Abstract
Tyramine, an endogenous ligand for mammalian trace amine-associated receptors, may act as a neuromodulator that regulates neuronal activity in basal ganglia. Using whole-cell patch recordings of subthalamic nucleus (STN) neurons in rat brain slices, we found that bath application of tyramine evoked an inward current in voltage-clamp in over 60% of all STN neurons. The inward current induced by tyramine was mimicked by the D2-like dopamine receptor agonist quinpirole, but was only partially blocked by the D2-like receptor antagonist sulpiride. In contrast, the D1-like receptor agonist SKF38393 evoked no current in STN neurons. Inward current evoked by tyramine was significantly reduced by the catecholamine uptake inhibitor nomifensine, and by exhausting catecholamines in the brain via pre-treatment with reserpine. Tyramine also reduced the amplitude of GABAA receptor-mediated IPSCs that were evoked by focal electrical stimulation of the slice. Inhibition of IPSCs by tyramine was mimicked by quinpirole and was blocked by sulpiride but not by SCH23390, a D1 receptor antagonist. Moreover, tyramine-induced inhibition of IPSCs was reduced in slices pre-treated with reserpine, and this inhibition could be restored by briefly superfusing the slice with dopamine. These results suggest that tyramine acts as an indirect dopamine agonist in the STN. Although inhibition of IPSCs are mediated by D2-like receptors, the dopamine-dependent inward currents evoked by tyramine do not fit a typical dopamine receptor pharmacological profile.
Keywords: tyramine, dopamine, subthalamic neurons, inward current, synaptic current
1. Introduction
Trace amines are a family of compounds that are chemically related to classical biogenic monoamines. In general, trace amines are produced by decarboxylation of those amino acids that serve as precursors for the classical monoamines dopamine, noradrenaline, and serotonin (Berry, 2004). Consequently, it is not surprising that trace amines and their binding sites are more abundant in monoamine-containing cells such as locus ceruleus and substantia nigra, as well as in areas of the brain that receive monoaminergic innervation, such as the basal ganglia (Durden and Davis, 1993; Vaccari, 1986; Boulton et al., 1977). Although trace amines normally exist in brain at relatively low concentration, levels of trace amines are increased in a variety of human conditions. For example, trace amine levels are greatly increased by levodopa that is used in the treatment of Parkinson’s disease (Edwards et al., 1981). Also, antidepressant agents that inhibit monoamine oxidase slow the metabolic degradation of trace amines and thereby increase levels of trace amines in brain (Branchek and Blackburn, 2003). So even though trace amines are not likely to be stored and released as classical neurotransmitters, there is growing appreciation that these substances may play important roles in human disease (Premont et al., 2001).
Tyramine is a trace amine that is structurally similar to dopamine. This structural similarity makes tyramine a good substrate for both the cytoplasmic dopamine transporter (Sitte et al., 1998; Parker and Cubeddu, 1988) and the vesicular monoamine transporter-2 (Partilla et al., 2006). Disruption of these transport mechanisms by tyramine causes release of dopamine into the extracellular space (Vaccari et al., 1991). Interest in tyramine has grown in recent years since it was suggested that tyramine might be an endogenous ligand for a newly cloned family of trace amine receptors (TARs) (Borowsky et al., 2001; Bunzow et al., 2001). These receptors are G protein-coupled, and their activation is positively coupled to adenylyl cyclase. Trace amines tyramine and β-phenylethylamine bind with nanomolar affinity to the TAR1 subtype, and its mRNA is widely expressed at low levels throughout the brain (Borowsky et al., 2001). Although the physiological consequence of TAR activation is not known, there is some evidence that TAR activation can alter G protein-dependent second messenger systems (Federici et al., 2005). However, pharmacological characterization of TARs has been hampered by the lack of selective antagonists.
Our laboratory has a long-standing interest in the role of dopamine in regulating neuronal activity in the subthalamic nucleus (STN). The STN is composed of glutamate-containing neurons that exert a strong excitatory influence on the major output nuclei of the basal ganglia (Parent and Hazrati, 1995). As a result, the STN is a key basal ganglia structure for the control of normal and abnormal movement. In Parkinson’s disease, the loss of dopamine causes STN neurons to exhibit excessive burst firing of action potentials, which is thought to contribute to symptoms of this disease (Bergman et al., 1998; Guridi and Obeso, 1998). Studies by our lab and elsewhere have shown that dopamine excites STN neurons by evoking an inward current that is mediated by a reduction in a resting K+ conductance (Mintz et al., 1986; Zhu et al., 2002a; Zhu et al., 2002b). However, it is thought that this excitatory effect of dopamine might benefit parkinsonism because membrane depolarization can convert the firing of action potentials from a bursting pattern to that of a single-spike firing pattern (Beurrier et al., 1999). In addition, dopamine has been shown to act presynaptically to inhibit GABA-mediated synaptic transmission in the STN (Shen and Johnson, 2000; Cragg et al., 2004). Based upon our previous experience with dopamine in the STN, we were interested in extending our studies to characterizing possible dopamine-like actions of a trace amine such as tyramine.
Therefore, we undertook the present study to characterize possible dopaminergic actions of tyramine on membrane properties and synaptic currents in STN neurons. A secondary goal was to identify possible non-dopaminergic actions of tyramine that might be mediated by the newly described TAR. Although our results largely support the conclusion that tyramine is an indirect dopamine agonist, our data also suggest that some actions of tyramine may follow an unconventional pharmacological profile.
2. Materials and Methods
2.1. Tissue preparation
Midbrain slices were prepared from male Sprague-Dawley rats (140–250 g; Simonsen, Gilroy, CA), as described previously (Shen and Johnson, 2000). Animals used in this study were treated in accordance with institutional guidelines and the National Institutes of Health (USA) regarding the care and use of animals for experimental procedures. Briefly, rats were anesthetized with isoflurane and sacrificed by severing major thoracic vessels. The brain was rapidly removed, and horizontal slices (300 μm thick) containing caudal diencephalon and rostral mesencephalon were cut in cold physiological saline with a vibratome (Series 1000, Technical Products International Inc., St Louis, MO). A slice containing the STN was then transferred to a recording chamber (volume 500 μl) and immobilized with an electron microscopy grid. The slice was submerged and superfused (1.5–2 ml/min) with a standard extracellular solution (aCSF) containing (in mM): NaCl, 126; KCl, 2.5; MgCl2, 1.2; NaH2PO4, 1.2; CaCl2, 2.4; glucose, 11; NaHCO3, 21. This solution was saturated with 95% O2 and 5% CO2, and had a pH 7.35 at 35–36 °C. Using a dissection microscope for visual guidance, the STN was located as grey matter about 2.7 mm lateral to the midline and 2.0 mm rostral from the center of the substantia nigra pars reticulata (Paxinos and Watson, 1986).
2.2. Dopamine depletion
In some experiments, brain slices were superfused with the tyrosine hydroxylase inhibitor α-methyl-DL-p-tyrosine (AMPT, 30 μM) for at least three hours before recording in order to deplete dopamine from the cytosolic pool. This treatment interferes with effects of dopamine releasing agents such as amphetamine, as has been shown previously by Mercuri et al. (1989). In other experiments, we also used reserpine in order to interfere with vesicular storage of dopamine (Carlsson, 1975). Rats were given an injection of reserpine (5 mg/kg i.p.) 24 hrs prior to the preparation of brain slices. Thereafter, slices were superfused with 10 μM reserpine and 30 μM AMPT.
2.3. Electrophysiological recordings
Slices were allowed to recover for 1–2 h before starting recordings. “Blind” patch-clamp recordings in whole-cell configuration were conducted in STN neurons with pipettes that had a resistance of 6–8 M when filled with (in mM): K-gluconate, 135; NaCl, 10; CaCl2, 1; EGTA, 10; HEPES, 10; MgATP, 2; Na3GTP, 0.5 (pH 7.3, 290–300 mOsmol/l). Recordings were made in voltage- or current-clamp mode with an Axopatch-1D amplifier and Digidata 1200 digitizer controlled with pClamp 9 software (Molecular Devices, Sunnyvale, CA, USA). Holding currents were measured at −70 mV recorded continuously using a MacLab digitizer controlled with Chart software (AD Instruments, Colorado Springs, CO, USA). Series resistance was monitored intermittently and data were discarded if values changed by more than 20%. Membrane potentials were corrected for the liquid junction potential (−10 mV).
2.4. Synaptic currents
Bipolar stimulation electrodes (tip separation 300–500 μm) were placed in the slice immediately rostral (100–200 μm) to the STN to evoke synaptic responses. A single rectangular pulse (0.1 ms duration) of constant current (0.1–1 mA) was delivered every 10 s. The amplitude of evoked synaptic currents was measured from the average of three responses. An IPSC mediated by GABAA receptors was isolated pharmacologically by recording in the presence of (±)-2-amino-5-phosphonopentanoic acid (AP5, 25 μM) and 6-cyano-7-nitro-quinoxalone (CNQX, 10 μM) in order to block ionotropic glutamate receptors. The IPSC was confirmed as being mediated by GABAA receptors by blocking the response with the antagonist bicuculline methiodide (BMI, 30 μM).
2.5. Drug application
All drugs added to the superfusate were first dissolved as aqueous stock solutions with the exception of CNQX, sulpiride and nomifensine, which were dissolved in dimethyl sulfoxide (DMSO). Each stock solution was diluted at least 1:1000 to the desired concentration in superfusate immediately prior to its use. DMSO, diluted 1:1000 in superfusate, had no effect on either holding current or synaptic currents. Approximately 30 s were required for the drug solution to enter the recording chamber due to passage of the perfusate through a heat exchanger. Complete exchange of the bath solution occurred within 2 min. D-amphetamine sulphate (amphetamine), AMPT, BMI, dopamine, noradrenaline, nomifensine, quinpirole, reserpine, SCH23390, SKF82958, SKF38393, sulpiride and para-tyramine (tyramine) were obtained from Sigma Chemical Co. (St Louis, MO, USA). AP5, CNQX, prazosin and tetrodotoxin (TTX) were purchased from Tocris Cookson Inc. (Ellisville, MO, USA).
2.6. Data analysis
Numerical data in the text and error bars in figures are expressed as mean ± SEM. Statistical significance was tested using the Student’s two-tailed t-test that was performed using SigmaStat statistical software (Systat Software, Point Richmond, CA, USA). Statistical tests were unpaired except when stated otherwise. A difference was accepted as significant when P < 0.05.
3. Results
3.1. Types of current evoked by tyramine
Voltage-clamp recordings in TTX (0.5 μM) showed that tyramine (100 μM) evoked inward currents, outward currents, or biphasic responses, as measured at a holding potential of −70 mV (see Fig. 1A). However, tyramine most commonly evoked inward current (11.2 ± 0.6 pA), which was observed in 61% (38/62) of cells tested. This inward current began within 2 min of starting perfusion with tyramine, and it reversed within 5 minutes after washout. Inward currents could be evoked repeatedly when tyramine was applied at 20 min intervals. Because tyramine at concentrations ≤ 10 μM produced no response (n = 4), all subsequent studies utilized the 100 μM concentration.
Fig. 1.
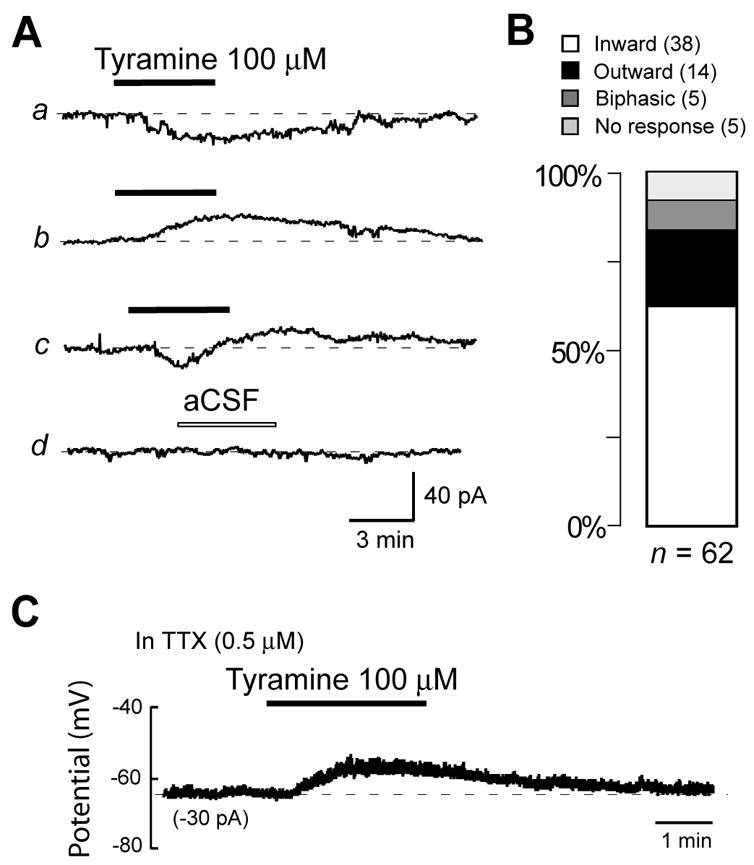
Tyramine evokes currents in STN neurons recorded under voltage-clamp (−70 mV). (A) Tyramine evokes inward currents (a), outward currents (b), and biphasic responses (c), whereas superfusion with aCSF produces no response (d). (B) Histogram showing that inward current is the most common type of current induced by tyramine (100 μM) in a population of STN neurons (n = 62). Voltage-clamp recordings were performed in TTX (0.5 μM) or in the presence of AP5 (25 μM), CNQX (10 μM) and BMI (30 μM). (C) Voltage trace recorded under current-clamp showing that tyramine depolarizes an STN neuron in the presence of TTX (0.5 μM).
Tyramine could also evoke outward currents (12.6 ± 1.6 pA), although it was observed in only 22% (14/62) of cells tested. Of the remainder of cells tested, 8% showed biphasic responses, and 8% showed no significant (< 2 pA) response to tyramine (Fig. 1B). When recording under current-clamp conditions, tyramine (100 μM) most frequently evoked membrane depolarization, as shown in Fig. 1C. Membrane depolarization was observed in the presence of either TTX (0.5 μM; n = 5) or blockers of glutamate and GABA receptors (25 μM AP5, 10 μM CNQX, 30 μM BMI; n = 11). These results suggest that the membrane depolarizing action of tyramine is not action potential-dependent and is not mediated by changes in glutamate or GABA release. Because inward current (or membrane depolarization) was the most common type of response to tyramine, our subsequent studies of tyramine focused on inward currents in voltage-clamp and on membrane depolarization recorded in current-clamp mode.
3.2. Tyramine-induced inward current is dopamine-dependent
In order to explore the pharmacological basis for inward current evoked by tyramine, we first tested actions of selective dopamine agonists. As shown in Fig. 2A, tyramine (100 μM) was mimicked by the dopamine D2 receptor agonist quinpirole (10 μM), which evoked an average inward current of 17.0 ± 2.2 pA (n = 8). In contrast, the dopamine D1 agonist SKF38393 (10 μM) produced no significant inward current (2.1 ± 1.5 pA; n = 10). Comparable results were found when recording membrane voltage under current-clamp mode (Fig. 2B). Tyramine caused an average membrane depolarization of 5.4 ± 0.5 mV (n = 11), which was similar to that evoked by quinpirole (6.2 ± 0.4 mV; n = 8). In contrast, the selective dopamine D1-like receptor agonist SKF82958 (5 μM) caused no significant change in membrane potential (n = 4). These data suggest that tyramine has an action similar to that of dopamine, which has been shown previously to evoke inward currents in STN neurons by reducing a resting K+ conductance (Zhu et al., 2002b).
Fig. 2.
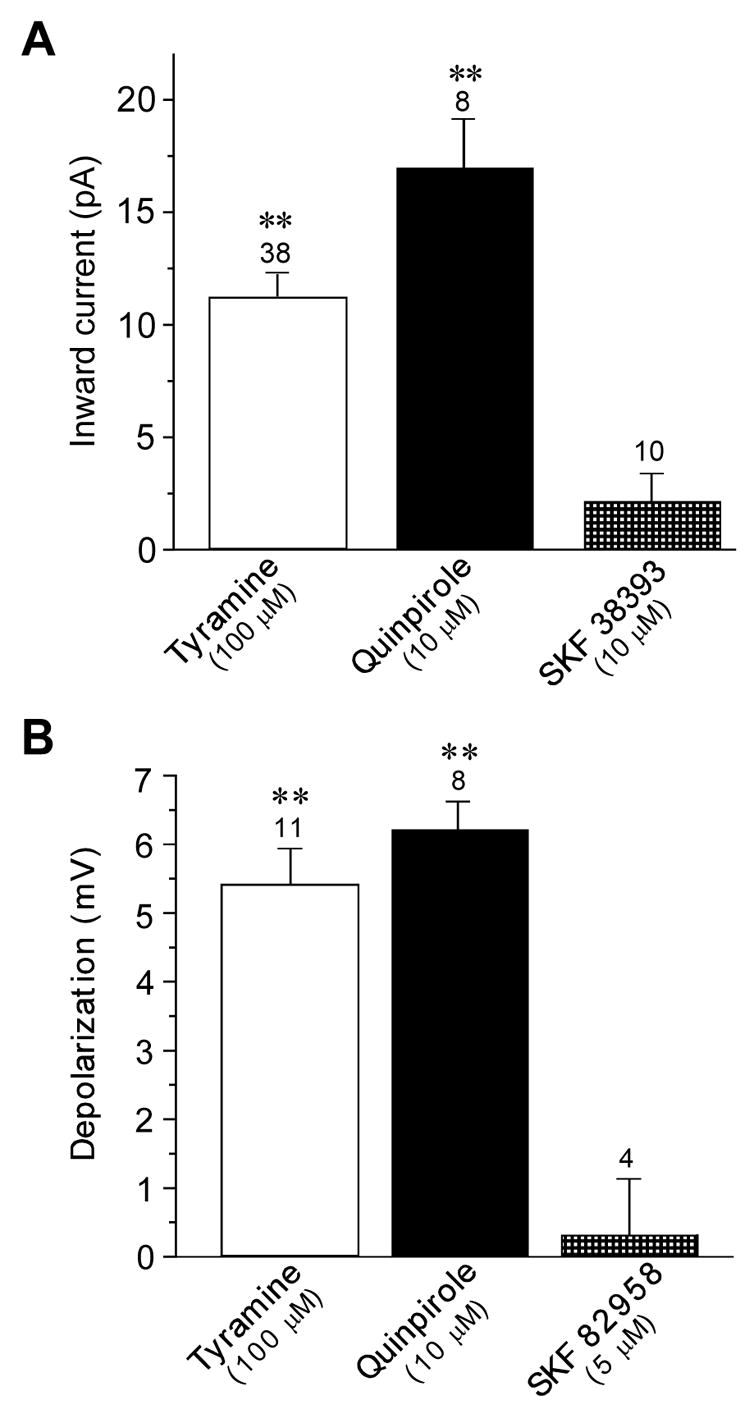
Actions of tyramine are mimicked by the dopamine D2 receptor agonist quinpirole. (A) Histogram shows that tyramine and quinpirole produced significant inward currents recorded under voltage-clamp, whereas the dopamine D1 receptor agonist SKF38393 did not evoke significant current. (B) Histogram shows that tyramine and quinpirole cause significant membrane depolarization, whereas the dopamine D1 receptor agonist SKF82958 did not. The number above each bar indicates the number of neurons tested. Double asterisks indicate significant differences (P < 0.01) Currents were measured at −70 mV.
To further investigate a possible dopamine-mediated action of tyramine, we tested the ability of dopamine to occlude inward current generated by tyramine. In the presence of dopamine (100 μM), tyramine (100 μM) only evoked 0.4 ± 0.5 pA of inward current (n = 4). However, when applied alone, dopamine evoked 10.2 ± 3.4 pA and tyramine evoked 8.5 ± 1.0 pA of inward current in these same four neurons. Because currents generated by dopamine occluded the currents induced by tyramine, these data further support the conclusion that actions of tyramine are mediated by the same receptors that are stimulated by dopamine.
Because inward currents evoked by tyramine were mimicked by the D2 agonist quinpirole, we next explored whether or not the D2 receptor antagonist sulpiride could block the effect of tyramine. In these experiments, slices were superfused with sulpiride at least 5 min before tyramine. Although Fig. 3A shows that sulpiride (3 μM) reduced inward currents evoked by tyramine (100 μM), we never observed a complete block of current. On average, sulpiride reduced tyramine-induced inward currents by only 32 ± 3%, from a control value of 13.5 ± 1.8 pA (n = 13). These data suggest that D2 receptor stimulation alone does not completely account for inward currents induced by tyramine.
Fig. 3.
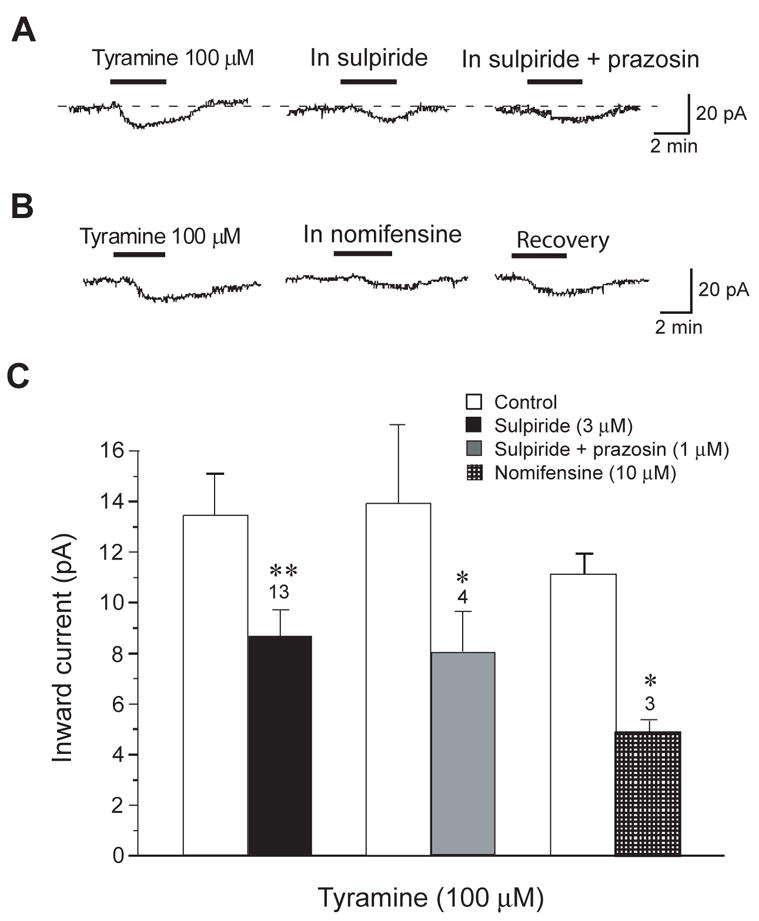
Endogenous dopamine contributes to tyramine-induced inward currents recorded under voltage-clamp (−70 mV). (A) Tyramine-induced inward current is reduced by sulpiride (3 μM), a selective D2 dopamine receptor antagonist. Addition of the adrenergic α1 blocker prazosin (1 μM) failed to further reduce tyramine current. (B) Tyramine-induced inward current is reduced reversibly by the catecholamine transporter blocker nomifensine (10 μM). (C) Histogram summarizing experiments with sulpiride, prazosin, and nomifensine. Although experimental treatments reduced tyramine-induced currents, note that none of the treatments completely blocked the effect of tyramine. Numbers above bars indicate number of cells; asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; paired t-tests).
Tyramine is also known to be a high affinity substrate for dopamine and noradrenaline transporters, and interactions with these transporters can cause release and/or block reuptake of catecholamines (Rothman et al., 2001). Therefore, we next tested the hypothesis that tyramine-induced inward currents require a catecholamine transporter. As seen in Fig. 3B, the catecholamine transporter inhibitor nomifensine (10 μM) significantly reduced tyramine-induced inward currents, although a complete block of current was never observed. In these experiments, slices were superfused with nomifensine at least 5 min before tyramine. On average, nomifensine reduced tyramine-dependent inward current by 56 ± 3%, from a control value of 10.5 ± 0.8 pA (n = 3). Although these data suggest a significant role for a catecholamine transporter in this action of tyramine, the incomplete block of currents by nomifensine suggests that alternate mechanisms of action may contribute the tyramine effect.
Because nomifensine inhibits the uptake of both dopamine and noradrenaline, either of these transmitters could contribute to inward currents evoked by tyramine. In fact, tyramine has been shown to potently release noradrenaline and inhibit its uptake (Rothman et al., 2001). Moreover, noradrenaline has been shown to excite STN neurons by stimulation of adrenergic α1 receptors (Arcos et al., 2003). In confirming this finding, we found that noradrenaline (30 μM) evoked 12.9 ± 1.7 pA of inward current in STN neurons (n = 7). Therefore, we used prazosin, a selective adrenergic α1 receptor antagonist, to investigate possible adrenergic actions of tyramine. But as seen in Fig. 3A, the addition of prazosin (1 μM) failed to add to the inhibitory action of sulpiride (3 μM) on inward currents evoked by tyramine. On average, prazosin plus sulpiride inhibited tyramine-induced currents by 42% (8.0 ± 1.4 pA v. control current of 13.9 ± 3.2 pA), which was not significantly different from the magnitude of inhibition produced by sulpiride alone (n = 4; paired t-test). Therefore, it does not appear that an adrenergic action contributes significantly to inward currents generated by tyramine. Data on prazosin, sulpiride and nomifensine are summarized in Fig. 3C.
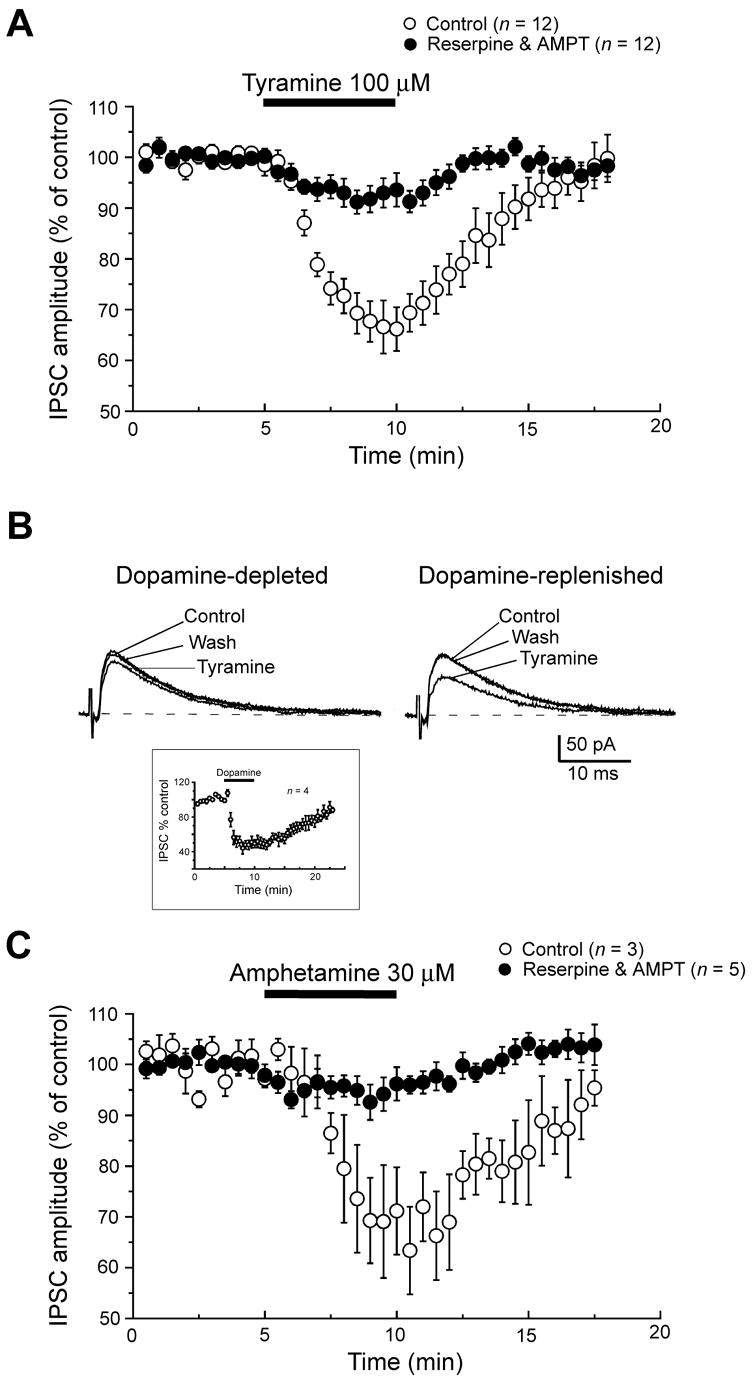
In order to further explore a possible role for endogenous dopamine in effects of tyramine, we performed experiments in brain slices pretreated with drugs to cause dopamine depletion. Therefore, we recorded from STN neurons in slices that had been perfused for at least three hours with the tyrosine hydroxylase inhibitor AMPT (30 μM). We (Munhall and Johnson, 2006) and others (Mercuri et al., 1989) have shown previously that this AMPT treatment greatly reduces the effect sympathomimetic agents that depend upon the presence of cytosolic dopamine to induce membrane currents. However, despite superfusion with AMPT, we found that tyramine (100 μM) evoked an average inward current of 12.5 ± 2.2 pA (n = 8), which was not significantly different the 10.5 ± 0.9 pA (n = 32) of inward current evoked in slices without AMPT (see Fig. 2A). We therefore repeated experiments with rats that had been treated 24 hours prior with reserpine (5 mg/kg, i.p.), which is known to cause depletion of dopamine from synaptic vesicles (Carlsson, 1975). In addition, slices were superfused with AMPT (30 μM) and reserpine (10 μM) three hrs prior to tyramine application. As seen in Fig. 4A, the combined AMPT and reserpine treatments caused a large reduction in tyramine-induced inward current. On average, tyramine (100 μM) only induced 3.9 ± 0.5 pA of inward current in the presence of AMPT and reserpine, which was a significant reduction compared to current evoked in AMPT alone (P < 0.01; see Fig. 4B). Because tyramine-induced inward currents were reduced by reserpine but not by AMPT, these data suggest that tyramine evokes inward currents by causing dopamine release from vesicular storage sites.
Fig. 4.
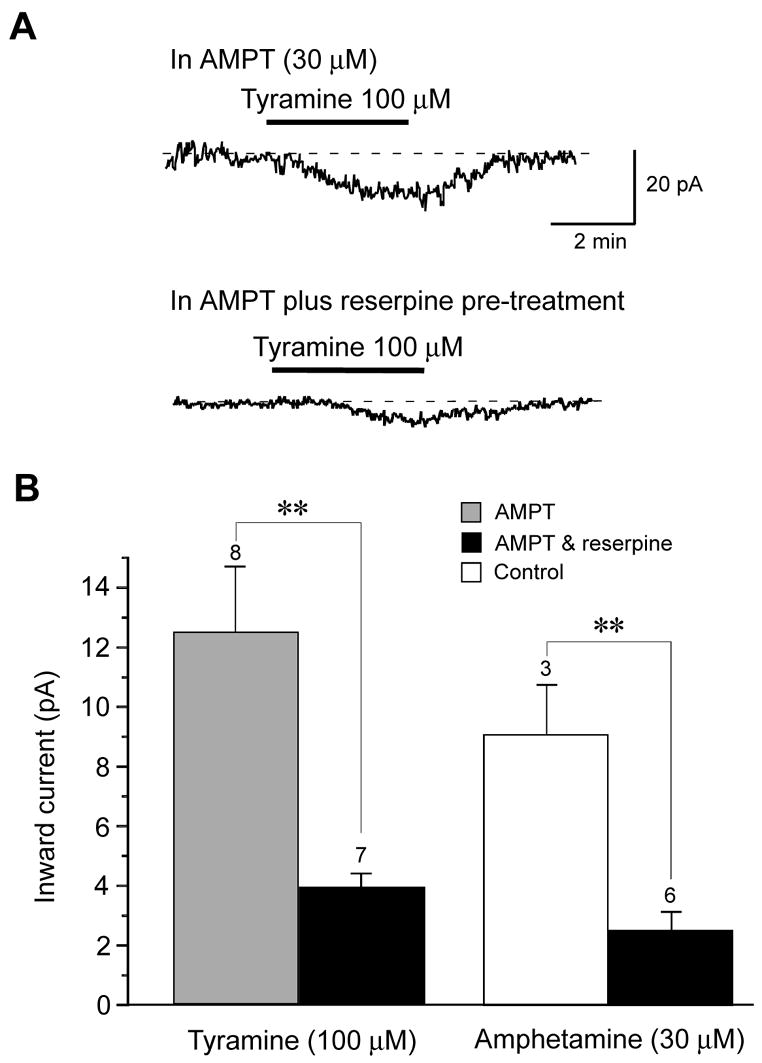
Catecholamine depletion reduces inward currents evoked by tyramine. (A) Despite superfusing the slice for three hours with the dopamine synthesis inhibitor α-methyl-DL-p-tyrosine (AMPT, 30 μM), tyramine was able to evoke inward currents similar to control condition. However, injection of reserpine (5 mg/kg, i.p.) 24 hours prior to brain slice preparation plus superfusion with AMPT (30 μM) and reserpine (10 μM) caused a significant reduction in tyramine-induced inward current. Dashed lines indicates zero current; −70 mV holding potential. (B) Summary histogram showing that reserpine pretreatment significantly reduces tyramine-induced inward currents compared to treatment with AMPT alone. The histogram also shows that inward currents evoked by amphetamine are also significantly reduced by treatment with reserpine plus AMPT, compared to currents evoked in control (untreated) brain slices. The number above each bar represents the number of neurons tested. The double asterisk indicates statistical significance (P < 0.01).
As a control experiment, we also tested the effect of amphetamine because this agent is known to be an effective releaser of dopamine from both cytoplasmic and vesicular pools (Sulzer et al., 1995). As shown in Fig. 4B, pretreatment with AMPT and reserpine reduced inward currents evoked by amphetamine (30 μM) by an average of 72% (P < 0.01). This finding shows that pretreatment with AMPT and reserpine effectively depletes dopamine from brain slice preparations.
3.3. Inhibition of GABAA IPSCs by tyramine
We next examined the ability of tyramine to inhibit IPSCs evoked by focal electrical stimulation of the brain slice. Tyramine (100 μM) reversibly reduced the amplitude of IPSCs, as seen in Fig. 5A. On average, tyramine reduced the IPSC amplitude by 34 ± 5% (n = 12). This inhibitory effect of tyramine was mimicked by the dopamine D2 agonist quinpirole (10 μM), which reduced IPSCs by 39 ± 6% (n = 3). However, the D1 agonist SKF38393 (10 μM) produced no significant effect on IPSC amplitudes (n = 2). Figure 5B shows the time-course for inhibition of IPSCs by tyramine and dopamine agonists. All IPSCs were recorded in AP5 (25 μM) and CNQX (10 μM) in order to block excitatory synaptic transmission. BMI (30 μM) abolished IPSCs, which signifies mediation by GABAA receptors (data not shown).
Fig. 5.
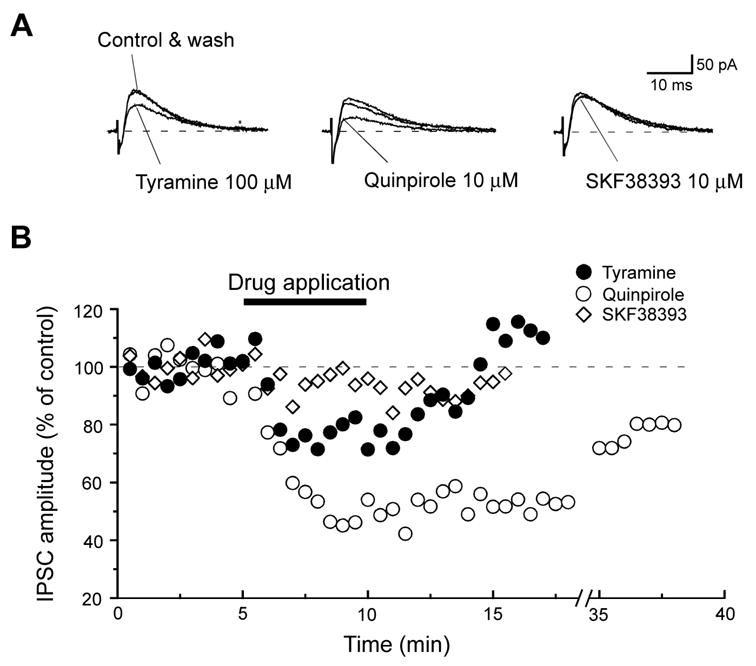
Tyramine inhibits GABAA receptor-mediated IPSCs in a STN neuron. (A) Superimposed current traces showing that both tyramine and quinpirole reversibly inhibit the stimulus-evoked IPSC, but SKF38393 does not. Dashed lines indicate zero current; holding potential was −70 mV. (B) Time-dependent effects of tyramine (100 μM), quinpirole (10 μM), and SKF38393 (10 μM) on IPSCs recorded in the same neuron. All recordings were made in AP5 (25 μM) and CNQX (10 μM).
We used selective dopamine antagonists to characterize the receptor pharmacology of tyramine on IPSCs. As seen in Fig. 6A, the tyramine-induced reduction in IPSC amplitude was largely prevented by superfusing the slice preparation with sulpiride (3 μM). The time-course for tyramine-induced inhibition of IPSCs is shown in Fig. 6B. Under control conditions, a five min application of tyramine (100 μM) reduced IPSC amplitudes by 32 ± 6%, whereas tyramine reduced IPSCs by only 14 ± 3% in the presence of sulpiride (P < 0.01, n = 6; paired t-test). In contrast, SCH23390 (2 μM), an antagonist at D1-like dopamine receptors, failed to block the inhibitory action of tyramine on GABAA IPSCs (Fig. 6C). A summary of data on SCH23390 is shown in Fig. 6D. Neither SCH23390 nor sulpiride changed IPSC amplitude when applied alone. These data suggest that the tyramine action to reduce IPSCs can largely be attributed to activation of dopamine D2-like – rather than D1-like – receptors. Moreover, this conclusion is consistent with a previous report showing that dopamine inhibits GABA-mediated IPSCs via activation of presynaptic dopamine D2 receptors in the STN (Shen and Johnson, 2000).
Fig. 6.
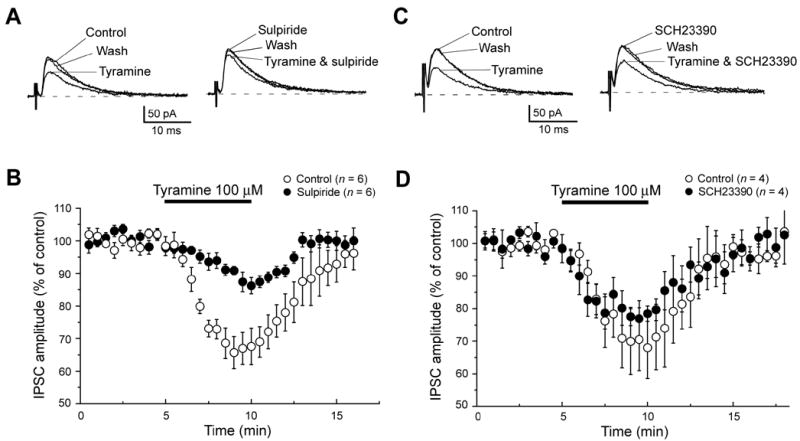
Tyramine-induced inhibition of GABAA IPSCs is reduced by sulpiride. (A) Superimposed current traces show that inhibition of IPSCs by tyramine (100 μM) is reduced by the dopamine D2 receptor antagonist sulpiride (3 μM). (B) Averaged time-dependent data showing that sulpiride (3 μM) reduces but does not completely block the ability of tyramine (100 μM) to reduce IPSC amplitude. (C) Superimposed current traces show that inhibition of IPSCs by tyramine (100 μM) not reduced by the dopamine D1 receptor antagonist SCH23390 (2 μM). (D) Averaged data showing lack of effect of SCH23390 (2 μM) on the ability of tyramine to reduce IPSC amplitude. Currents were measured at −70 mV.
3.4. Tyramine-induced inhibition of IPSCs requires endogenous dopamine
To ascertain whether or not the action of tyramine on GABAA IPSCs is dependent upon release of endogenous dopamine, we examined the effect of tyramine after AMPT and reserpine pretreatment. As seen in Fig. 7A, pretreatment of rats with reserpine (5 mg/kg) and superfusion of slices with reserpine (10 μM) and AMPT (30 μM) significantly reduced the ability of tyramine (100 μM) to inhibit IPSCs. After AMPT and reserpine treatment, tyramine reduced IPSC amplitudes by only 7 ± 3% (n = 12); this effect was markedly smaller than the 34 ± 5% reduction seen in control conditions (P < 0.01).
Fig. 7.
Tyramine-induced inhibition of IPSCs is dependent upon endogenous dopamine. (A) Summary data showing that injection of reserpine (5 mg/kg, i.p.) 24 hours prior to brain slice preparation plus superfusing slices with AMPT (30 μM) and reserpine (10 μM) caused a significant reduction in the ability of tyramine to inhibit IPSC amplitudes, compared to tyramine effects in STN neurons from control slices. (B) Superimposed current traces from dopamine-depleted slices showing that tyramine effectively inhibits IPSCs after superfusing the slice with dopamine (30 μM) for 5 min. Slices were prepared from rats pretreated with reserpine and superfused with AMPT and reserpine, as in “A”. Inset shows the time-course of averaged IPSC amplitudes in response to superfusing the slice with dopamine. (C) Summary data showing that amphetamine inhibits IPSCs in slices from control rats, and that this inhibition is blocked in recordings from slices prepared from rats pretreated with reserpine and superfused with AMPT and reserpine. All currents were measured at −70 mV.
Despite the failure of tyramine to inhibit IPSCs after reserpine and AMPT treatment, the current traces in Fig. 7B show that exposure of slices to exogenous dopamine was able to re-establish the inhibitory effect of tyramine on IPSCs. In these experiments, the first application of tyramine was followed by a 5 min superfusion with dopamine (30 μM). As seen in the inset in Fig. 7B, dopamine caused a reversible inhibition of IPSC amplitude, as has been reported previously (Shen and Johnson, 2000). Tyramine was then reapplied to the slice at least 30 min after dopamine washout, which allowed for complete recovery of IPSC amplitude. As seen in Fig. 7B, tyramine (100 μM) reduced IPSC amplitudes by 30 ± 2% after dopamine application (n = 3); this effect was significantly greater than the magnitude of inhibition produced by tyramine before dopamine application (P < 0.05; paired t-test). Taken together, these results suggest that the inhibition of GABAA-mediated IPSCs by tyramine requires the presence of endogenous dopamine.
As a control experiment, we examined the ability of amphetamine to inhibit IPSCs after reserpine and AMPT treatment. Figure 7C shows that a five min superfusion of amphetamine (30 μM) inhibited IPSC amplitude by 37 ± 8% when recording under control conditions (n = 3). However, Fig. 7C also shows that amphetamine only reduced IPSCs by 6 ± 3% in slices with reserpine and AMPT pretreatment (n = 5; P < 0.01). This result with amphetamine further substantiates our use of reserpine and AMPT as an effective method for causing dopamine depletion in the brain slice.
4. Discussion
4.1. Tyramine inhibits IPSCs via an indirect dopaminergic mechanism
Our finding that tyramine-induced inhibition of IPSCs could be nearly completely blocked by pretreatment with AMPT and reserpine strongly suggests that this action of tyramine depends upon the presence of endogenous catecholamine. Moreover, our finding that a brief exposure of the slice to exogenous dopamine can re-establish the inhibitory effect of tyramine despite the continued presence of AMPT and reserpine is evidence in support of a dopaminergic mechanism for tyramine. An indirect dopaminergic action of tyramine is quite feasible because detailed histologic and neurophysiological studies have demonstrated that the STN receives functional dopaminergic innervation (Cragg et al., 2004; Brown et al., 1979; Hassani et al., 1997). It is useful to note that the inhibition of IPSCs by tyramine was mimicked by amphetamine, and this effect of amphetamine was also blocked by pretreatment with AMPT plus reserpine. This suggests that tyramine has an amphetamine-like effect in which IPSCs are inhibited by release of dopamine and perhaps by blocking reuptake as well. The fact that inhibition of IPSCs by tyramine was mimicked by quinpirole and largely blocked by sulpiride suggests involvement of dopamine D2-like receptors. This receptor pharmacology is consistent with the report by Shen and Johnson (2000) in which dopamine was found to activate presynaptic D2-like receptors to inhibit GABAA IPSCs in STN neurons. Thus, we conclude that tyramine acts as an indirect dopamine agonist to inhibit GABA release in the STN.
4.2. Membrane currents evoked by tyramine
Inward currents evoked by tyramine appear to be largely mediated by endogenous dopamine, although significant questions remain. In support of a dopaminergic mechanism, we found that inward currents evoked by tyramine and amphetamine were reduced about 70% by AMPT and reserpine pretreatment. The catecholamine uptake inhibitor nomifensine significantly reduced tyramine-induced inward currents, which is consistent with the hypothesis that tyramine must bind to the dopamine transporter to cause dopamine release (Sitte et al., 1998). Moreover, mediation by noradrenaline is unlikely because we showed that prazosin failed to block tyramine inward currents. On the other hand, we also found that tyramine evokes outward currents in a minority of STN neurons. This is important, because dopamine has not been reported to evoke outward currents in STN neurons (Mintz et al., 1986; Zhu et al., 2002a; Cragg et al., 2004). Therefore, it appears that tyramine may have multiple actions in the STN, and outward currents may be mediated by a non-dopaminergic action of tyramine.
4.3. Pharmacology of tyramine-induced inward currents
Because we found that inward currents evoked by tyramine were mimicked by quinpirole but not SKF38393, this suggests that this effect of tyramine is mediated by dopamine D2-like receptors. This result would agree with Geracitano et al. (2004) who reported that dopaminergic effects of tyramine could be mimicked by D2 agonists and blocked by the D2 antagonist sulpiride (1 μM) in midbrain dopamine neurons. Our finding that dopamine occluded inward current produced by tyramine also supports the conclusion that tyramine activates dopamine receptors. However, in the present study, we found that 3 μM sulpiride reduced tyramine-induced inward currents by only about 36%. This is surprising, because others (Bowery et al., 1994; Calabresi et al., 1992) and we (Shen and Johnson, 2000) have shown that this concentration of sulpiride is maximally effective in blocking D2-like responses in brain slice preparations. Thus, it is possible that the dopamine receptor mediating the tyramine effect may possess an unconventional pharmacological profile. Indeed, this is consistent with the study by Tofighy et al. (2003) who showed that excitatory effects of dopamine on STN neurons could be mimicked by D2-like agonists, and yet a variety of selective D2 antagonists showed little or no efficacy in blocking this effect of dopamine. In agreement, we therefore conclude that inward currents evoked by tyramine are mediated by dopamine receptors that have an unconventional pharmacology. In contrast, the dopamine-mediated inhibitory effect of tyramine on GABAA IPSCs appears to follow a more conventional D2 receptor profile.
4.4. Proposed mechanism of dopamine release by tyramine
Tyramine has been well characterized as a substrate for the dopamine transporter that causes dopamine release as well as inhibition of uptake (Rothman et al., 2001; Sitte et al., 1998). Similar to amphetamine, data suggest that tyramine acts at the transporter to cause release of dopamine from the cytoplasmic pool (Parker and Cubeddu, 1988). Because newly synthesized dopamine is a major source of dopamine in the cytoplasmic pool, it was surprising that we found that treatment of slices with the tyrosine hydroxylase inhibitor AMPT did not reduce inward currents evoked by tyramine. However, tyramine is also known to bind to reserpine-sensitive sites on dopaminergic synaptic vesicles (Vaccari, 1986). In addition, a recent report has shown that tyramine and related amphetamine-like compounds are substrates of the vesicular monoamine transporter-2 that cause dopamine release from vesicles as well as inhibition of uptake (Partilla et al., 2006). Therefore, our finding that reserpine treatment greatly reduces the actions of tyramine in the STN suggests that tyramine acts primarily on synaptic vesicles to cause release of dopamine into the cytoplasm where it is then available for release through the dopamine transporter. In contrast, our finding that AMPT failed to reduce the tyramine effect suggests that inward currents induced by tyramine are not mediated by newly synthesized dopamine. It is interesting to note that in midbrain dopamine neurons, Geracitano et al. (2004) found that inhibiting new synthesis of dopamine with carbidopa treatment could block dopamine-mediated actions of tyramine, whereas reserpine failed to block tyramine actions (Geracitano et al., 2004). Thus, it appears that tyramine can either release newly synthesized dopamine from the cytoplasmic pool or it can release stored dopamine from the vesicular pool, and the predominant source of dopamine differs according to the neuronal type under investigation.
4.5. Functional implications
Although we have shown that tyramine is an effective indirect dopamine agonist in the STN, the high concentration required suggests that this action of tyramine is likely to be non-physiological. However, under special circumstances, it is possible that dopaminergic actions of tyramine and other trace amines may have functional importance. For example, brain levels of tyramine and other trace amines are greatly increased by monoamine oxidase inhibitors that are sometimes used for treatment of depression (Branchek and Blackburn, 2003). Thus, it is possible that the dopamine releasing properties of trace amines may contribute to the therapeutic effect of monoamine oxidase inhibitors. Trace amines such as tyramine may also have functional significance when levodopa is used to treat symptoms of Parkinson’s disease. Levodopa is used to treat Parkinson’s disease because this drug is readily decarboxylated to dopamine, which thereby replaces the deficient transmitter. However, levodopa treatment and the subsequent metabolism of dopamine increase the levels of many trace amines including tyramine (Edwards et al., 1981; Boulton and Quan, 1970). Furthermore, the loss of dopamine uptake sites in severe Parkinson’s disease causes levodopa treatment to increase the levels of dopamine and dopamine metabolites to a much greater extent compared to levels produced by levodopa in control subjects (de la Fuente-Fernández et al., 2001; Abercrombie et al., 1990). From the standpoint of efficacy, it has been suggested that elevated levels of trace amines might contribute to the superiority of levodopa over synthetic dopamine agonist treatment of Parkinson’s disease (Mercuri and Bernardi, 2005). But it is also possible that the ability of trace amines to release dopamine might contribute to adverse effects of levodopa treatment, such as motor fluctuations and dyskinesia. Moreover, dopamine release from mesocorticolimbic brain regions could contribute to hallucinations and behavioral changes that are often produced by levodopa treatment in subjects with severe Parkinson’s disease. Future studies will be needed to characterize the possible role of trace amines in the therapeutic and adverse effects of levodopa in Parkinson’s disease.
Acknowledgments
This work was supported by NIH/NINDS grant NS38715 and by the Parkinson’s Disease Research, Education, and Clinical Center (PADRECC) at the Portland VA Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Research. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Arcos D, Sierra A, Nuñez A, Flores G, Aceves J, Arias-Montaño JA. Noradrenaline increases the firing rate of a subpopulation of rat subthalamic neurones through the activation of α1-adrenoceptors. Neuropharmacology. 2003;45:1070–1079. doi: 10.1016/s0028-3908(03)00315-0. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends in Neuroscience. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. Journal of Neurochemistry. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. Journal of Neuroscience. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin Mm, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranaova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Procedings of the National Academy of Sciences (USA) 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA, Juorio AV, Philips SR, Wu PH. The effects of reserpine and 6-hydroxydopamine on the concentrations of some arylakylamines in rat brain. British Journal of Pharmacology. 1977;59:209–214. doi: 10.1111/j.1476-5381.1977.tb06996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA, Quan L. Formation of p-tyramine from DOPA and dopamine in rat brain. Canadian Journal of Biochemistry. 1970;48:1287–1291. doi: 10.1139/o70-199. [DOI] [PubMed] [Google Scholar]
- Bowery B, Rothwell LA, Seabrook GR. Comparison between the pharmacology of dopamine receptors mediating the inhibition of cell firing in rat brain slices through the substantia nigra pars compacta and ventral tegmental area. British Journal of Pharmacology. 1994;112:873–880. doi: 10.1111/j.1476-5381.1994.tb13161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Current Opinions in Pharmacology. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Brown LL, Makman MH, Wolfson LI, Dvorkin B, Warner C, Katzman R. A direct role of dopamine in the rat subthalamic nucleus and an adjacent intrapeduncular area. Science. 1979;206:1416–1418. doi: 10.1126/science.505015. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular Pharmacology. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: Physiological and pharmacological characterization. Journal of Neuroscience. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. Monoamine-depleting drugs. Pharmacology and Therapeutics. 1975;1:393–400. doi: 10.1016/0306-039x(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD. Synaptic release of dopamine in the subthalamic nucleus. European Journal of Neuroscience. 2004;20:1788–1802. doi: 10.1111/j.1460-9568.2004.03629.x. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Lu JQ, Sossi V, Jivan S, Schulzer M, Holden JE, Lee CS, Ruth TJ, Calne DB, Stoessl AJ. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson's disease: PET evidence of increased dopamine turnover. Annals of Neurology. 2001;49:298–303. doi: 10.1002/ana.65.abs. [DOI] [PubMed] [Google Scholar]
- Durden DA, Davis BA. Determination of regional distributions of phenylethylamine and meta- and para-tyramine in rat brain regions and presence in human and dog plasma by an ultra-sensitive negative chemical ion gas chromatography-mass spectrometric (NCI-GC-MS) method. Neurochemical Research. 1993;18:995–1002. doi: 10.1007/BF00966759. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Rizk M, Spiker DG. Effects of L-DOPA on the excretion of alcoholic metabolites of catecholamines and trace amines in rat and human urine. Biochemical Medicine. 1981;25:135–148. doi: 10.1016/0006-2944(81)90070-3. [DOI] [PubMed] [Google Scholar]
- Federici M, Geracitano R, Tozzi A, Longone P, Di Angelantonio S, Bengtson CP, Bernardi G, Mercuri NB. Trace amines depress GABAB response in dopaminergic neurons by inhibiting G-βγ-gated inwardly rectifying potassium channels. Molecular Pharmacology. 2005;67:1283–1290. doi: 10.1124/mol.104.007427. [DOI] [PubMed] [Google Scholar]
- Geracitano R, Federici M, Prisco S, Bernardi G, Mercuri NB. Inhibitory effects of trace amines on rat midbrain dopaminergic neurons. Neuropharmacology. 2004;46:807–814. doi: 10.1016/j.neuropharm.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Guridi J, Obeso JA. The role of the subthalamic nucleus in the origin of hemiballism and parkinsonism: new surgical perspectives. Advances in Neurology. 1998;74:235–247. [PubMed] [Google Scholar]
- Hassani O-K, Francois C, Yelnik J, Feger J. Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Research. 1997;749:88–94. doi: 10.1016/s0006-8993(96)01167-5. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bernardi G. The "magic" of L-dopa: why is it the gold standard Parkinson's disease therapy? Trends in Pharmacological Sciences. 2005;26:341–344. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Bernardi G. The mechanism of amphetamine-induced inhibition of rat substantia nigra compacta neurones investigated with intracellular recording in vitro. British Journal of Pharmacology. 1989;98:127–134. doi: 10.1111/j.1476-5381.1989.tb16872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I, Hammond C, Feger J. Excitatory effect of iontophoretically applied dopamine on identified neurons of the rat subthalamic nucleus. Brain Research. 1986;375:172–175. doi: 10.1016/0006-8993(86)90971-6. [DOI] [PubMed] [Google Scholar]
- Munhall AC, Johnson SW. Dopamine-mediated actions of ephedrine in the rat substantia nigra. Brain Research. 2006;1069:96–103. doi: 10.1016/j.brainres.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research Reviews. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parker EM, Cubeddu LX. Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding. Journal of Pharmacology and Experimental Therapeutics. 1988;245:199–210. [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. Journal of Pharmacology and Experimental Therapeutics. 2006;319:237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Procedings of the National Academy of Sciences (USA) 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll I, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Presynaptic dopamine D-2 and muscarine M-3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. Journal of Physiology (London) 2000;525:331–341. doi: 10.1111/j.1469-7793.2000.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. Journal of Neurochemistry. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen T-K, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. Journal of Neuroscience. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighy A, Abbott A, Centonze D, Cooper AJ, Noor E, Pearce SM, Puntis M, Stanford IM, Lacey MG. Excitation by dopamine of rat subthalamic nucleus neurones in vitro -- a direct action with unconventional pharmacology. Neuroscience. 2003;116:157–166. doi: 10.1016/s0306-4522(02)00546-8. [DOI] [PubMed] [Google Scholar]
- Vaccari A. High affinity binding of [3H]-tyramine in the central nervous system. British Journal of Pharmacology. 1986;89:15–25. doi: 10.1111/j.1476-5381.1986.tb11116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari A, del Zompo M, Melis F, Gessa GL, Rossetti ZL. Interaction of 1-methyl-4-phenylpyridinium ion and tyramine with a site putatively involved in the striatal vesicular release of dopamine. British Journal of Pharmacology. 1991;104:573–574. doi: 10.1111/j.1476-5381.1991.tb12470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bartol M, Shen K-Z, Johnson SW. Excitatory effects of dopamine on subthalamic nucleus neurons: in vitro study of rats pretreated with 6-hydroxydopamine and levodopa. Brain Research. 2002a;945:31–40. doi: 10.1016/s0006-8993(02)02543-x. [DOI] [PubMed] [Google Scholar]
- Zhu Z-T, Shen K-Z, Johnson SW. Pharmacological identification of inward current evoked by dopamine in rat subthalamic neurons in vitro. Neuropharmacology. 2002b;42:772–781. doi: 10.1016/s0028-3908(02)00035-7. [DOI] [PubMed] [Google Scholar]


