Abstract
Introduction
Pancreatic ductal adenocarcinoma has a high mortality rate with limited treatment options. One option is pancreaticoduodenectomy, although complete resection may require venous resection. Pancreaticoduodenectomy with venous resection and reconstruction is becoming a more common practice with many choices for venous reconstruction. We describe the technique of using the left renal vein as a conduit for venous reconstruction during pancreaticoduodenectomy.
Methods
The technique for use of the left renal vein as an interposition graft for venous reconstruction during pancreaticoduodenectomy is described as well as outcomes for nine patients that have undergone the procedure.
Results
Nine patients, seven men, with a mean age of 57 years, have undergone the operation. There were eight interposition grafts and one patch graft. Mean operating time was 7.8 hours, and mean tumor size was 3.4 cm. Eight patients had node-positive disease, and six had involvement of the vein. Mean hospital stay was 14 days and perioperative morbidity included a superficial wound infection, delayed gastric emptying, ascites, and gastrointestinal bleeding in one patient each. Creatinine ranged from 0.8–1.1 mg/dl preoperatively and from 0.7–1.3 mg/dl at discharge. Mean follow-up was 6.8 months with normal creatinine values noted through the follow-up period. Two patients had died during follow-up from recurrent disease at 8.3 and 18.2 months after the operation.
Conclusions
The left renal vein provides an additional choice for an autologous graft during pancreaticoduodenectomy with venous resection. The ease of harvesting the graft and maintenance of renal function distinguish its use.
Keywords: Pancreatic cancer, Pancreaticoduodenectomy, Venous resection, Portal vein, Superior mesenteric vein, Left renal vein
Introduction
Pancreatic ductal adenocarcinoma has a high mortality rate1, which approaches the incidence, and treatment options remain limited. For those patients diagnosed with pancreatic ductal adenocarcinoma, resection continues to offer the only chance for cure. Historically, involvement of local vasculature was considered a contraindication to pancreaticoduodenectomy (PD), with early experience associated with prohibitively high morbidity and mortality rates.2 As surgeon experience has grown, morbidity and mortality rates have decreased, and at high-volume pancreatic surgery centers, invasion of local mesenteric venous structures is no longer a contraindication to resection. Venous resection occurs in up to 25% of patients undergoing PD at several centers.3,4 Several techniques are described for reconstruction of the venous system after PD when necessary: primary lateral venorrhaphy, primary end-to-end anastomosis, and interposition grafting.5,6 Both synthetic grafts and autologous vein grafts have been used, with several donor sites available.3,7
The goal of this report is to describe the use of the left renal vein as a conduit for venous reconstruction after PD with venous resection. Historically, the left renal vein has been used to repair the portal vein, hepatic vein, or inferior vena cava during resection of hepatic hilar carcinomas,8–11 and its use offers distinct advantages for venous reconstruction during PD. Importantly, previous studies have demonstrated the safety of left renal vein ligation, specifically in relation to renal function.12 We describe the technique of mesenteric venous reconstruction after PD with venous resection using a left renal vein graft and report on a group of patients that have undergone this repair.
Patient Selection and Technique
Contrast-enhanced computed tomography (CT) is used to evaluate a pancreatic head mass and its relation to vascular structures. CT accurately diagnoses mesenteric vein involvement, aiding in operative planning. The CT is also used to assess the length and caliber of the left renal vein, the status of the kidneys bilaterally, and the presence of the left gonadal and adrenal veins, which serve as collateral venous drainage. Additional imaging of the pancreas with endoscopic ultrasound (EUS) is frequently used to further evaluate the location and extent of any venous involvement.
After initiation of the operation, a Kocher maneuver allows assessment of tumor location and its relationship to the superior mesenteric artery (SMA), further assessing resectability. The lesser sac is entered by mobilizing the greater omentum off of the transverse colon through an avascular plane. The middle colic vein is followed centrally to identify the superior mesenteric vein (SMV) and the gastrocolic venous trunk. The gastrocolic venous trunk is routinely divided. (Fig. 1a) If venous resection is anticipated, to increase mobility, the middle colic vein and several other tributaries are ligated and divided. (Fig. 1b)
Figure 1.
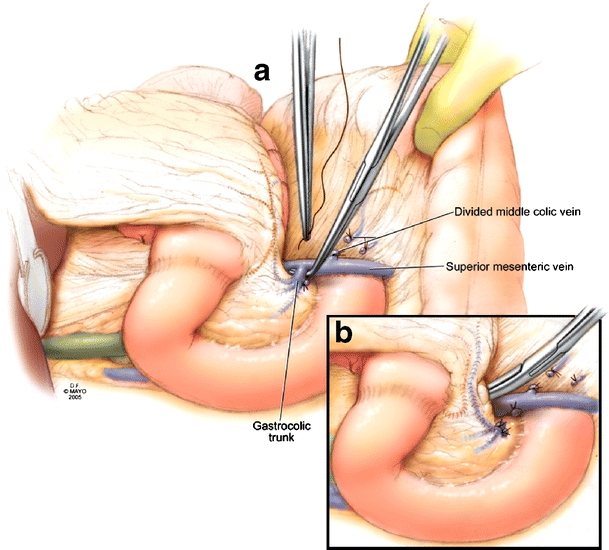
a Division of gastrocolic venous trunk and middle colic vein. b Mobilization of superior mesenteric vein in preparation of venous reconstruction.
The superior border of the pancreas is approached by incising the gastrohepatic ligament. The right gastric and gastroduodenal arteries are routinely ligated and divided. Retraction of the common hepatic artery cephalad allows dissection of the portal vein (PV), thereby, allowing completion of the plane between the pancreas and the PV-SMV.
The gallbladder is dissected from the liver, and the hepatic duct is encircled near the cystic duct junction. If a pylorus-preserving PD is planned, the superior and inferior aspects of the duodenum are skeletonized, individually ligating the right gastroepiploic vessels. The ligament of Treitz is mobilized, and the proximal jejunum is divided. Sequential ligation and division of the bowel mesentery exposes the uncinate process. The mobilized duodenum and jejunum are passed beneath the superior mesenteric vessels into the right upper quadrant.
Mobilization is now complete and transection begins at the duodenum approximately 3 cm distal to the pylorus. The bile duct is transected, and the margin is evaluated for malignancy. Stay sutures are placed on the superior and inferior borders of the pancreas to aid in retraction and hemostasis. The neck of the pancreas is transected over a clamp to protect the portal vein. (Fig. 2a) Reflection laterally allows visualization and ligation of venous tributaries. (Fig. 2b)
Figure 2.
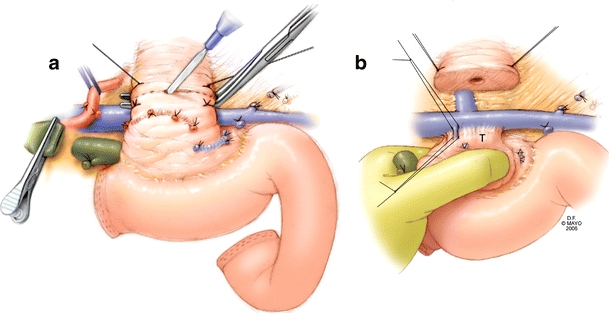
a The neck of the pancreas is transected over a clamp, thereby, protecting the portal vein from injury. b Venous tributaries to the portal vein are individually ligated while mobilizing the head and uncinate process of the pancreas.
Often, venous invasion is not discovered until this juncture, and although some resections can be limited to tangential excision and primary lateral venorrhaphy, there are oncologic and vascular considerations that make other options, including segmental resection with primary end-to-end anastomosis or interposition grafting with autologous or synthetic material, advantageous. Early in our experience, we completed venous resection before division of the arterial branches and soft tissue along the right lateral aspect of the superior mesenteric artery (SMA) (Fig. 3a). More recently, we have altered our technique by performing the dissection of the retroperitoneal margin before venous resection. The advantages of this are to avoid the need for venous anastomosis before removal of the specimen, minimize venous occlusion time, and allow preservation of the splenic vein. This is accomplished by performance of a generous Kocher maneuver and isolation of the superior mesenteric artery both at its origin and caudad to the uncinate process. The Kocher maneuver orients the superior mesenteric artery posterior to the PV-SMV and allows access for completion of the retroperitoneal dissection (Fig. 3b). Arterial branches coursing into the uncinate are sequentially clamped, divided, and ligated, thereby, completely freeing the pancreas from the SMA. The pancreatic head is then rotated back to its normal anatomic orientation, and it is at this juncture that a decision is made for primary end-to-end venous reconstruction or renal vein interposition grafting.
Figure 3.
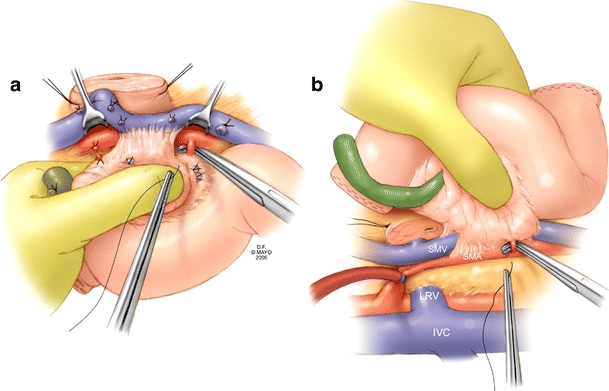
Dissection of the superior mesenteric artery proximal and distal to the area of venous invasion will limit total venous occlusion time after resection is performed. This can be approached anteriorly (a) or posteriorly (b) as necessary. Inflow occlusion of the SMA during posterior dissection is used selectively if maintenance of hemostasis is problematic.
Mobilization of the portal vein superior to the pancreas and the peritoneum along the root of the small bowel mesentery may provide length for the SMV or PV segment. This is accomplished by ligating and dividing small branches to the SMV, PV, and splenic vein (SV). Although primary end-to-end anastomosis is preferred, if interposition grafting is necessary, autologous vein and specifically the left renal vein is utilized for two reasons. First, the vein may be exposed within the same operative field, thereby, eliminating a second operative field and dissection. Second, the caliber and wall thickness of the vein is similar to the portal vein in most instances, providing good handling and suturing properties. Harvest of the left renal vein is undertaken after the retroperitoneal dissection when the specimen remains attached to the portal vein segment only (before venous resection). This allows the best assessment of the need for interposition grafting and minimizes the amount of clamp time by harvesting the graft before SMA and venous occlusion.
The left renal vein is optimally exposed by extending the Kocher maneuver to the left and elevating the head of the pancreas. (Fig. 4a) The vein is divided at the junction of the left gonadal and left adrenal veins, always preserving these vessels as collateral venous outflow for the left kidney. The vein is divided again flush with the inferior vena cava.(Fig. 4b) This typically provides a 3- to 4-cm venous segment. For venous division, we prefer a linear stapling device. The vein segment is placed in heparinized saline and then can be used as an interposition graft. (Fig. 4c)
Figure 4.
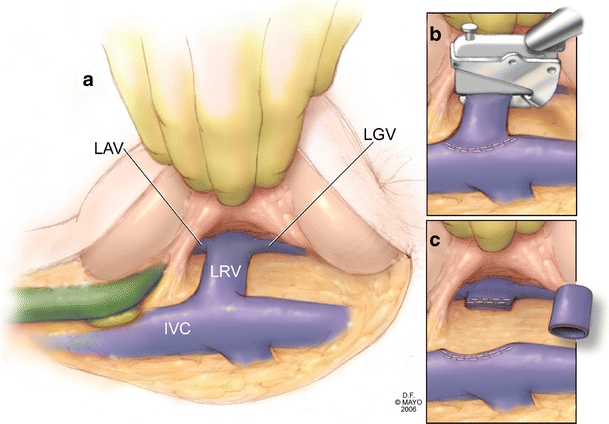
a The Kocher maneuver is extended to the left and elevation of the pancreatic head allows exposure of the entire left renal vein and the left adrenal and gonadal veins. b The vein is transected with a linear stapling device distal to the insertion of the left adrenal and gonadal veins and again flush with the inferior vena cava. c The left renal vein is used as an interposition graft to restore continuity to the mesenteric venous system.
Vascular control includes the SMA with placement of a Rummel tourniquet (in addition to venous occlusion proximal and distal) and is obtained immediately before resection. This allows inflow occlusion during the resection and reconstruction, decreasing the amount of intestinal engorgement, thereby, facilitating an easier pancreaticojejunostomy. The patient is not systemically heparinized. Venous resection is done sharply to obtain a margin.(Fig. 5) If the specimen has not been entirely freed from the SMA, the remaining branches are now ligated. Communication between the surgical and pathological teams is crucial to fully evaluate the specimen. Of specific importance is the venous segment for margin status and invasion and the retroperitoneal (uncinate) margin. (Fig. 6) Frozen section analysis allows additional margin to be obtained if necessary before reconstruction. After completion of both anastomoses, intraoperative ultrasound is routinely utilized to evaluate the reconstruction for patency. After satisfactory venous reconstruction, the remainder of the gastrointestinal reconstruction is completed. (Fig. 7)
Figure 5.
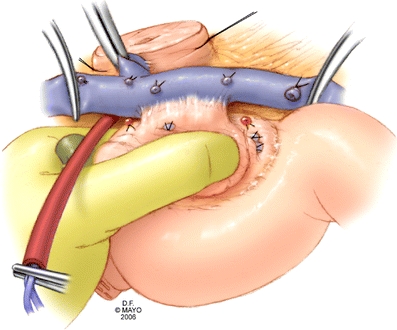
Vascular clamps are used to control the superior mesenteric vein, splenic vein, and portal vein before sharp dissection and resection of the involved venous segment. Inflow occlusion of the superior mesenteric artery during reconstruction reduces bowel engorgement.
Figure 6.
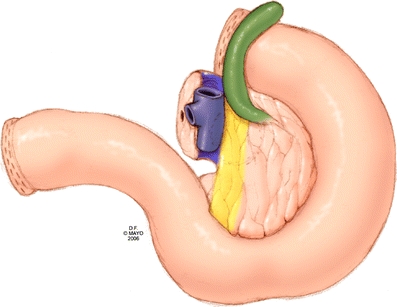
The specimen is carefully marked for all margins, including the venous segment margin. The portal vein groove and the retroperitoneal margin should be inked, and the venous segment should be evaluated histologically for malignant invasion (posterior view).
Figure 7.
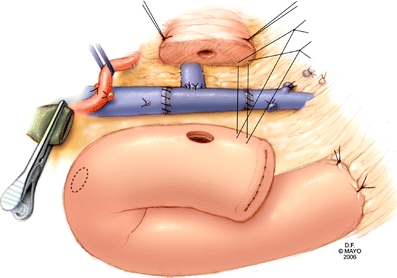
In the example shown, reconstruction of the PV-SMV confluence with an interposition graft utilizing left renal vein was performed initially, followed by reimplantation of the splenic vein into the graft (end-to-side) Gastrointestinal reconstruction is performed in a standard fashion after completion of venous reconstruction.
Results
Nine patients have undergone reconstruction of the SMV-PV during PD with an autologous left renal vein graft. There were seven men and two women with a mean age of 57 years (range, 31–77). Preoperative abdominal CT had suggested mesenteric vein involvement in seven of the nine patients. EUS was completed in three patients. In one patient, EUS suggested there was no vein involvement, while in the remaining two patients, EUS did suggest involvement. Preoperative serum creatinine levels ranged from 0.8 to 1.1 mg/dl in these patients (normal values 0.9–1.4 mg/dl ). Mean follow-up was 6.8 months.
The procedure consisted of three standard PD and six pylorus-preserving PD. Venous reconstruction consisted of eight interposition grafts and one patch graft. The patch graft was located on the lateral edge of the SMV and PV. Five of the interposition grafts were placed in the SMV, inferior to the confluence. One interposition graft was placed between the SMV and PV with reimplantation of the splenic vein; an additional was placed between the SMV and PV without reimplantation of the splenic vein, and the final graft was in the portal vein. The mean operating time was 7.8 hours (range, 6.5–9.5). The mean tumor size was 3.4 cm (range, 2.2–5). The mean estimated blood loss was 1,300 ml (range, 350–2,500). Eight patients were found to have node-positive disease with six of these patients noted to have histological involvement of the venous segment, while one additional patient had pathologically negative lymph nodes and no evidence of malignant invasion of the vein. In two patients, the uncinate margin was microscopically positive.
One patient was monitored overnight in the intensive care unit. There were no operative mortalities, and reoperation was not required in any of the patients. The mean length of hospitalization was 14 days (range, 9–29). Immediate perioperative morbidity included a superficial wound infection in one patient, delayed gastric emptying in one patient, and postoperative gastrointestinal bleeding in one patient. None of the patients experienced a pancreatic leak. No hematuria was noted. One patient was diagnosed with ascites and stenosis of the left renal vein interposition graft anastomosis 1 month after the operation. This patient had a congenitally cystic (nonfunctioning) left kidney. The left renal vein was reduced in caliber, but felt to be adequate for grafting at the time of the original operation. The patient underwent stenting of the graft by interventional radiology with resolution of her symptoms. Eight patients underwent adjuvant treatment, which included radiation therapy in six patients. None of the six patients receiving radiation therapy experienced a decrement in renal function after radiation therapy. Two patients had died 8.3 and 18.2 months after the operation of recurrent disease. Median survival has not been reached.
After discharge, all patients were evaluated with contrast-enhanced CT. All grafts were patent, and both kidneys were perfused well. Renal function was monitored by following serum creatinine levels. After the operation, creatinine levels transiently increased, but normalized by discharge. (Table 1) Creatinine values were available for a mean of 6.8 months postoperatively, with all levels within the normal range. One patient was anticoagulated with clopidogrel, while subsequent patients were treated with aspirin. Our current protocol is to treat all patients with daily aspirin if no clot is noted on the postoperative imaging, and heparin transitioned to coumadin if clot is noted.
Table 1.
A Comparison of Creatinine Levels
| Serum Creatinine Concentrations (mg/dL) | |||
|---|---|---|---|
| Patient | Preoperative | Peak | Time of Discharge |
| 1 | 1.1 | 1.1 | 1.1 |
| 2 | 1.1 | 1.4 | 1.1 |
| 3 | 1 | 1.5 | 1 |
| 4 | 0.9 | 1.3 | 1.1 |
| 5 | 1 | 1.3 | 1 |
| 6 | 0.9 | 1.3 | 1.3 |
| 7 | 0.8 | 0.9 | 0.9 |
| 8 | 0.8 | 1.1 | 0.9 |
| 9 | 0.8 | 0.8 | 0.7 |
Discussion
Venous resection and reconstruction is not uncommon during PD at high volume centers.3,4 Involvement of the vein by malignancy is not always suggested preoperatively by imaging and is often discovered at a time during the operation after commitment has already been made to resection. This fact necessitates that the surgeon has a plan for completing the resection and reconstruction of the venous system.13 Whereas many such cases can be completed with segmental resection and primary end-to-end anastomosis, a number will require either patch repair or interposition grafting. Other groups routinely use the internal jugular vein3 or superficial femoral vein14 as a conduit. While these groups have demonstrated good results with these conduits, the use of the left renal vein for autologous grafting offers some significant advantages and avoids the handling difficulties that can be encountered with the internal jugular vein and the risk of lymphedema or venous thrombosis that can be encountered with use of the superficial femoral vein.
The left renal vein provides a graft with good length, good caliber, and is easily accessible. The left renal vein typically provides a graft of 3–4 cm in length when harvested from the junction of the left gonadal and left adrenal vein proximally and the inferior vena cava distally, although some reports have indicated lengths up to 6 cm.9 The caliber of the graft allows for excellent flow, as demonstrated by CT and Doppler ultrasound. The ease of harvesting the graft also is an important consideration. Exposure of the left renal vein can be accomplished through a standard PD incision, without requiring any further prepping, an additional incision, or the need for an additional operating team. Furthermore, use of the left renal vein leaves the patient with all possible routes of central venous access.
Importantly, the operation is tolerated well from a renal standpoint. Previous work demonstrated that good collateral flow and functional capacity of the left kidney is preserved despite ligation of the left renal vein. McCullough and colleagues reported that after a right nephrectomy and ligation of the left renal vein for malignancy, only one of three patients experienced transient renal insufficiency.12 In our series, serum creatinine levels transiently increased after operation, but all normalized before discharge. Creatinine levels remained normal throughout follow-up.
Conclusions
Resection offers the only chance at cure for patients with pancreatic cancer, and potentially curative resection may require venous resection. When reconstruction of the venous system necessitates the use of interposition grafting, autologous vein interposition grafts are preferred. The left renal vein provides an additional choice for an autologous graft, and its use is distinguished by ease of harvest and maintenance of renal function. The use of the left renal vein for interposition grafting and patch repair should be considered by surgeons experienced in SMV-PV reconstruction during PD.
Acknowledgments
The authors wish to thank David Factor for the illustrations.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer Statistics, 2005. CA: A Cancer Journal for Clinicians 2005;55:10–30. [DOI] [PubMed]
- 2.Fortner JG. Regional resection of cancer of the pancreas: A new surgical approach. Surgery 1973;73(2):307–320. [PubMed]
- 3.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: Margin status and survival duration. J Gastrointest Surg 2004;8(8):935–950. [DOI] [PubMed]
- 4.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996;223(3):273–279. [DOI] [PMC free article] [PubMed]
- 5.van Geenen RC, ten Kate FJ, de Wit LT, van Gulik TM, Obertop H, Gouma DJ. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery 2001;129(2):158–163. [DOI] [PubMed]
- 6.Nakagohri T, Kinoshita T, Konishi M, Inoue K, Takahashi S. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg 2003;186(2):149–153. [DOI] [PubMed]
- 7.Howard TJ, Villanustre N, Moore SA, et al. Efficacy of venous reconstruction in patients with adenocarcinoma of the pancreatic head. J Gastrointest Surg 2003;7(8):1089–1095. [DOI] [PubMed]
- 8.Kurosawa H, Kimura F, Ito H, et al. Right hepatectomy combined with retrohepatic caval resection, using a left renal vein patch graft for advance cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2004;11:362–365. [DOI] [PubMed]
- 9.Miyazaki M, Itoh H, Kaiho T, et al. Portal vein reconstruction at the hepatic hilus using a left renal vein graft. J Am Coll Surg 1995;180(4):497–498. [PubMed]
- 10.Miyazaki M, Ito H, Nakagawa K, et al. Vascular reconstruction using left renal vein graft in advanced hepatobiliary malignancy. Hepato-Gastroenterology 1997;44(18):1619–1623. [PubMed]
- 11.Ohwada S, Takeyoshi I, Ogawa T, et al. Hepatic vein reconstruction at inferior vena cava confluence using left renal vein graft. Hepato-Gastroenterology 1998;45(23):1833–1836. [PubMed]
- 12.McCullough DL, Gittes RF. Ligation of the renal vein in the solitary kidney: Effects on renal function. J Urol 1975;113(3):295–298. [DOI] [PubMed]
- 13.Cusack JC, Jr., Fuhrman GM, Lee JE, Evans DB. Managing unsuspected tumor invasion of the superior mesenteric-portal venous confluence during pancreaticoduodenectomy. Am J Surg 1994;168(4):352–354. [DOI] [PubMed]
- 14.Fleming JB, Barnett CC, Clagett GP. Superficial femoral vein as a conduit for portal vein reconstruction during pancreaticoduodenectomy. Arch Surg 2005;140(7):698–701. [DOI] [PubMed]


