Abstract
CD24 has been described as an adverse prognostic marker in several malignancies. This study evaluates CD24 expression in cholangiocarcinoma and correlates the findings with clinicopathologic data and patient survival. Between 1996 and 2002, 22 consecutive patients with cholangiocarcinoma were treated at our institution. Demographic data, SEER stage, pathologic data, treatment, expression of CD24, mitogen-activated protein kinase (MAPK), phosphorylated MAPK, and survival were analyzed. The majority of the tumors demonstrated CD24 (81.8%) and p-MAPK (87%) expression. A negative association was noted between the expression of CD24 and p-MAPK. Median survival for patients with low expression of CD24 was 36 months and high expression was 8 months. Median survival for patients who received chemotherapy with low CD24 expression was 163 months, and for seven patients with high CD24 expression, it was 17 months (p = 0.04). With the addition of radiation therapy, median survival for patients with low expression of CD24 was 52 months and high expression was 17 months (p = 0.08). On multivariate analysis, the use of chemotherapy (p = 0.0014, hazard ratio 0.069) and the CD24 overexpression (p = 0.02, hazard ratio 7.528) were predictive of survival. CD24 is commonly expressed in cholangiocarcinoma, and overexpression is predictive of poor survival and possibly of lack of response to chemotherapy and radiation therapy. These findings may improve selection of patients for the appropriate treatment modality and the development of CD24-targeted therapy.
Keywords: CD24 expression, Cholangiocarcinoma, Survival
Introduction
Malignancies of the biliary tract are uncommon in the Western world. Two-thirds arise in the gallbladder and the remainder in the biliary tree or periampullary region. Cholangiocarcinoma or bile duct cancer is a rare but lethal malignancy with an incidence of 1–2 cases per 100,000 patients in the United States.1 Clinicopathologic factors predictive of survival include curative resection, tumor stage and grade, serum bilirubin level <10 mg/dl, low CA19-9 level, hepatitis viral infection,2–4 lymphovascular or portal vein invasion,5 intrahepatic satellite lesions, inraductal papillary compvonent, tumor angiogenesis,6 and DNA ploidy.7 Reports of molecular markers predictive of survival in cholangiocarcinoma include cluster of differentiation CD24,8 MMP-2, TIMP-2,9 cholinesterase level,10 MUC-4,11 cyclin D1,12 VEGF-C,13 p27,14 p53, and p73.15
Recently, CD24 has been described in a wide variety of malignancies and shown to be a prognostic marker in several solid tumors including colorectal, stomach, lung, prostate, ovarian, and breast.16–21 CD24 is a small, heavily glycosylated, mucin-like, cell-surface protein expressed in developing cells including pre-B cells, keratinocytes, and renal tubular epithelium.22–24 It functions as an alternative ligand of P-selectin, an adhesion receptor expressed on activated endothelial cells and platelets which can enhance the metastatic potential of CD24-expressing tumor cells.25–28 CD24 has apoptotic activity, and its cross-linking induces the sustained activation of p38 MAPK (mitogen-activated protein kinases)—the magnitude of which may determine the survival or death of pre-B cells.29 An improved understanding of the molecular pathways involved in the pathogenesis and progression of cholangiocarcinoma will contribute to the development of targeted therapy.
This study correlates CD24 and MAPK expression with patient survival in cholangiocarcinoma with the objective of identifying a subset of patients who may benefit from targeted molecular therapy.
Patients and Methods
Clinical Data
After obtaining approval of the Institutional Review Board, a review of the tumor registry at Roswell Park Cancer Institute identified 31 consecutive patients with histologically proven cholangiocarcinoma between 1996 and 2002. Twenty-two patients had adequate tissue for further histopathologic studies and constitute the basis of this study. Medical records of these patients were reviewed for demographic data including age; gender; surveillance, epidemiology, and end results (SEER) stage at presentation; treatment; and survival from the time of diagnosis.
Immunohistochemical Staining
For most of the patients, diagnosis was established by examination of conventional hematoxylin and eosin (H&E)-stained slides and, in the remainder diagnosis, was confirmed with ancillary techniques including immunohistochemistry and special histochemistry with mucin and PAS stains. Uniform tissue fixation techniques were used for all patients. For each patient, a representative block containing adequate neoplastic and nonneoplastic tissue was selected. Five-micrometer tissue sections from these blocks were placed on charged slides and dried in a 60°C oven for 1 h. Upon return to room temperature, the slides were deparaffinized in three changes of xylene and rehydrated using graded alcohols. Endogenous peroxidase was quenched with 3% aqueous H2O2 for 15 min and washed with phosphate buffered saline with 0.05% Tween-20 (PBS/T). CD24 primary antibody was obtained from BD Biosciences (clone ML5) and used with the recommended incubation time and antigen retrieval procedures. The primary antibody used for MAPK was obtained from Cell Signaling and for phosphorylated/activated MAPK from Santa Cruz. After a PBS/T wash, 0.03% casein (in PBS/T) was used as a block for 30 min followed by the application of the primary antibody to the slides for an hour or overnight. Another PBS/T wash was followed by exposure to the biotinylated secondary antibody for 30 min. A third PBS/T wash was followed by exposure to the streptavidin–peroxidase complex for 30 min. A PBS/T wash was followed by the application of the chromogen DAB (DAKO, Carpinteria CA, USA) for 5 min. The slides were then counterstained with hematoxylin, rinsed with water, dehydrated, and cleared, and a coverslip was placed. The use of biomarkers, related controls, and interpretation of results using the Histoscore system for quantification of results have been described previously by our group.30 Histoscore was defined as the product of the percentage of positive cells and the intensity of stain. The grade of positive staining depended upon the intensity of staining (0: no staining, 1: weak, 2: moderate, and 3: strong staining) and the percentage of cells stained. The final score was calculated as a sum of each stain intensity multiplied by the percentage of stained cells in the area of interest. For example: if tumor showed 50% weak, 30% moderate, and 20% strong staining, the score assigned was 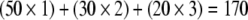 .
.
Histological Grading
The cholangiocarcinoma tissue specimens were also stained by routine H&E stains. The specimens were graded based on the degree of tumor differentiation using the World Health Organization (WHO) system.
Statistical Analysis
Association between biomarker expressions in tumor tissue was investigated using the Kendall’s tau. Biomarker expressions were classified as high and low based on whether their scores were above or below the median value, and survival between low and high expressions was compared using the log-rank test. The Kaplan–Meier method was also used to estimate the survival curves and median survival. The Cox’s proportional hazards survival analysis was used in the multivariate analysis of survival data to explain the effect of biomarker expressions together with other diagnostic parameters. Patient demographics including age, tumor grade, SEER stage, and treatment received were considered as possible parameters for explanatory variables in the model. All statistical tests were two-sided with statistical significance level at 5%.
Results
Patient Characteristics
Of the 22 patients included in the study, 7 were males and 15 females. The median age was 66.5 years (range: 35–77). SEER staging was local in 1 (4.6%), regional in 14 (63.6%), and distant in 7 (31.8%) patients. Differentiation of the tumor was classified as grade 1 in 3 (13.6%), grade 2 in 9 (40.9%), and grade 3 in 10 (45.5%) patients according to the WHO classification. Treatment for cholangiocarcinoma included surgery only (n = 8), surgery and chemotherapy (n = 5), surgery and radiation (n = 1), chemotherapy only (n = 2), and all three treatment modalities (n = 5), and one patient did not receive any treatment.
Immunohistochemical Staining
Normal bile duct staining was used to set the score intensity. Most of the bile ducts were negative. Occasionally, they demonstrated weak focal and incomplete staining as seen in Fig. 1. The cholangiocarcinoma cells were scored as 1+ when they demonstrated weak expression of CD24, 2+ for moderate expression, and 3+ for strong expression of CD24. Figure 1 depicts cholangiocarcinoma positive for CD24 expression adjacent to normal biliary epithelium. The majority of the tumors demonstrated CD24 (81.8%) and p-MAPK (87%) expression. Immunohistochemical staining for these proteins was higher in malignant tissue in comparison to normal biliary epithelium. The pattern of staining was usually a combination of cytoplasmic and apical, and few specimens demonstrated the apical pattern only.
Figure 1.
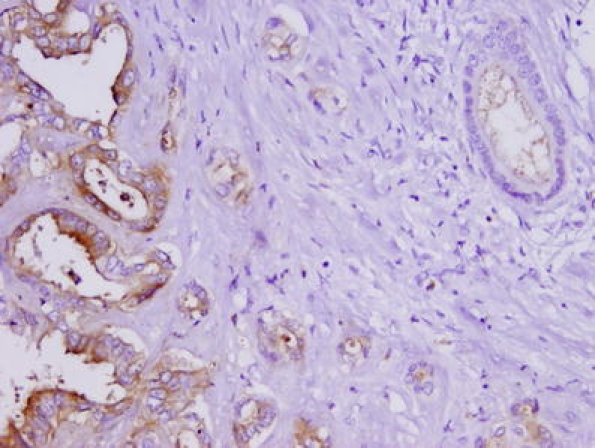
Moderately differentiated cholangiocarcinoma with overexpression of CD24. Normal bile duct (right upper corner) demonstrated weak apical staining, whereas neoplastic cells had strong apical and cytoplasmic staining (20×).
Relationship Between Biomarkers
A negative association was suggested between the expression of CD24 and phosphorylated/activated p-MAPK (Kendall’s τ = −0.32408, p = 0.0501).
Survival
Median survival was 36 months for nine patients with low expression of CD24 and 8 months for 13 patients with high expression of CD24 as shown in Fig. 2. The median survival for five patients who received chemotherapy with low CD24 expression was 163 months, and for seven patients with high CD24 expression, it was 17 months (Fig. 3, p = 0.04). Median survival for four patients treated with radiation in the presence of low CD24 expression was 52 months, and it was 17 months for two patients with overexpression of CD24 (Fig. 4, p = 0.08). Overexpression of CD24 continued to affect survival adversely despite the overall improvement noted with the addition of radiation therapy. Multivariate analysis using the Cox’s proportional hazards survival analysis demonstrated that overexpression of CD24 (p = 0.02, hazard ratio 7.528) and use of chemotherapy (p = 0.0014, hazard ratio 0.069) were predictive of survival (Table 1). There was no significant association noted between survival and patient’s age, sex, SEER stage, grade of the tumor, surgery, radiation therapy, or expression of MAPK.
Figure 2.
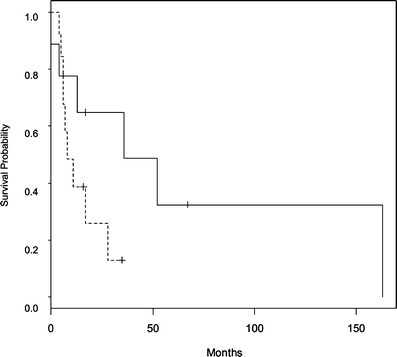
Kaplan–Meier survival curve for patients of cholangiocarcinoma with low and high levels of CD24 expression (n = 22). p = 0.02. Low CD24 (–––––––), high CD24 (- - - - - - - - - - - -).
Figure 3.
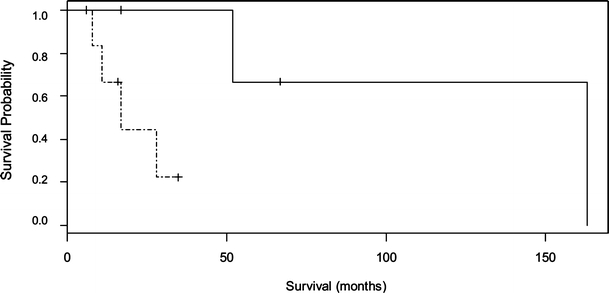
Kaplan–Meier survival curve for patients who received chemotherapy with low and high levels of CD24 expression (n = 12). p = 0.04. Low CD24 (–––––––), high CD24 (– - – - – - –).
Figure 4.
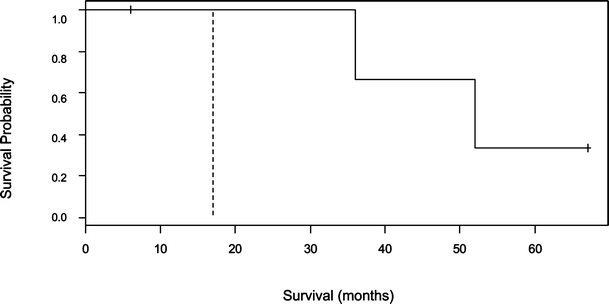
Kaplan–Meier survival curve for patients who received radiation therapy with low and high levels of CD24 expression (n = 6). p = 0.08. Low CD24 (–––––––), high CD24 (– - – - – - –).
Table 1.
Prognostic Variables for Survival in 22 Patients with Cholangiocarcinoma
| Variable | n | Median survival (months) | Survival (p value) | Multivariate analysis (p value) |
|---|---|---|---|---|
| Age | ||||
| <68 | 11 | 17 (8–36) | 0.60 | |
| >68 | 11 | 11 (5–52) | ||
| Gender | ||||
| Male | 7 | 12 (8–163) | 0.52 | |
| Female | 15 | 17 (6–52) | ||
| Grade | ||||
| 1, 2 | 12 | 15 (6–52) | 0.99 | |
| 3 | 10 | 11 (8–*) | ||
| SEER stage | ||||
| 1, 2 | 15 | 11 (6–52) | 0.48 | |
| 3 | 7 | 28 (6–163) | ||
| Chemotherapy | ||||
| No | 10 | 6 (4–13) | 0.0005 | 0.0014 |
| Yes | 12 | 52 (17–163) | ||
| Radiation | ||||
| No | 16 | 8 (6–28) | 0.12 | |
| Yes | 6 | 44 (17–*) | ||
| Surgery | ||||
| No | 3 | 8 (0–28) | 0.17 | |
| Yes | 19 | 17 (7–52) | ||
| MAPK | ||||
| Low | 10 | 17 (8–36) | 0.68 | |
| High | 11 | 28 (5–163) | ||
| P-MAPK | ||||
| Low | 10 | 13 (8–52) | 0.34 | |
| High | 11 | 36 (4–163) | ||
| CD24 | ||||
| <120 | 9 | 36 (13–163) | 0.10 | 0.02 |
| >120 | 13 | 8 (6–28) | ||
*The estimate was not provided because the upper limit of the survival curve had not reached a 50% failure rate.
CD denotes cluster of differentiation.
p-MAPK denotes phosphorylated form of mitogen-activated protein kinase.
Discussion
The physiologic function of CD24 is incompletely understood but it has been shown to increase tumor proliferation, cell adhesion, motility, invasion, and apoptosis.22–24,31 Selectins are cell adhesion molecules involved in the rolling adhesion of leukocytes to endothelial cells and platelets under the shear forces of circulation, and P-selectin expressed by thrombin-activated platelets and endothelial cells is a major ligand for CD24 on carcinoma cells.26,27 This suggests that CD24-expressing tumor cells can disseminate more readily due to their capacity to form thrombi with activated platelets or to adhere to endothelial cells. Friederichs et al.28 have demonstrated that the carbohydrate sialylLex abundantly expressed on human cancers is essential for CD24-mediated rolling of tumor cells on P-selectin, and in its absence, human adenocarcinoma cells failed to arrest and colonize the lungs. CD24, a metastasis-associated protein, has been recently identified as a downstream target of Ral signaling.32 Ral GTPases are important mediators of transformation, tumorigenesis, and cancer progression. Microarray by immunohistochemistry of a human bladder cancer identified CD24 as a novel Ral-regulated target and a prognostic biological marker.
In this study, 81.8% of patients with cholangiocarcinoma expressed CD24. Median survival for patients with overexpression of CD24 was significantly shorter, and the addition of chemotherapy improved survival. A negative association was noted between the expression of CD24 and p-MAPK. The use of chemotherapy in patients with low expression of CD24 was associated with a median survival of 163 months compared to 17 months in patients with a high CD24 expression (p = 0.04). The use of radiation therapy in patients with low expression of CD24 was also associated with an improved survival than with overexpression of CD24 although the data did not attain statistical significance possibly due to the small number of patients in this series.
It has been reported by Taguchi et al.29 that the cross-linking of CD24 induces apoptosis in Burkitt’s lymphoma enhanced by a B-cell antigen receptor (BCR)-mediated signal. They observed that simultaneous cross-linking of pre-BCR clearly inhibited CD24-mediated apoptosis in pre-B cells. CD24 cross-linking also induces the sustained activation of p38 MAPK, and whether pre-B cells survive or die may be determined by the magnitude of MAPK activation. Consistent with these observations, the present study suggests an inverse association between CD24 and p-MAPK, and eventual cellular proliferation or apoptosis might be a consequence of the dominant effect in a complex interplay of opposing influences.33
Our data indicate that high expression of CD24 remains an adverse prognosticator despite the use of additional therapy. Chemotherapy and radiation were noted to provide maximal survival benefit to low expressors of CD24 although the data for the use of radiation was statistically insignificant probably due to the small number of patients in this study. Furthermore, correlation between CD24 expression and radiation sensitivity has been noted to vary with the cell type as in human small cell lung cancer, and radiation doses required to induce apoptosis of CD24-negative human ALL (acute lymphoblastic leukemia) cells were higher than those required for CD24-positive cells, suggesting that lack of CD24 surface antigen expression is associated with intrinsic radiation resistance.34,35
Hypoxia is a characteristic feature of tumor cells due to the sustained proliferation which progressively results in an acidic, nutrient-deprived, and hypoxic tumor microenvironment. Tumor oxygenation has been identified as an independent prognostic variable for locoregional control and overall survival following definitive irradiation for squamous cell carcinoma of the head, neck, and uterine cervix.36,37 Recent reports have indicated decreased efficacy of chemotherapy under hypoxic conditions in several tumor types including pancreatic cancer and testicular tumors.38,39 Because treatment failure was a consequence of hypoxia, the authors recommend novel treatment strategies aimed at improving tumor oxygenation or enhancing the treatment sensitivity of hypoxic tumor cells. Aimed at identifying potential oxygen-dependent markers in vascular endothelial cells for therapeutic intervention in tumor angiogenesis, Scheurer et al.40 performed a broad-range transcriptomic analysis of selected extracellular matrix protein gene expression levels in human umbilical cord vein endothelial cells exposed in vitro to hypoxia for different time periods. They noted several genes transcriptionally upregulated including CD24 at late times of exposure to hypoxia, indicating that it was a useful marker of hypoxic activation in vascular endothelial cells. In the present series, low expressors of CD24 demonstrated greater survival benefit from chemotherapy and radiation than the high expressors, suggesting that its expression may be a marker for tumor hypoxia and response to therapy. This finding that shows that patients with low expression of CD24 may benefit from chemotherapy or radiation is of importance because it has been previously reported that adjuvant or palliative radiation had no effect on survival in patients with cholangiocarcinoma.41 However, the small number of patients in the present series limits interpretation of data suggesting that CD24 overexpression may be predictive of lack of response to radiation or chemotherapy.
CD24 has been shown to be a prognostic marker for shortened survival and disease progression in several malignancies including colorectal, stomach, lung, prostate, ovarian, and breast cancers.16–21 Weichert et al. report that in colorectal cancer, only the subset of patients with exceptionally strong cytoplasmic CD24 staining comprising 10% of their study group demonstrated a markedly shortened mean survival of 31.5 months compared to 67.5 months for the remaining patients.16 They also reported that cytoplasmic CD24 staining pattern is prognostically more significant than the membranous pattern—the biological significance of which was unclear. Su et al. noted a 51% expression of CD24 by immunohistochemistry in intrahepatic cholangiocarcinoma as compared to the 81.8% in the present series. They reported CD24 expression and tumor stage as independently predictive of survival on multivariate analysis and suggested membrane-bound CD24 protein as a potential target for immunotherapy.8
In conclusion, overexpression of the molecular marker CD24 in cholangiocarcinoma is predictive of poor survival. CD24 overexpressors demonstrated a lack of response to chemotherapy and possibly radiation therapy although these observations were limited by the small sample size. Additional properties of tumor proliferation, invasion, metastasis, and apoptosis make CD24 a potent target for specifically directed molecular therapy and its overexpression a potential criterion in the selection of patients for the appropriate conventional treatment modality.
References
- 1.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar cholangiocarcinoma: patterns of spread, importance of hepatic resection for curative operation and a presurgical clinical staging system. Ann Surg 1998;228:385–394. [DOI] [PMC free article] [PubMed]
- 2.Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993;128(8):871–877. [DOI] [PubMed]
- 3.Klempnauer J, Rideer GJ, von Wasielewski R, Werner M, Weimann A, Pichmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol 1997;15(3):947–954. [DOI] [PubMed]
- 4.Abdel Wahab M, Fathy O, Elghwalby N, Sultan A, Elebidy E, Abdalla T, Elshobary M, Mostafa M, Foad A, Kandeel T, Abdel Raouf A, Salah T, Abu Zeid M, Abu Elenein A, Gad Elhak N, ElFiky A, Ezzat F. Resectability and prognostic factors after resection of hilar cholangiocarcinoma. Hepatogastroenterology 2006;53(67):5–10. [PubMed]
- 5.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg 2003;238(5):720–727. [DOI] [PMC free article] [PubMed]
- 6.Shirabe K, Shimada M, Tsujita E et al. Prognostic factors in node-negative intrahepatic cholangiocarcinoma with special reference to angiogenesis. Am J Surg 2004;187(4):538–542. [DOI] [PubMed]
- 7.Abou-Rebyeh H, Al-Abadi H, Jonas S, Rotter I, Bechstein WO, Neuhaus P. DNA analysis of cholangiocarcinoma cells: prognostic and clinical importance. Cancer Detect Prev 2002;26(4):313–319. [DOI] [PubMed]
- 8.Su MC, Hsu C, Kao HL, Jeng YM. CD24 expression is a prognostic factor in intrahepatic cholangiocarcinoma. Cancer Lett 2006;235(1):34–349. [DOI] [PubMed]
- 9.Xiao M, Zhou NX, Huang ZQ, Lu YL, Chen LH, Wang DJ, Chang WL. The ratio of MMP-2 to TIMP-2 in hilar cholangiocarcinoma: a semi-quantitative study. Hepatobiliary Pancreat Dis Int 2004;3(4):599–602. [PubMed]
- 10.Hamamoto Y, Niino K, Ishiyama H, Hosoya T. Impact of pretreatment cholinesterase level on survival of inoperable intrahepatic or hepatic–hilar carcinomas treated with three-dimensional conformal radiotherapy. Radiat Med 2004;22(5):316–323. [PubMed]
- 11.Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology 2004;39(1):220–229. [DOI] [PubMed]
- 12.Sugimachi K, Aishima S, Taguchi K, Tanaka S, Shimada M, Kajiyama K, Sugimachi K, Tsuneyoshi M. The role of overexpression and gene amplification of cyclin D1 in intrahepatic cholangiocarcinoma. J Hepatol 2001;35(1):74–79. [DOI] [PubMed]
- 13.Park BK, Paik YH, Park JY, Park KH, Bang S, Park SW, Chung JB, Park YN, Song SY. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol 2006;29(2):138–142. [DOI] [PubMed]
- 14.Fiorentino M, Altimari A, D’Errico A, Gabusi E, Chieco P, Masetti M, Grigioni WF. Low p27 expression is an independent predictor of survival for patients with either hilar or peripheral intrahepatic cholangiocarcinoma. Clin Cancer Res 2001;7(12):3994–3999. [PubMed]
- 15.Tannapfel A, Engeland K, Weinans L, Katalinic A, Hauss J, Mossner J, Wittekind C. Expression of p73, a novel protein related to the p53 tumour suppressor p53, and apoptosis in cholangiocellular carcinoma of the liver. Br J Cancer 1999;80(7):1069–1074. [DOI] [PMC free article] [PubMed]
- 16.Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, Altevogt P, Dietel M, Kristansen G. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res 2005;11(18):6572–6581. [DOI] [PubMed]
- 17.Lim SC, Oh SH. The role of CD24 in various human epithelial neoplasias. Pathol Res Pract 2005;201(7):479–486. [DOI] [PubMed]
- 18.Kristiansen G, Schluns K, Yongwei Y, Denkert C, Dietel M, Petersen I. CD24 is an independent prognostic marker of survival in nonsmall cell lung cancer patients. Brit J Cancer 2003;88:231–236. [DOI] [PMC free article] [PubMed]
- 19.Kristiansen G, Pilarsky C, Pervan J, Sturzebecher B, Stephan C, Jung K, Loening S, Rosenthal A, Dietel M. CD24 expression is a significant predictor of PSA relapse and poor prognosis in low grade or organ confined prostate cancer. Prostate 2004;58:183–192. [DOI] [PubMed]
- 20.Kristiansen G, Denkert C, Schluns K, Dahl E, Pilarsky C, Hauptmann S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol 2002;161(4):1215–1221. [DOI] [PMC free article] [PubMed]
- 21.Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schluns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H, Dietel M. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res 2003;9:4906–4913. [PubMed]
- 22.Pirrucello SJ, LeBien TW. The human B cell-association antigen CD24 is a single chain sialoglycoprotein. J Immunol 1986;136:3779–3784. [PubMed]
- 23.Fischer GF, Majdic O, Gadd S, Knapp W. Signal transduction in lymphocytic and myeloid cells via CD24, a new member of phosphoinositol-anchored membrane molecules. J Immunol 1990;144:638–641. [PubMed]
- 24.Akashi T, Shirasawa T, Hirokawa K. Gene expression of CD24 core polypeptide molecule in normal rat tissues and human tumor cell lines. Virchows Arch 1994;425:399–406. [DOI] [PubMed]
- 25.Aigner S, Ruppert M, Hubbe M, Sammar M, Sthoeger Z, Butcher EC, Vestweber D, Altevogt P. Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol 1995;7(10):1557–1565. [DOI] [PubMed]
- 26.Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P. CD24 a mucin-type glycoprotein is a ligand for P-selectin on human tumor cells. Blood 1997;89(9):3385–3395. [PubMed]
- 27.Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J 1998;12(12):1241–1251. [DOI] [PubMed]
- 28.Friederichs J, Zeller Y, Hafezi-Moghadam A, Grone HJ, Ley K, Altevogt P. The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res 2000;60(23):6714–6722. [PubMed]
- 29.Taguchi T, Kiyokawa N, Mimori K, Suzuki T, Sekino T, Nakajima H, Saito M, Katagiri YU, Matsuo N, Matsuo Y, Karasuyama H, Fujimoto J. Pre-B cell antigen receptor-mediated signal inhibits CD24-induced apoptosis in human pre-B cells. J Immunol 2003;170(1):252–260. [DOI] [PubMed]
- 30.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda R, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res 2002;8:1168–1171. [PubMed]
- 31.Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yaqita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res 2005;65(23):10783–10793. [DOI] [PubMed]
- 32.Smith SC, Oxford G, Wu Z, Nitz MD, Conaway M, Frierson HF, Hampton G, Theodorescu D. The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res 2006;66(4):1917–1922. [DOI] [PubMed]
- 33.Javle MM, Yu J, Khoury T. AKT expression may predict favorable prognosis in cholangiocarcinoma. J Gastroenterol Hepatol 21:1744–1751. [DOI] [PubMed]
- 34.Uckun FM, Song CW. Lack of CD24 antigen expression in B-lineage acute lymphoblastic leukemia is associated with intrinsic radiation resistance of primary clonogenic blasts. Blood 1993;81(5):1323–1332. [PubMed]
- 35.Weber E, Schmitter D, Resch H, Zarn JA, Waibel R, Mabry M, Huquenin P, Stahel RA. Radiation studies on B cell differentiation marker CD24/SCLC Cluster-4 antigen expressing and non-expressing lung cancer cell lines and mouse fibroblasts. Int J Radiat Biol 1995;68(2):205–213. [DOI] [PubMed]
- 36.Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol 2004;43(4):396–403. [DOI] [PubMed]
- 37.Rofstad EK, Sundfor K, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer 2000;83(3):354–359. [DOI] [PMC free article] [PubMed]
- 38.Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res 2004;10(7):2299–2306. [DOI] [PubMed]
- 39.Koch S, Mayer F, Honecker F, Schittenhelm M, Bokemeyer C. Efficacy of cytotoxic agents used in the treatment of testicular germ cell tumours under normoxic and hypoxic conditions in vitro. Br J Cancer 2003;89(11):2133–2139. [DOI] [PMC free article] [PubMed]
- 40.Scheurer SB, Rybak JN, Rosli C, Neri D, Elia G. Modulation of gene expression by hypoxia in human umbilical cord vein endothelial cells: a transcriptomic and proteomic study. Proteomics 2004;4:1737–1760. [DOI] [PubMed]
- 41.Pitt HA, Nakeeb A, Abrams RA et al. Perihilar cholangiocarcinoma: postoperative radiotherapy does not improve survival. Ann Surg 1995;221(6):788–798. [DOI] [PMC free article] [PubMed]


