Summary
Cyclin K and the closely related cyclins T1, T2a, and T2b interact with cyclin-dependent kinase 9 (CDK9) forming multiple nuclear complexes, collectively referred to as positive transcription elongation factor b (P-TEFb). Through phosphorylation of the C-terminal domain of the RNA polymerase II largest subunit, distinct P-TEFb species regulate the transcriptional elongation of specific genes that play central roles in human physiology and disease development, including cardiac hypertrophy and human immunodeficiency virus-1 pathogenesis. We have determined the crystal structure of human cyclin K (residues 11-267) at 1.5 Å resolution, which represents the first atomic structure of a P-TEFb subunit. The cyclin K fold comprises two typical cyclin boxes with two short helices preceding the N-terminal box. A prominent feature of cyclin K is an additional helix (H4a) in the first cyclin box that obstructs the binding pocket for the cell cycle inhibitor p27Kip1. Modeling of CDK9 bound to cyclin K provides insights into the structural determinants underlying the formation and regulation of this complex. A homology model of human cyclin T1 generated using the cyclin K as a template reveals that the two proteins have similar structures, as expected from their high sequence identity. Nevertheless, their CDK9-interacting surfaces display significant structural differences, which could potentially be exploited for the design of cyclin-targeted inhibitors of the CDK9–cyclin K and CDK9–cyclin T1 complexes.
Keywords: Cyclin K, cyclin T1, CDK9, P-TEFb, crystal structure
Introduction
Cyclins were originally discovered as proteins whose accumulation during the interphase and destruction at mitosis played a critical role in the progression through the eukaryotic cell cycle.1 It was subsequently found that cyclins control the cell cycle by binding to Ser/Thr cyclin-dependent kinases (CDKs) and regulating their activities.1,2 The transient association of CDKs with their specific regulatory cyclin subunits controls several cell cycle processes, including cell growth (CDK4–cyclin D and CDK6–cyclin D complexes in G1 phase), DNA replication (CDK2–cyclin E in G1/S and CDK2–cyclin A in S), and cell division (CDK1–cyclin A and CDK1–cyclin B in G2 and M).1,2 CDKs are inactive in the apo form but upon binding to their cyclin partners they undergo conformational changes leading to partial activation. Subsequent phosphorylation of a conserved threonine in the CDK T-loop (T160 in CDK2) results in full kinase activity. The structural basis underlying cyclin-mediated CDK activation has been revealed by the crystal structures of the CDK2–cyclin A,3-5 CDK2–cyclin E1,6 CDK2–M-cyclin,7 and CDK6–V-cyclin8 complexes.
A second group of CDK–cyclin pairs that includes CDK7–cyclin H, CDK8–cyclin C, CDK9–cyclin K, and CDK9–cyclins T (T1, T2a, and T2b), function as transcriptional regulators of gene expression by phosphorylating the C-terminal domain (CTD) of the largest subunit of RNA polymerase II (RNAPII).9,10 The CDK7–cyclin H complex is a component of the general transcription factor TFIIH,11 the CDK8–cyclin C pair is part of the transcriptional Mediator,12 whereas the complexes of CDK9 with cyclins K, T1, T2a, and T2b constitute the positive transcription elongation factor b (P-TEFb).13-16 The elongation step of eukaryotic mRNA synthesis is precisely regulated by the interactions between RNAPII and transcription elongation factors, which include the negative transcription elongation factor (N-TEF) and P-TEFb.17 RNAPII initiates transcription but the elongation step is halted by its interaction with N-TEF, resulting in abortive initiation and accumulation of premature transcripts. P-TEFb is then recruited to the transcription complex, where the CDK9 subunit phosphorylates the residues S2 and S5 of the RNAPII CTD heptapeptide repeats YSPTSPS18,19 and induces processive elongation.
Human cyclin K was first identified as a protein that could substitute for the function of a G1 phase cyclin in Saccharomyces cerevisiae, and it was classified as a member of the transcriptional cyclin family.20 Its N-terminal region is predicted to contain two canonical cyclin boxes21 that share extensive sequence similarity with human cyclin T1 (Figure 1(a)). Subsequently, it was found that cyclin K interacts with CDK9 in vitro and in vivo and functions as a regulatory subunit of CDK9.22 Remarkably, cyclin K activates transcription only when tethered to RNA but not DNA, suggesting that the CDK9–cyclin K complex may be required by RNA-bound proteins for their transcriptional activity.23 Notably, the cyclin K gene is transcriptionally activated by the tumor suppressor p53 in response to genotoxic stresses, such as adriamycin treatment, ultraviolet and γ radiation.24
Figure 1.
(a) Sequence comparison of human cyclin K (residues 11-267) and cyclin T1 (residues 1-272). The protein sequences were aligned using the program CLUSTALW.59 Hyphens represent gaps inserted for optimum alignment. Identical residues are shown in white on blue background and similar residues are highlighted in yellow. The secondary structure elements of cyclin K, assigned by the program STRIDE60 using both the hydrogen bonding and backbone torsion angles, are indicated at the top. Helical regions in the N- and C-terminal cyclin boxes are colored green and red, respectively. The cyclin T1-specifc motif TRM is boxed and residue C261 that is critical for binding to HIV-1 Tat is shown in red. (b) Stereo view of a weighted 2Fobs−Fcalc electron density map calculated at 1.5 Å and contoured at 2.9 σ. Carbon, nitrogen, oxygen, and sulfur atoms are shown in magenta, blue, red, and yellow, respectively. The figure was made using BOBSCRIPT61 and POV-Ray (www.povray.org).
Two isoforms of CDK9 have been identified in mammalian cells, CDK942 and CDK955, which differ in their N-terminal domains.25,26 The association of these isoforms with cyclins K, T1, T2a, and T2b, results in the formation of eight structurally and functionally distinct P-TEFb complexes. Importantly, sequence differences among cyclins K, T1, T2a, and T2b permit interactions with different regulatory proteins, giving rise to specialized functions of P-TEFb species in the transcriptional control of specific genes, as exemplified by the regulation of human immunodeficiency virus-1 (HIV-1) gene expression by CDK9–cyclin T1 through its association with the viral transactivator Tat.16,27 Tat binds to the Tat-response RNA element (TAR), a stem-loop structure located at the 5′ ends of nascent HIV-1 transcripts, and recruits CDK9–cyclin T1 to stimulate the productive elongation of HIV-1 mRNAs. This interaction requires both cyclin boxes of human cyclin T1 and the Tat–TAR recognition motif (TRM) downstream of the second box harboring the critical residue C261 (Figure 1(a)), which is not present in cyclins K, T2a, or T2b.15,16 In addition to HIV-1 Tat, cellular transcription factors known to associate with P-TEFb complexes include the androgen receptor,28 the aryl hydrocarbon receptor,29 the peroxisome proliferator-activated receptor γ,30 c-Myc,31 MyoD,32 STAT3,33 and NF-κB.34 Through such interactions, distinct P-TEFb species regulate specific genetic networks and participate in the control of cell growth, proliferation, differentiation, apoptosis, adipogenesis, HIV-1 pathogenesis,15,16 and heart hypertrophy.35,36
As a first step towards the elucidation of the structural basis for the CDK9 activation by cyclins K and T1, we determined the crystal structure of human cyclin K in the apo form at 1.5 Å resolution, the highest resolution for a cyclin to date. Despite the overall structural similarity of cyclin K with other cyclins, it displays significant differences, including an additional helix (H4a) in the first cyclin box, which apparently occludes the binding pocket for the cell cycle inhibitor p27Kip1.37 Modeling studies using the cyclin K structure as a template provided insights into the human cyclin T1 and CDK9–cyclin K structures. Given the role of P-TEFb in diverse cellular processes and the pathogenesis of serious human diseases, the present work will facilitate the dissection of the molecular mechanisms underlying gene regulation by distinct P-TEFb species and will provide a framework for the development of specific P-TEFb inhibitors with potential biomedical applications.
Results and Discussion
Structure determination
We expressed a protein fragment spanning residues 11-267 of human cyclin K as a GST fusion in Escherichia coli cells, purified it using affinity chromatography, and released the cyclin K from the GST moiety with thrombin digestion. The cyclin K protein was further purified using ion exchange chromatography, and crystallized by the sitting drop vapor diffusion method. Initial attempts to solve the structure by molecular replacement using other cyclin structures as search models failed (data not shown). The structure was determined by single-wavelength anomalous data from SeMet-substituted crystals and multiple isomorphous replacement with anomalous scattering (MIRAS) from two heavy atom derivatives. The model was refined to 1.5 Å resolution with a crystallographic R factor of 18.3% and an Rfree of 21.8%. The structure is well ordered and has an excellent electron density map (Figure 1(b)).
Cyclin K structure
The overall topology of cyclin K is similar to previously determined cyclin structures,38-42 and consists of fifteen helices, ten of which are arranged in two typical cyclin domains or boxes (Figure 2(a)). The five helices comprising the N- and C-terminal cyclin domains are designated H1–H5 and H1′–H5′, respectively, with the central helices H3 or H3′ being surrounded by the remaining four helices. In spite of an extremely low sequence identity (10.2%), the N- and C- terminal domains have very similar topology and they can be superimposed with an RMSD of 1.69 Å for 49 Cα atoms. However, there are distinct structural differences between the two cyclin domains. At the N terminus, the 310 helix HNa (residues 28-32) and the α-helix HNb (35-39) precede the first cyclin box. Helix H1 is eleven residues longer than H1′, whereas H2 is four residues shorter than H2′. A short 310 helical span H2″ (residues 197-199) exists in the H2′–H3′ loop. Notably, the N-terminal cyclin box has two helices, H4 (113-123) and H4a (126-132), whereas the C-terminal box contains two short remnants of an H4′ helix with 310 geometry (residues 222-224 and 234-237). Helix H5 (136-151) is two residues longer than H5′ (244-257).
Figure 2.
Structural and topological comparison of cyclins K, C, and H. Ribbon and topology diagrams of human cyclin K (a), S. pompe cyclin C (b), and human cyclin H (c). In the ribbon diagrams the corresponding helices in the three cyclins have identical colors. In the topology diagrams the helices are shown as cylinders, with the N-terminal cyclin box helices H1–H5 colored green and the C-terminal box helices H1′–H5′ colored red. The figure was made using PYMOL (www.pymol.org) and TOPDRAW.62
The stability of the cyclin box structure is achieved by hydrophobic interactions and is enhanced by several intradomain hydrogen bonds and salt bridges between residues in the helices. In the N-terminal box these include the interactions between Q37 (HNb) and E46 (H1), E52 (H1) and R92 (H3), R63 (H1) and S122 (H4), T73 (H2) and E107 (H3), Y82 (H2) and T95 (H3), Y93 (H3) and Q131 (H4a), K105 (H3) and E144 (H5), and R121 (H4) and D126 (H4a). In the C-terminal box stabilizing hydrogen bonds are formed between Y161 (H1′) and N190 (H2′), and between Y212 (H3′) and E248 (H5′). The two cyclin boxes pack against each other burying a surface area of 1,465 Å2. Several interdomain contacts at the cyclin box interface stabilize the overall fold, including those between K28 (HNa) and D249 (H5′), T73 (H2) and H159 (H5–H1′ loop), and H79 (H2) and L193 (H2′). Significantly, the residues T73, K105, E144, N190, and L199 that are involved in the stabilization of the cyclin fold and/or participate in interdomain interactions, are invariant in cyclins K, T1 (Figure 1(a)), H,39,40 and C.42
Structural comparison of the transcriptional cyclins K, C, and H
The overall secondary structures and topologies of human cyclin K and Schizosaccharomyces pombe cyclin C are quite similar and their crystal structures can be superimposed with an RMSD of 1.73 Å for 164 Cα atoms (data not shown). Both cyclins have additional N-terminal helices which are disposed differently but their polypeptide chains coincide at the beginning of H1 (Figures 2(a) and 2(b)). Two structural features in cyclin K that are absent in cyclin C include the presence of a short helix H4a in the H4–H5 loop and the disruption of helix H4′ by an insertion of residues 225-233 that results in a greater surface exposure of these residues.
Likewise, human cyclins K and H have overall similar topologies and three-dimensional structures but they also exhibit distinct differences (Figures 2(a) and 2(c)). Specifically, cyclin H has a long helix HN instead of the short helices HNa and HNb in cyclin K, and it has an additional short helix between H1′ and H2′ and a long C-terminal helix HC but lacks the helix H4a and the first helical span H4′. Notably, the polypeptide chains enter and exit nearby in cyclins K, C, and H but their N and C termini follow different paths in each of the structures.
A model for the CDK9–cyclin K complex
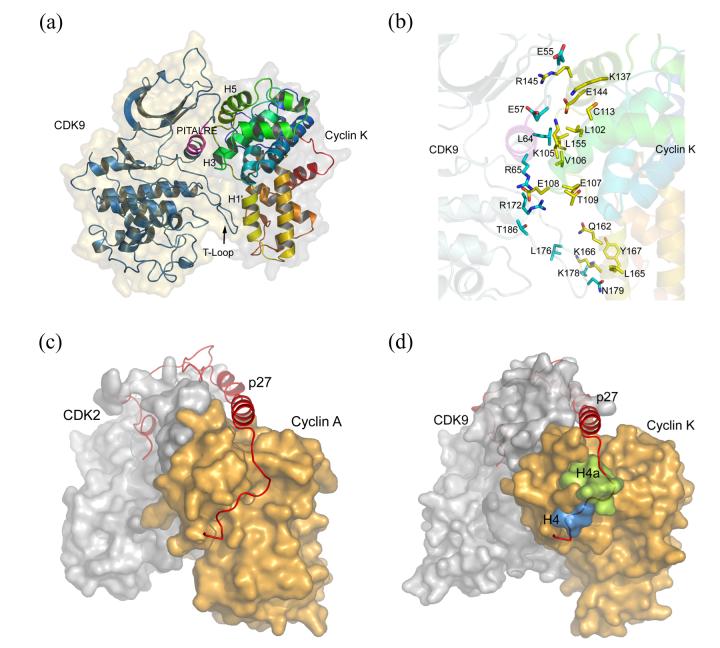
To obtain mechanistic insights into the CDK9 activation by cyclin K, we generated a CDK9–cyclin K model using the crystal structures of cyclin K and the fully-activated CDK2–cyclin A complex (PDB code 1QMZ) as templates (see Experimental Procedures). In the resulting CDK9–cyclin K model many of the intermolecular hydrogen bonds and hydrophobic interactions have their exact counterparts in CDK2–cyclin A, whereas the cyclin K helices HNa and HNb do not interact with CDK9. The critical helix PSTAIRE in CDK2 is replaced by the PITALRE sequence (residues 60-66) in CDK9 (Figure 3(a)). Four of the eleven residues in CDK2 that bind to cyclin A (E40, E42, R50, and R150) have equivalents in CDK9 (E55, E57, R65, and R172, respectively), that are also predicted to interact with cyclin K (Figure 3(b)). In addition, the CDK9 residue L64 likely makes hydrophobic contacts with cyclin K in a manner similar to its corresponding residue in CDK2 (I49) that fits tightly into a hydrophobic pocket of cyclin A.3
Figure 3.
(a) Model of the CDK9–cyclin K complex generated using the program MODELLER based on the CDK2–cyclin A structure (PDB code 1QMZ). The proteins are depicted as ribbon models with superimposed semitransparent molecular surfaces. CDK9 is colored blue and the PITALRE region is colored magenta. Cyclin K is colored as in Figure 2(a). (b) Amino acids at the CDK9–cyclin K interface. Side chains of residues ideally situated for binding are colored cyan (CDK9) and yellow (cyclin K). (c) Surface representation of the CDK2–cyclin A complex bound to p27Kip1 (red ribbon) (PDB code 1JSU). (d) p27Kip1 is shown in the modeled CDK9–cyclin K complex. Binding of the N-terminal p27Kip1 region to cyclin K is obstructed by the C-terminal end of helix H4 (cyan) and helix H4a (green).
The T-loop of CDK9 extends from F174 to T191 and is longer than the T-loop of CDK2. The phosphorylated T160 in CDK2 corresponds to T186 in CDK9, which is situated at the end of the substrate binding groove (Figure 3(b)). Phosphorylation of T186 is essential for CDK9 kinase activity.43 The side chain of T186 is favorably positioned for intramolecular hydrogen bonding of its phosphate group with the guanidino groups of R148 and R172, and the hydroxyl group of Y206, corresponding to a similar arrangement in CDK2.4 The side chain of R148 in CDK9 likely forms intramolecular hydrogen bonds with the carbonyl oxygen of L170 and the hydroxyl group of Y206, further stabilizing this conformation of T-loop, as it is observed in CDK2, where R126 hydrogen bonds with the carbonyl oxygen of L148 and the hydroxyl group of Y180.4 Additional intermolecular contacts may be formed by the side chain of R184 in CDK9, which is suitably placed to interact with H67, E107, and T109 in cyclin K.
Helix H4a obstructs binding of p27Kip1
The protein p27Kip1 was identified as an inhibitor of the CDK2–cyclin A and CDK2–cyclin E complexes in G1 phase of the cell cycle.37 In the crystal structure of CDK2–cyclin A–p27Kip1,44 the N-terminal region of p27Kip1 spans both CDK2 and cyclin A, interacting with both of them (Figure 3(c)). The N-terminal sequence SACRNLFG of p27Kip1 forms a rigid coil that inserts into a shallow groove of cyclin A formed by helices H1, H3 and H4, with the conserved LFG region making many van der Waals contacts to the sequence MRAILVDW in helix H1. Superposition of the CDK9–cyclin K model onto the CDK2–cyclin A–p27Kip1 structure reveals that similar binding of the p27Kip1 to cyclin K is physically blocked by the presence of helix H4a (Figure 3(d)). The p27Kip1 binding groove is also occluded by R63 at the C-terminal end of H1, and residues S122-N125 at the C-terminal end of H4 and the beginning of the H4–H4a loop. Notably, cyclin K lacks the motif MRAILVDW in helix H1 (Figure 1(a)), providing additional structural evidence for the ability of CDK9–cyclin K to evade inhibition by p27Kip1.45
A homology model for human cyclin T1
The lack of structural information on cyclin T1 impedes a thorough understanding of the molecular determinants underlying its interaction with target macromolecules, including CDK9, and the HEXIM–7SK RNA and HIV-1 Tat–TAR nucleoprotein complexes.16 In previous studies, the cyclin H structure was used as a template to generate a cyclin T1 model,46 despite their low sequence similarity (14.2% identity in the cyclin boxes). The high sequence similarity between cyclins K and T1 (32% identity in the cyclin boxes) enabled us to compute a more accurate model of human cyclin T1. The secondary structure, folding, and relative orientation of the cyclin boxes in the cyclin T1 model is virtually identical to cyclin K, and the two molecules superimpose with an RMSD of 0.44 Å (Figure 4(a)). Cyclin T1 has the helices: HNa (residues 16-20), HNb (23-27), H1 (31-52), H2 (56-72), H3 (80-95), H4 (101-111), H4a (121-125), H5 (129-144), H1′ (153-163), H2′ (168-181), H2″ (186-190), H3′ (193-208), H4′ (212-215 and 221-225), and H5′ (231-245). Most of these are identical in length with the corresponding helices of cyclin K, except for H2′, which is five residues shorter in cyclin T1. Notably, there are two modified surface loops in cyclin T1 compared to their counterparts in cyclin K, i.e. a longer H4–H4a loop and a shorter loop inserted in helix H4′ (Figures 1(a) and 4(a)). As in cyclin K, the H4a helix in cyclin T1 also seems to occlude the p27Kip1 binding groove. However, it was not possible to model the TRM region of cyclin T1 because a corresponding region does not exist in cyclin K. Importantly, recent studies provided evidence that the TRM is disordered in the apo form and adopts a structure upon binding to Tat and TAR.47 Therefore, the structural basis for the cyclin T1 interaction with HIV-1 Tat and TAR will require future structural analysis of this ternary complex.
Figure 4.
(a) Stereo view of a human cyclin T1 model (colored gray) superimposed to the cyclin K structure (colored orange). (b) and (c) Surface representation of the CDK9-interacting surfaces of cyclins K and T1, respectively. Identical, similar, and different cyclin residues at the interface with CDK9 are colored blue, yellow, and red, respectively. Figure (a) was made using PYMOL and Figures (b) and (c) using GRASP.63
Comparison of the CDK9-interacting surfaces of cyclins K and T1
The two boxes of cyclins K and T1 have eighty identical residues, fifty-three of which are located in the first cyclin box that interacts with CDK9 (Figure 1(a)). In both cyclins, many of these residues form a continuous binding interface for CDK9, including F101, L102, K105, V106, E107, E108, P110, K111, K112, E144, I146, L148, Q149, F153, and L155 in cyclin K, which correspond to F89, L90, K93, V94, E95, E96, P98, K99, K100, E137, I139, L141, Q142, F146, and L148 in cyclin T1, respectively (Figures 4(b) and (c)). In addition, the surface loop between H2 and H3 (S74-P79) and the short 310 helix H2″, which acts as the primary interface between the N- and the C-terminal cyclin boxes, are conserved in cyclins K and T1.
Strikingly, despite the large number of conserved residues that likely mediate the interaction of cyclins K and T1 with CDK9, the two cyclin surfaces display significant structural differences (Figures 4(b) and (c)). Specifically, two prominent clusters of non-identical residues include C113, K137, E138, R145 (cluster I), and H67, D69, T109, Q156, Q162, F163, Y167, Q170 (cluster II) in cyclin K, corresponding to L101, V130, Q131, S138 (cluster I), and S55, A57, Q97, T149, T155, H156, C160, L163 (cluster II) in cyclin T1, respectively. It is therefore conceivable that these structural differences could be exploited for the development of inhibitors that could bind preferentially to the surfaces of cyclins K or T1 and disrupt their interaction with CDK9 in a cyclin-dependent manner. A similar approach was taken for the design of peptide inhibitors of the CDK2–cyclin A pair by targeting the cyclin A recruitment site.48 In contrast to CDK9 inhibitors that block indiscriminately all P-TEFb complexes,49 peptidomimetics or small molecules targeting cyclins K or T could selectively affect distinct P-TEFb species and modulate the expression of specific downstream genetic networks, offering new therapeutic approaches for heart hypertrophy, AIDS, and other diseases.
Experimental Procedures
Protein expression and purification
A DNA fragment encoding the human cyclin K region spanning residues 11-267 was amplified by the polymerase chain reaction using the cDNA clone BC015935 (ATCC MGC-9113) as a template and was cloned into a modified pGEX-2T vector (GE Healthcare). Recombinant cyclin K was expressed in E. coli BL21 cells as a glutathione S-transferase (GST) fusion protein by growing the cells at 37°C until they reached an OD600 of 0.5, followed by induction with 0.5 mM IPTG at 30°C for 15 hrs. The cells were harvested by centrifugation, resuspended in phosphate buffered saline (PBS) supplemented with protease inhibitor cocktail tablets (Roche Applied Science) on ice, and lysed on an EmulsiFlex-C3 homogenizer (Avestin). Cell debris was pelleted by centrifugation and the supernatant was loaded onto a glutathione Sepharose 4B column (GE Healthcare). The column was washed with PBS and the fusion protein was eluted with 50 mM Tris-HCl, pH 8.0, 10 mM reduced glutathione. Fractions containing the fusion protein were pooled and digested overnight at room temperature with thrombin (Haematologic Technologies). Following centrifugation at 15,000 × g for 1 hr at 4°C, the clear supernatant was loaded onto a UNO Q ion-exchange column (Bio-Rad), equilibrated with loading buffer (50 mM Tris, pH 7.4 and 1 mM DTT). The column was washed with loading buffer and cyclin K was eluted with a NaCl gradient. Pooled fractions of eluted cyclin K protein were dialyzed overnight in 10 mM acetate, pH 4.8, 25 mM NaCl, 2 mM DTT at 4°C, and were concentrated by ultracentrifugation to 4 mg/ml. Selenomethionine (SeMet)-substituted cyclin K(11-267) was produced in E. coli B834 cells (Novagen) grown in M9 medium supplemented with 40 mg/l SeMet (Sigma), and the protein was purified in the same manner as the unlabeled protein.
Crystallization and data collection
Initial crystallization conditions were obtained using sparse matrix crystal screens (Hampton Research and Nextal Biotechnologies) by the sitting drop vapor diffusion technique. Cyclin K crystals were grown from 2 μl of protein (4 mg/ml) and 2 μl of 0.1 M HEPES, pH 7.5, 2.15 M ammonium sulfate, 3% polyethylene glycol 400, and 5 mM DTT at 20°C. Crystals were cryoprotected by adding stepwise glycerol (15% final concentration), followed by mounting in nylon loops and freezing in liquid nitrogen. A native diffraction data set consisting of high (15 sec exposure time) and low resolution (2 sec exposure time) data from two crystals was collected at 100 K on a Quantum-Q315 CCD detector, beamline X29, at the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory, Long Island, NY. High-resolution data for the SeMet derivative were collected at the Se peak wavelength (0.97887 Å) on a Quantum-210 detector, beamline F2, at the Cornell High Energy Synchrotron Source (CHESS), Ithaca, NY. Heavy atom derivatives were collected at 100 K on an in-house R-AXIS IV image plate system using CuKα radiation from a Rigaku RU-300 X-ray generator. All data sets were processed using the HKL package.50 The crystals belong to space group P21 with unit cell dimensions a = 40.77 Å, b = 69.19 Å, c = 50.51 Å, and β = 93.3°. There is one molecule in the asymmetric unit. Data collection and processing statistics are given in Table 1.
Table 1.
Statistics of structure determination and refinement
| Nativea (λ=0.9793) |
SeMetb (λ=0.97887) |
PCMBSc (λ=1.5418) |
UO2OAc2d (λ=1.5418) |
|
|---|---|---|---|---|
| Resolution (Å) | 25–1.5 | 50–2.0 | 25–2.2 | 25–2.5 |
| Completeness (%) | 99.9 (99.3)e | 99.8 (99.4) | 99.8 (99.6) | 93.2 (93.9) |
| Rsym (%)f | 7.1 (21.7) | 5.5 (21.9) | 6.9 (47.7) | 5.6 (28.7) |
| Riso (%)g | 31.2 | 19.2 | ||
| Number of sites | 6 | 3 | 1 | |
| Refined structure | ||||
| R (%)h | 18.3 | |||
| Rfree (%)h | 21.8 | |||
| RMSD from ideal values | ||||
| Bonds (Å) | 0.022 | |||
| Angles (°) | 1.982 | |||
| Ramachandran plot (%) | ||||
| Most favored | 92.6 | |||
| Allowed regions | 6.1 | |||
| Generous | 0.9 | |||
| Disallowed | 0.4 | |||
| Number of atomsi | 2337 |
Collected at NSLS.
Collected at CHESS.
PCMBS: 4-(Chloromercuri)benzene sulfonic acid, sodium salt; collected in-house on an R-AXIS IV system using CuKα radiation.
UO2OAc2: Uranyl acetate dihydrate; collected in-house on an R-AXIS IV system using CuKα radiation.
Numbers in parentheses correspond to the highest resolution shell (1.54–1.50 Å for Native, 2.07–2.00 Å for SeMet, 2.28–2.20 Å for PCMBS, and 2.59–2.50 Å for UO2OAc2).
Rsym = Σ|I – <I>|/Σ(I), where I is the observed intensity and <I> is the average intensity of symmetry related reflections, respectively.
Riso = Σ|FPH – FP|/ΣFPH, where FPH and FP are the derivative and native structure factors, respectively.
R and Rfree = Σ||Fobs|– k|Fcalc||/ Σ|Fobs|, where 5% of the reflections were omitted for the calculation of Rfree.
254 protein residues (14-267), 3 acetate and 226 water molecules.
Structure determination and refinement
All six Se positions present in the recombinant cyclin K, three mercury sites, and one uranium site were found using SOLVE.51 Initial MIRAS phases were used for automated model building with RESOLVE51 and ARP/wARP,52 followed by cycles of refinement with REFMAC5.53 Inspection of the electron density maps showed very good side chain density and manual model building was carried out using O.54 The complete polypeptide chains of the molecules were examined with Fobs−Fcalc, 2Fobs−Fcalc and omit maps, except for the four N-terminal residues (the vector-derived G and the cyclin K residues S11, V12, and T13), which are disordered and not included in the final model. Solvent molecules were added using the ARP/wARP program in the CCP4 package.55 Three acetate molecules were found associated with the structure and the refinement was continued until convergence was reached. PROCHECK56 and WHATCHECK57 were used for analysis and validation of the refined structure (Table 1). One residue, A15, occurs in the disallowed region of the Ramachandran plot (φ = 74.6°, ψ = −61.8°) and is part of a sharp turn at the N-terminus of cyclin K, which is stabilized by a hydrogen bond between the carbonyl oxygen of S14 and the main chain amide of N16.
Molecular modeling
We used the program MODELLER 8v258 to derive a homology model of human cyclin T1 (residues 10-254) using the cyclin K structure as a template. To generate a CDK9–cyclin K model, the cyclin K was first superimposed with cyclin A in the fully-activated CDK2–cyclin A complex (PDB code 1QMZ), using the Cα atoms in four regions with corresponding topology: residues 47-86, 92-107, 111-120, and 137-155 of cyclin K and residues 208-247, 253-268, 273-282, and 288-306 of cyclin A, respectively. The best superposition of the N-terminal cyclin boxes of cyclins K and A was chosen automatically by the program Superpose in the CCP4 package using the topologies of the proteins. The two structures were superimposed well with a root mean square deviation (RMSD) of 1.04 Å. Subsequently, the cyclin A was removed to generate an intermediate CDK2-cyclin K complex. In parallel, a homology model of CDK9 was generated using MODELLER and the crystal structure of CDK2 (PDB code 1QMZ) as a template, since the CDK2 and CDK9 sequences are 39% identical. The derived CDK9 model was then superimposed onto the CDK2–cyclin K complex using the program LSQKAB in the CCP4 package, followed by removal of the CDK2 molecule. Optimization and refinement of the obtained models were carried out by automated simulated annealing and molecular dynamics. Final homology models for cyclin T1 and CDK9 were selected from five computed models based upon the lowest values of their respective MODELLER objective functions and minimal differences in their discrete optimized protein energy (DOPE) profiles for the loop regions.
Acknowledgements
We thank Aditi Soni and the staff at NSLS and CHESS for assistance with data collection. This work was supported by grants GM065520, DK062162, and AG021964 from the National Institutes of Health, DAMD170210300, DAMD170310563, and W81XWH0510622 from the US Department of Defense, 0275730RGT from the American Foundation for AIDS Research, and the Temple Foundation Discovery Award TLL035927 from the Alzheimer's Association to J.A.A.L.
Abbreviations
- CDK9
cyclin-dependent kinase 9
- CTD
C-terminal domain
- GST
glutathione S-transferase
- HIV-1
human immunodeficiency virus type-1
- N-TEF
negative transcription elongation factor
- PDB
protein data bank
- P-TEFb
positive transcription elongation factor b
- RMSD
root mean square deviation
- RNAPII
RNA polymerase II
- SeMet
selenomethionine
- TAR
Tat-response RNA element
- TRM
Tat–TAR recognition motif
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Coordinates
The atomic coordinates and structure factors of cyclin K have been deposited in the Protein Data Bank with accession code 2I53. The coordinates of the cyclin T1 and CDK9–cyclin K models are available from the authors upon request.
References
- 1.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 2.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 4.Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nature Struct. Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 5.Brown NR, Noble ME, Endicott JA, Johnson LN. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nature Cell Biol. 1999;1:438–443. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]
- 6.Honda R, Lowe ED, Dubinina E, Skamnaki V, Cook A, Brown NR, Johnson LN. The structure of cyclin E1/CDK2: implications for CDK2 activation and CDK2-independent roles. EMBO J. 2005;24:452–463. doi: 10.1038/sj.emboj.7600554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card GL, Knowles P, Laman H, Jones N, McDonald NQ. Crystal structure of a gamma-herpesvirus cyclin-cdk complex. EMBO J. 2000;19:2877–2888. doi: 10.1093/emboj/19.12.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze-Gahmen U, Kim SH. Structural basis for CDK6 activation by a virus-encoded cyclin. Nature Struct. Biol. 2002;9:177–181. doi: 10.1038/nsb756. [DOI] [PubMed] [Google Scholar]
- 9.Oelgeschlager T. Regulation of RNA polymerase II activity by CTD phosphorylation and cell cycle control. J. Cell. Physiol. 2002;190:160–169. doi: 10.1002/jcp.10058. [DOI] [PubMed] [Google Scholar]
- 10.Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RP. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J. Cell Sci. 2005;118:5171–5180. doi: 10.1242/jcs.02718. [DOI] [PubMed] [Google Scholar]
- 12.Boube M, Joulia L, Cribbs DL, Bourbon HM. Evidence for a mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell. 2002;110:143–151. doi: 10.1016/s0092-8674(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 13.Peng J, Zhu Y, Milton JT, Price DH. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price DH. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 2000;20:2629–2634. doi: 10.1128/mcb.20.8.2629-2634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca A, De Falco M, Baldi A, Paggi MG. Cyclin T: three forms for different roles in physiological and pathological functions. J. Cell. Physiol. 2003;194:101–107. doi: 10.1002/jcp.10196. [DOI] [PubMed] [Google Scholar]
- 16.Garriga J, Grana X. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene. 2004;337:15–23. doi: 10.1016/j.gene.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Conaway JW, Shilatifard A, Dvir A, Conaway RC. Control of elongation by RNA polymerase II. Trends Biochem. Sci. 2000;25:375–380. doi: 10.1016/s0968-0004(00)01615-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhou M, Halanski MA, Radonovich MF, Kashanchi F, Peng J, Price DH, Brady JN. Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 2000;20:5077–5086. doi: 10.1128/mcb.20.14.5077-5086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanathan Y, Rajpara SM, Reza SM, Lees E, Shuman S, Mathews MB, Pe'ery T. Three RNA polymerase II carboxyl-terminal domain kinases display distinct substrate preferences. J. Biol. Chem. 2001;276:10913–10920. doi: 10.1074/jbc.M010975200. [DOI] [PubMed] [Google Scholar]
- 20.Edwards MC, Wong C, Elledge SJ. Human cyclin K, a novel RNA polymerase II-associated cyclin possessing both carboxy-terminal domain kinase and Cdk-activating kinase activity. Mol. Cell. Biol. 1998;18:4291–4300. doi: 10.1128/mcb.18.7.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble ME, Endicott JA, Brown NR, Johnson LN. The cyclin box fold: protein recognition in cell-cycle and transcription control. Trends Biochem. Sci. 1997;22:482–487. doi: 10.1016/s0968-0004(97)01144-4. [DOI] [PubMed] [Google Scholar]
- 22.Fu TJ, Peng J, Lee G, Price DH, Flores O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 1999;274:34527–34530. doi: 10.1074/jbc.274.49.34527. [DOI] [PubMed] [Google Scholar]
- 23.Lin X, Taube R, Fujinaga K, Peterlin BM. P-TEFb containing cyclin K and CDK9 can activate transcription via RNA. J. Biol. Chem. 2002;277:16873–16878. doi: 10.1074/jbc.M200117200. [DOI] [PubMed] [Google Scholar]
- 24.Mori T, Anazawa Y, Matsui K, Fukuda S, Nakamura Y, Arakawa H. Cyclin K as a direct transcriptional target of the p53 tumor suppressor. Neoplasia. 2002;4:268–274. doi: 10.1038/sj.neo.7900235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grana X, De Luca A, Sang N, Fu Y, Claudio PP, Rosenblatt J, Morgan DO, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc. Natl. Acad. Sci. USA. 1994;91:3834–3838. doi: 10.1073/pnas.91.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shore SM, Byers SA, Maury W, Price DH. Identification of a novel isoform of CDK9. Gene. 2003;307:175–182. doi: 10.1016/s0378-1119(03)00466-9. [DOI] [PubMed] [Google Scholar]
- 27.Barboric M, Peterlin BM. A new paradigm in eukaryotic biology: HIV Tat and the control of transcriptional elongation. PLoS Biol. 2005;3:e76. doi: 10.1371/journal.pbio.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DK, Duan HO, Chang C. Androgen receptor interacts with the positive elongation factor P-TEFb and enhances the efficiency of transcriptional elongation. J. Biol. Chem. 2001;276:9978–9984. doi: 10.1074/jbc.M002285200. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, Ke S, Chen M, Sheng T. Interactions between the aryl hydrocarbon receptor and P-TEFb. Sequential recruitment of transcription factors and differential phosphorylation of C-terminal domain of RNA polymerase II at cyp1a1 promoter. J. Biol. Chem. 2003;278:44041–44048. doi: 10.1074/jbc.M306443200. [DOI] [PubMed] [Google Scholar]
- 30.Iankova I, Petersen RK, Annicotte JS, Chavey C, Hansen JB, Kratchmarova I, Sarruf D, Benkirane M, Kristiansen K, Fajas L. PPARγ recruits the P-TEFb complex to activate transcription and promote adipogenesis. Mol. Endocrinol. 2006;20:1494–1505. doi: 10.1210/me.2005-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanazawa S, Soucek L, Evan G, Okamoto T, Peterlin BM. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene. 2003;22:5707–5711. doi: 10.1038/sj.onc.1206800. [DOI] [PubMed] [Google Scholar]
- 32.Simone C, Stiegler P, Bagella L, Pucci B, Bellan C, De Falco G, De Luca A, Guanti G, Puri PL, Giordano A. Activation of MyoD-dependent transcription by cdk9/cyclin T2. Oncogene. 2002;21:4137–4148. doi: 10.1038/sj.onc.1205493. [DOI] [PubMed] [Google Scholar]
- 33.Giraud S, Hurlstone A, Avril S, Coqueret O. Implication of BRG1 and CDK9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene. 2004;23:7391–7398. doi: 10.1038/sj.onc.1207972. [DOI] [PubMed] [Google Scholar]
- 34.Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell. 2001;8:327–337. doi: 10.1016/s1097-2765(01)00314-8. [DOI] [PubMed] [Google Scholar]
- 35.Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, Schneider MD. Activation and function of cyclin T-CDK9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nature Med. 2002;8:1310–1317. doi: 10.1038/nm778. [DOI] [PubMed] [Google Scholar]
- 36.Sano M, Schneider MD. Cyclin-dependent kinase-9: an RNAPII kinase at the nexus of cardiac growth and death cascades. Circ. Res. 2004;95:867–876. doi: 10.1161/01.RES.0000146675.88354.04. [DOI] [PubMed] [Google Scholar]
- 37.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 38.Brown NR, Noble ME, Endicott JA, Garman EF, Wakatsuki S, Mitchell E, Rasmussen B, Hunt T, Johnson LN. The crystal structure of cyclin A. Structure. 1995;3:1235–1247. doi: 10.1016/s0969-2126(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 39.Kim KK, Chamberlin HM, Morgan DO, Kim SH. Three-dimensional structure of human cyclin H, a positive regulator of the CDK-activating kinase. Nature Struct. Biol. 1996;3:849–855. doi: 10.1038/nsb1096-849. [DOI] [PubMed] [Google Scholar]
- 40.Andersen G, Busso D, Poterszman A, Hwang JR, Wurtz JM, Ripp R, Thierry JC, Egly JM, Moras D. The structure of cyclin H: common mode of kinase activation and specific features. EMBO J. 1997;16:958–967. doi: 10.1093/emboj/16.5.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze-Gahmen U, Jung JU, Kim SH. Crystal structure of a viral cyclin, a positive regulator of cyclin-dependent kinase 6. Structure. 1999;7:245–254. doi: 10.1016/s0969-2126(99)80035-5. [DOI] [PubMed] [Google Scholar]
- 42.Hoeppner S, Baumli S, Cramer P. Structure of the mediator subunit cyclin C and its implications for CDK8 function. J. Mol. Biol. 2005;350:833–842. doi: 10.1016/j.jmb.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Price JP, Byers SA, Cheng D, Peng J, Price DH. Analysis of the large inactive P-TEFb complex indicates that it contains one 7SK molecule, a dimer of HEXIM1 or HEXIM2, and two P-TEFb molecules containing CDK9 phosphorylated at threonine 186. J. Biol. Chem. 2005;280:28819–28826. doi: 10.1074/jbc.M502712200. [DOI] [PubMed] [Google Scholar]
- 44.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 45.Garriga J, Bhattacharya S, Calbo J, Marshall RM, Truongcao M, Haines DS, Grana X. CDK9 is constitutively expressed throughout the cell cycle, and its steady-state expression is independent of SKP2. Mol. Cell. Biol. 2003;23:5165–5173. doi: 10.1128/MCB.23.15.5165-5173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taube R, Fujinaga K, Irwin D, Wimmer J, Geyer M, Peterlin BM. Interactions between equine cyclin T1, Tat, and TAR are disrupted by a leucine-to-valine substitution found in human cyclin T1. J. Virol. 2000;74:892–898. doi: 10.1128/jvi.74.2.892-898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das C, Edgcomb SP, Peteranderl R, Chen L, Frankel AD. Evidence for conformational flexibility in the Tat-TAR recognition motif of cyclin T1. Virology. 2004;318:306–317. doi: 10.1016/j.virol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Atkinson GE, Cowan A, McInnes C, Zheleva DI, Fischer PM, Chan WC. Peptide inhibitors of CDK2-cyclin A that target the cyclin recruitment-site: structural variants of the C-terminal Phe. Bioorg. Med. Chem. Lett. 2002;12:2501–2505. doi: 10.1016/s0960-894x(02)00508-5. [DOI] [PubMed] [Google Scholar]
- 49.Sausville EA. Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends Mol. Med. 2002;8:S32–S37. doi: 10.1016/s1471-4914(02)02308-0. [DOI] [PubMed] [Google Scholar]
- 50.Otwinowski Z, Minor W. Processing of X-ray crystallographic data in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 51.Terwilliger TC, Berendzen J. Automated MAD and MIR structure solution. Acta Crystallog. sect. D. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 53.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallog. sect. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 54.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallog. sect. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 55.Collaborative Computational Project Number 4 The CCP4 suite: programs for protein crystallography. Acta Crystallog. sect. D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 56.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallog. 1993;26:283–291. [Google Scholar]
- 57.Hooft RW, Vriend G, Sander C, Abola EE. Errors in protein structures. Nature. 1996;381:272. doi: 10.1038/381272a0. [DOI] [PubMed] [Google Scholar]
- 58.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 59.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frishman D, Argos P. Knowledge-based protein secondary structure assignment. Proteins: Struct. Funct. Genet. 1995;23:566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- 61.Esnouf RM. An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graph. Model. 1997;15:132–134. doi: 10.1016/S1093-3263(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 62.Bond CS. TopDraw: a sketchpad for protein structure topology cartoons. Bioinformatics. 2003;19:311–312. doi: 10.1093/bioinformatics/19.2.311. [DOI] [PubMed] [Google Scholar]
- 63.Nicholls A, Sharp KA, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins: Struct. Funct. Genet. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]