), 1124–1133. [DOI] [PubMed]
- 15.Lo, C. M., Shaked, A., Drazan, K., Yersiz, H., Jurim, O., & Busutil, R. W. (1995). Variables affecting transplantation across ABO blood group. Transplantation Proceedings, 27, 1159. [PubMed]
- 16.Casanueva, M., Valdes, M. D., & Ribera, M. C. (1994). Lack of alloimmunization to D antigen in D-negative immunosuppressed liver transplant recipients. Transfusion, 34, 570–572. [DOI] [PubMed]
- 17.Bryan, C. F., Mitchell, S. I., Lin, J. M., Nelson, P. W., Shield, C. F., III, Luger, A. M., et al. (1998). Influence of the Rh (D) blood group system on graft survival in renal transplantation. Transplantation, 65, 588–592. [DOI] [PubMed]


