Abstract
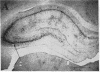
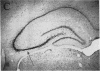
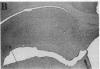
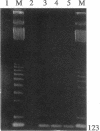
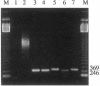
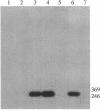
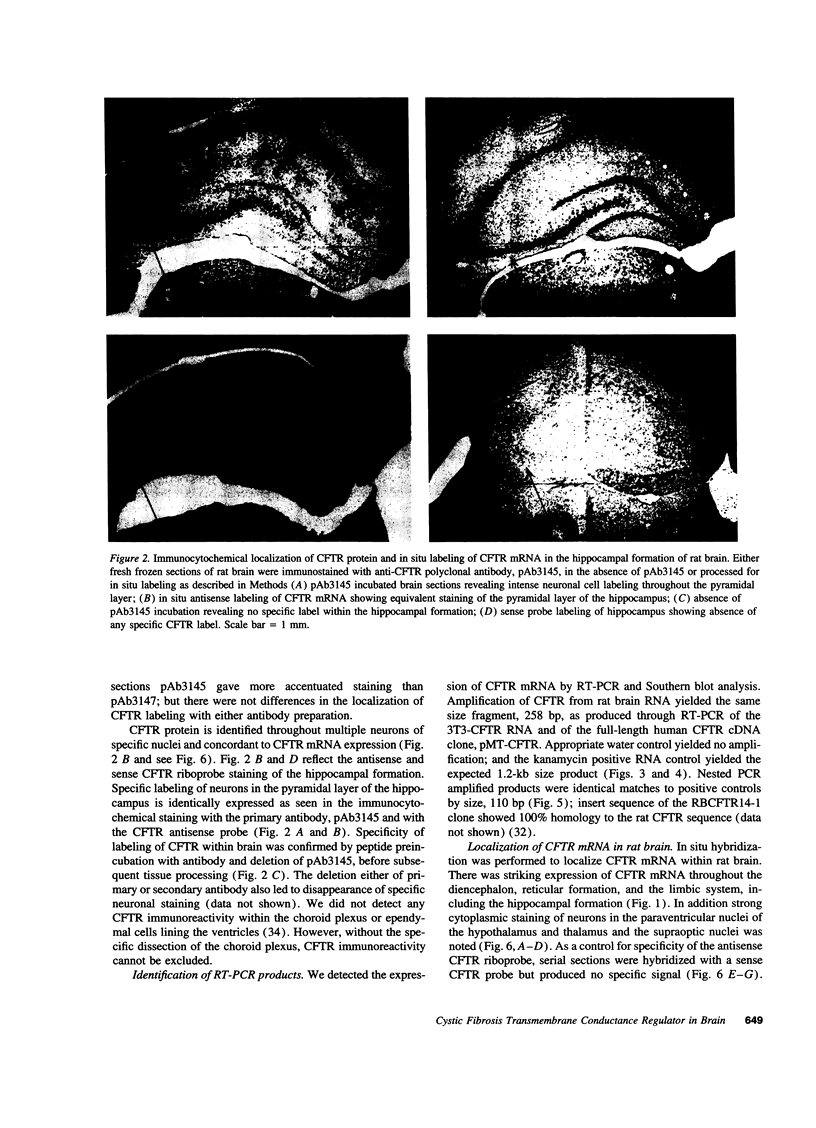
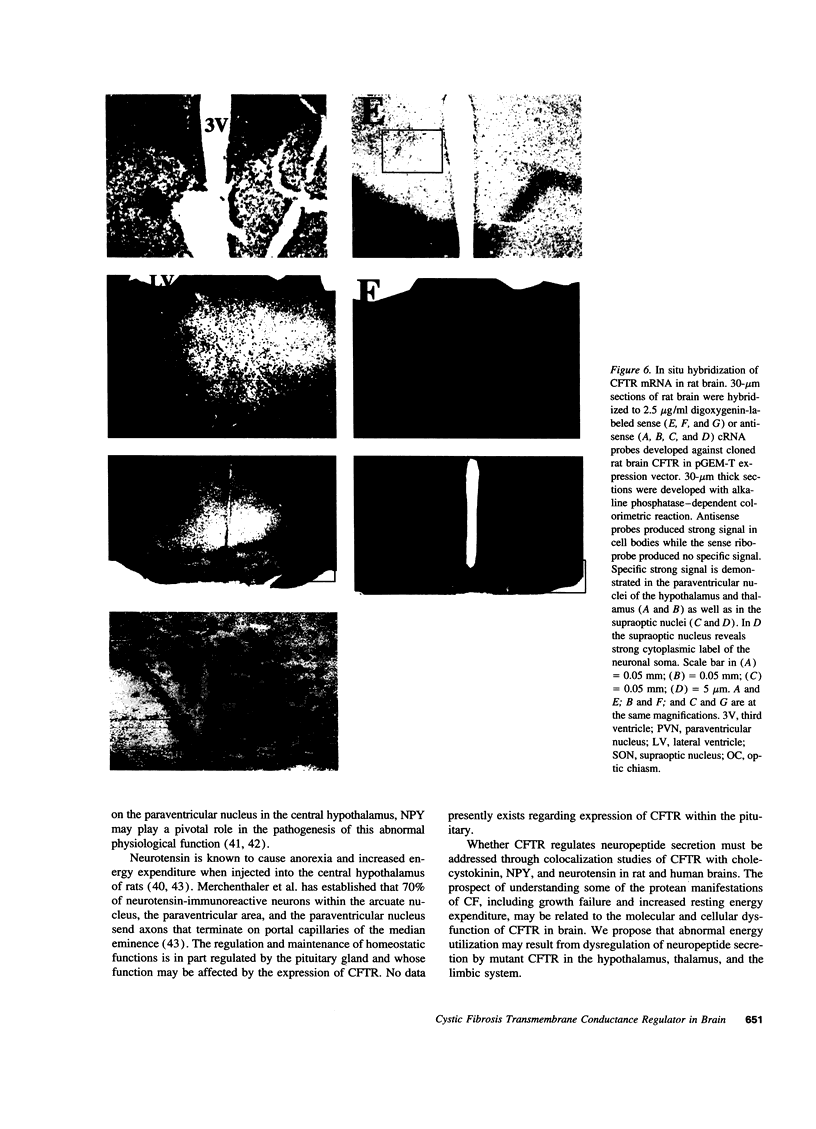
In previous studies we have characterized the expression of the cystic fibrosis transmembrane conductance regulator (CFTR) protein in clathrin-coated vesicles derived from bovine brain and in neurons of rat brain. In this study we have further characterized the expression of the CFTR protein mRNA and protein in rat brain with reverse transcriptase polymerase chain reaction amplification (RT-PCR), in situ hybridization, and immunocytochemistry. The expression of CFTR mRNA and protein in discrete areas of brain, including the hypothalamus, thalamus, and amygdaloid nuclei, which are involved in regulation of appetite and resting energy expenditure, is identical. The presence of CFTR in neurons localized to these regions of brain controlling homeostasis and energy expenditure may elucidate the pathogenesis of other nonpulmonary and gastrointestinal manifestations which commonly are observed in children with cystic fibrosis. Dysregulation of normal neuropeptide vesicle trafficking by mutant CFTR in brain may serve as a pathogenic mechanism for disruption of homeostasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Barasch J., al-Awqati Q. Defective acidification of the biosynthetic pathway in cystic fibrosis. J Cell Sci Suppl. 1993;17:229–233. doi: 10.1242/jcs.1993.supplement_17.32. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Cohn J. A., Venglarik C. J., Bridges R. J. Biochemical and biophysical identification of cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J Biol Chem. 1994 Mar 18;269(11):8296–8302. [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. Regulation of plasma membrane recycling by CFTR. Science. 1992 Apr 24;256(5056):530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Kirk K. L., Bridges R. J. Regulated endocytosis in a chloride secretory epithelial cell line. Am J Physiol. 1992 Mar;262(3 Pt 1):C752–C759. doi: 10.1152/ajpcell.1992.262.3.C752. [DOI] [PubMed] [Google Scholar]
- Broussard D. L., Wiedner E. B., Li X., Altschuler S. M. NMDAR1 mRNA expression in the brainstem circuit controlling esophageal peristalsis. Brain Res Mol Brain Res. 1994 Dec;27(2):329–332. doi: 10.1016/0169-328x(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Buchdahl R. M., Cox M., Fulleylove C., Marchant J. L., Tomkins A. M., Brueton M. J., Warner J. O. Increased resting energy expenditure in cystic fibrosis. J Appl Physiol (1985) 1988 May;64(5):1810–1816. doi: 10.1152/jappl.1988.64.5.1810. [DOI] [PubMed] [Google Scholar]
- Buchdahl R. M., Fulleylove C., Marchant J. L., Warner J. O., Brueton M. J. Energy and nutrient intakes in cystic fibrosis. Arch Dis Child. 1989 Mar;64(3):373–378. doi: 10.1136/adc.64.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzá L., Giardino L., Battistini N., Zanni M., Galetti S., Protopapa F., Velardo A. Increase of neuropeptide Y-like immunoreactivity in the paraventricular nucleus of fasting rats. Neurosci Lett. 1989 Sep 25;104(1-2):99–104. doi: 10.1016/0304-3940(89)90336-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohn J. A., Nairn A. C., Marino C. R., Melhus O., Kole J. Characterization of the cystic fibrosis transmembrane conductance regulator in a colonocyte cell line. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2340–2344. doi: 10.1073/pnas.89.6.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M., Flotte T., Afione S., Solow R., Zeitlin P. L., Carter B. J., Guggino W. B. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992 Aug 13;358(6387):581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- Farouk M., Geoghegan J. G., Pruthi R. S., Thomson H. J., Pappas T. N., Meyers W. C. Intracerebroventricular neuropeptide Y stimulates bile secretion via a vagal mechanism. Gut. 1992 Nov;33(11):1562–1565. doi: 10.1136/gut.33.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M. A., Nemecz Z. K., Shull G. E. Cloning and sequence analysis of rat cystic fibrosis transmembrane conductance regulator. Am J Physiol. 1992 Jun;262(6 Pt 1):L779–L784. doi: 10.1152/ajplung.1992.262.6.L779. [DOI] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989 Jul;69(3):765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Skach W., Baker O., Calayag M. C., Lingappa V., Verkman A. S. A multifunctional aqueous channel formed by CFTR. Science. 1992 Nov 27;258(5087):1477–1479. doi: 10.1126/science.1279809. [DOI] [PubMed] [Google Scholar]
- Hyde T. M., Miselis R. R. Area postrema and adjacent nucleus of the solitary tract in water and sodium balance. Am J Physiol. 1984 Jul;247(1 Pt 2):R173–R182. doi: 10.1152/ajpregu.1984.247.1.R173. [DOI] [PubMed] [Google Scholar]
- Hyde T. M., Miselis R. R. Effects of area postrema/caudal medial nucleus of solitary tract lesions on food intake and body weight. Am J Physiol. 1983 Apr;244(4):R577–R587. doi: 10.1152/ajpregu.1983.244.4.R577. [DOI] [PubMed] [Google Scholar]
- Lee B. H., Lynn R. B., Lee H. S., Miselis R. R., Altschuler S. M. Calcitonin gene-related peptide in nucleus ambiguus motoneurons in rat: viscerotopic organization. J Comp Neurol. 1992 Jun 22;320(4):531–543. doi: 10.1002/cne.903200410. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Lee M. G., Cowan N. J. Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol. 1985 Sep;101(3):852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs G. L., Chang X. B., Kartner N., Rotstein O. D., Riordan J. R., Grinstein S. The cystic fibrosis transmembrane regulator is present and functional in endosomes. Role as a determinant of endosomal pH. J Biol Chem. 1992 Jul 25;267(21):14568–14572. [PubMed] [Google Scholar]
- Marino C. R., Matovcik L. M., Gorelick F. S., Cohn J. A. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991 Aug;88(2):712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. V., Nghiem P. T., Gardner P., Martens C. L. Human lymphocytes transcribe the cystic fibrosis transmembrane conductance regulator gene and exhibit CF-defective cAMP-regulated chloride current. J Biol Chem. 1992 Feb 15;267(5):3242–3248. [PubMed] [Google Scholar]
- Merchenthaler I., Lennard D. E. The hypophysiotropic neurotensin-immunoreactive neuronal system of the rat brain. Endocrinology. 1991 Dec;129(6):2875–2880. doi: 10.1210/endo-129-6-2875. [DOI] [PubMed] [Google Scholar]
- Miselis R. R., Hyde T. M., Shapiro R. E. Area postrema and adjacent solitary nucleus in water and energy balance. Fed Proc. 1984 Dec;43(15):2969–2971. [PubMed] [Google Scholar]
- Mulberg A. E., Tulk B. M., Forgac M. Modulation of coated vesicle chloride channel activity and acidification by reversible protein kinase A-dependent phosphorylation. J Biol Chem. 1991 Nov 5;266(31):20590–20593. [PubMed] [Google Scholar]
- Mulberg A. E., Wiedner E. B., Bao X., Marshall J., Jefferson D. M., Altschuler S. M. Cystic fibrosis transmembrane conductance regulator protein expression in brain. Neuroreport. 1994 Aug 15;5(13):1684–1688. doi: 10.1097/00001756-199408150-00035. [DOI] [PubMed] [Google Scholar]
- O'Rawe A., McIntosh I., Dodge J. A., Brock D. J., Redmond A. O., Ward R., Macpherson A. J. Increased energy expenditure in cystic fibrosis is associated with specific mutations. Clin Sci (Lond) 1992 Jan;82(1):71–76. doi: 10.1042/cs0820071. [DOI] [PubMed] [Google Scholar]
- Pages N., Orosco M., Rouch C., Yao O., Jacquot C., Bohuon C. Fasting affects more markedly neuropeptide Y than monoamines in the rat brain. Pharmacol Biochem Behav. 1993 Jan;44(1):71–75. doi: 10.1016/0091-3057(93)90282-x. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Shepherd R. W., Holt T. L., Vasques-Velasquez L., Coward W. A., Prentice A., Lucas A. Increased energy expenditure in young children with cystic fibrosis. Lancet. 1988 Jun 11;1(8598):1300–1303. doi: 10.1016/s0140-6736(88)92119-8. [DOI] [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Smith J. J., Welsh M. J. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992 Apr;89(4):1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomezsko J. L., Stallings V. A., Kawchak D. A., Goin J. E., Diamond G., Scanlin T. F. Energy expenditure and genotype of children with cystic fibrosis. Pediatr Res. 1994 Apr;35(4 Pt 1):451–460. doi: 10.1203/00006450-199404000-00013. [DOI] [PubMed] [Google Scholar]
- Tsui L. C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992 Nov;8(11):392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Williams G., Cardoso H., Lee Y. C., Ghatei M. A., Flatt P. R., Bailey C. J., Bloom S. R. Reduced hypothalamic neurotensin concentrations in the genetically obese diabetic (ob/ob) mouse: possible relationship to obesity. Metabolism. 1991 Oct;40(10):1112–1116. doi: 10.1016/0026-0495(91)90139-n. [DOI] [PubMed] [Google Scholar]