Abstract
The function of γδ T cells during ruminant paratuberculosis (Johne’s disease) is presently unknown. An ex vivo system was used to test the hypothesis that γδ T cells are capable of activating Mycobacterium avium subsp. paratuberculosis- (M. paratuberculosis)-infected macrophages. Peripheral blood-derived macrophages were infected in vitro with live M. paratuberculosis, and autologous LN-derived γδ T cells or CD4+ T cells were co-cultured with infected macrophages for 48 hours, at which time bacterial survival as well as production of nitrites and IFN-γ were evaluated. Incubation of M. paratuberculosis-infected macrophages with autologous γδ T cells did not result in reduced intracellular bacterial viability compared to infected macrophage cultures without added T cells. IFN-γ production by-infected cultures containing added γδ T cells was not enhanced compared to that of infected macrophages alone. Although infection of macrophage cultures caused increased production of nitrites at both post-infection day (PID) 0 and PID 60, the addition of γδ T cells did not further increase nitrite production. In contrast, addition of PPD-stimulated CD4+ T cells obtained at PID 60 to M. paratuberculosis-infected macrophages resulted in significantly increased IFN-γ production compared to cultures without added T cells or cultures containing unstimulated CD4+ T cells or unstimulated or antigen-stimulated γδ T cells. However, the increased production of IFN-γ by co-cultures containing PPD-stimulated CD4+ T cells did not result in increased bacterial killing or increased production of nitrites compared to cultures without added T cells. In additional in vitro experiments, M. paratuberculosis-infected macrophages, but not uninfected macrophages, were unable to increase nitrite production when stimulated with recombinant IFN-γ. Taken together, the data suggest that (1) γδ T cells do not produce significant IFN-γ and do not significantly increase NO production from M. paratuberculosis-infected macrophages in vitro, (2) the production of significant IFN-γ by antigen-stimulated CD4+ T cells from infected calves is insufficient to enhance mycobacterial killing or nitrite production by infected macrophages, and (3) macrophages may have an impaired NO response following intracellular M. paratuberculosis infection, even in the presence of significant concentrations of IFN-γ.
Keywords: Mycobacterium avium subsp. paratuberculosis, γδ T cells, CD4+ T cells, IFN-γ, macrophage activation
1. Introduction
Paratuberculosis (Johne’s disease) is a chronic, granulomatous enteritis of ruminants caused by infection with Mycobacterium avium subsp. paratuberculosis (M. paratuberculosis). M. paratuberculosis is transmitted from infected cattle to calves through fecal contamination within the environment; some animals apparently clear the infection (Chiodini et al., 1984), while in others, a long subclinical phase follows during which organisms can be intermittently shed in the feces (Whitlock and Buergelt, 1996). After 3–5 years of incubation, animals with clinical disease have progressive weight loss, chronic diarrhea, and decreased milk production (Whitlock and Buergelt, 1996).
The role of IFN-γ-producing CD4+ T cells in the protective immune response to infection with other mycobacteria has been firmly established. Mice deficient in MHC class II or those depleted of CD4+ T cells by monoclonal antibodies succumb much more rapidly to Mycobacterium tuberculosis infection than wild-type mice, and M. tuberculosis-infected IFN-γ knockout mice have significantly shorter survival times than their infected wild-type counterparts (Mogues et al., 2001). It is presumed in M. paratuberculosis infection of cattle that CD4+ T cells play a central role in controlling the infection, although what constitutes a protective response against M. paratuberculosis has yet to be defined. In calves experimentally inoculated via the intratonsillar route, antigen-specific CD4+ T cell proliferation was detected at 286 days post-inoculation, with production of IFN-γ by CD4+ T cells at 313 days post-inoculation (Waters et al., 2003). In orally-inoculated calves, an antigen-specific CD4+ T cell response developed 6 months after infection (Koo et al., 2004). Despite the initial CD4+ T cell response, cell-mediated immunity begins to wane late in the disease (Chiodini, 1996). Other investigators have shown that cattle with clinical paratuberculosis have decreased numbers of CD4+ T cells within the ileal lamina propria and ileocecal lymph node compared to control animals or those with subclinical Johne’s disease (Koets et al., 2002). Decreased expression of IFN-γ mRNA within the ileum and cecal lymph node in animals with clinical illness compared to subclinically-affected animals has also been demonstrated (Sweeney et al., 1998).
In contrast to what is known about CD4+ T cells, the role of γδ T cells in mycobacterial infection is less clear. In humans and non-human primates, γδ T cells proliferate in response to protein (Boom et al., 1994; Born et al., 1990) and non-protein (Morita et al., 1995; Rojas et al., 2002; Wang et al., 2003) antigens of mycobacteria in vitro. Human γδ T cells produce IFN-γ when stimulated with mycobacterial antigens (Garcia et al., 1997) and accumulate in mycobacterial lesions in vivo (Modlin et al., 1989). In Mycobacterium bovis-infected cattle, γδ T cells proliferate (Rhodes et al., 2001) and produce IFN-γ (Vesosky et al., 2004) in response to stimulation with M. bovis antigens. WC1+ γδ T cells appear to play a role in polarizing the developing immune response to M. bovis to a Th1-type response, as infected calves experimentally depleted of WC1+ cells by administration of mAbs had increased antigen-specific production of IL-4, negligible IgG2 production, and decreased innate production of IFN-γ (Kennedy et al., 2002). γδ T cells have been previously shown to accumulate in the granulomatous skin lesions induced by subcutaneous inoculation of calves with M. paratuberculosis (Simutis et al., 2005). Interestingly, acid-fast staining of histologic sections from these lesions showed that M. paratuberculosis was cleared from many of these sites, suggesting a role for γδ T cells in resolution of mycobacterial infection.
Despite the propensity of γδ T cells towards a Th1-type response (Yin et al., 2000), few studies have evaluated the role of γδ T cells in macrophage activation, and no published reports document macrophage activation by γδ T cells during mycobacterial disease. Studies in other experimental systems have suggested that γδ T cells are capable of upregulating effector molecules of macrophage activation. γδ T cells have been shown to enhance macrophage TNF-α production in response to stimulation with LPS (Nishimura et al., 1995) and upregulate macrophage NO production during mucosal infection with Candida albicans (Jones-Carson et al., 1995). The hypothesis for the present study was that γδ T cells draining the site of M. paratuberculosis infection are capable of activating infected macrophages to promote bacterial killing. To test this hypothesis, we co-cultured infected bovine macrophages with autologous γδ T cells and assessed nitrite production and bacterial killing. For these experiments, we used a previously-described subcutaneous infection system in calves to generate antigen-specific γδ T cells and CD4+ T cells (Simutis et al., 2005). Lymph node-derived T cells were co-cultured with autologous, M. paratuberculosis-infected blood-derived macrophages, and macrophage activation was assessed by measuring nitrite production and mycobacterial killing efficacy.
2. Materials and Methods
2.1. Monoclonal antibodies and antigens
The following mouse anti-bovine monoclonal antibodies were purchased from VMRD, Pullman, WA and used to characterize T cells and macrophages by flow cytometry: anti-γδ TCR (GB21A), anti-CD4 (IL-A11), and anti-CD14 (MM61A). Phycoerythrin-conjugated rat anti-mouse IgG (BD Pharmingen, San Diego, CA) was used as a secondary antibody. PPD from M. paratuberculosis strain 19698 (Steadham et al., 2002) used for in vitro lymphocyte stimulation and for in vivo delayed-type hypersensitivity (DTH) testing was a generous gift of Dr. Charles Thoen, Department of Veterinary Microbiology and Preventive Medicine, Iowa State University, Ames, IA.
2.2. Animals
All animal protocols were approved by the Iowa State University Committee on Animal Care. Eight male Holstein calves were purchased from the Iowa State University dairy research farm (Ankeny, IA). This herd has had no clinical cases of paratuberculosis for the five years prior to these experiments, and fecal cultures and antibody ELISA results from cows with chronic diarrhea have consistently been negative for M. paratuberculosis infection. At three weeks of age, calves were transported to biosafety level 2 housing for a one-week period of acclimation prior to use in experiments. Calves were housed in individual concrete rooms, fed milk replacer until eight weeks of age, and provided a complete grain mixture and water ad libitum. Peripheral blood was additionally obtained from two paratuberculosis-free steers maintained in isolation at the Iowa State University research farm. These animals are tested monthly for PPD-specific IFN-γ production by peripheral blood mononuclear cells (PBMC), fecal bacterial shedding, and for M. paratuberculosis-specific antibodies by routine methods.
2.3. Bacteria
M. paratuberculosis strain 19698 was cultured in Middlebrook 7H9 broth supplemented with mycobactin J. Bacteria were maintained in log phase growth by weekly passage; cultures 4 weeks of age were used in these experiments. Aliquots for inoculation into calves containing 100 mg (wet weight) of M. paratuberculosis were frozen at −80° C until use, thawed, and resuspended by gentle sonication prior to inoculation. Previously, an aliquot of this frozen inoculum was thawed and plated in 10-fold dilutions onto 7H10 agar plates; 100 mg wet weight of M. paratuberculosis contained approximately 5 x 108 colony-forming units (CFU) (Simutis et al., 2005). For in vitro experiments, bacteria were washed in sterile saline, and the concentration of bacteria was estimated by measuring the absorbance at 540 nm and comparing the absorbance against a standard curve as previously described (Chiodini and Buergelt, 1993). Bacteria were non-immune serum opsonized by resuspension in autologous pre-inoculation calf serum and incubated for 1 hour at 37° C. Bacteria were centrifuged and resuspended at a concentration of 107 CFU/mL in RPMI 1640 medium containing 10% fetal bovine serum, 0.5 mM 2-mercaptoethanol, and 2 mM L-glutamine.
2.4. Experimental design
For these experiments, a localized M. paratuberculosis infection system developed by our laboratory was utilized (Simutis et al., 2005), with modifications. With this system, antigen-specific T cells from the lymph node draining the site of M. paratuberculosis inoculation can be readily obtained for functional studies. Calves inoculated subcutaneously with live M. paratuberculosis develop a localized immune response by PID 60 characterized by antigen-specific lymphocyte proliferation but negligible antigen-specific IFN-γ production by draining lymph node cells. In addition, these calves develop a DTH response to M. paratuberculosis-PPD.
Superficial cervical lymph nodes were removed (one node prior to inoculation and one at PID 60) to obtain T cells for co-culture with autologous in vitro-infected, blood-derived macrophages. Following a previously-described surgical procedure (Cheville et al., 1992), the right superficial cervical LN (containing M. paratuberculosis-naïve T cells) was excised from each calf at four weeks of age and transported to the laboratory in cold RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin G (100 U/mL), streptomycin (100 μg/mL), amphotericin B (250 ng/mL), 0.5 mM 2-mercaptoethanol, and 2 mM L-glutamine (complete medium). Immediately after excision of the right superficial cervical LN, each calf was inoculated subcutaneously in the left cervical region with 5 x 108 CFU of live M. paratuberculosis in an area draining into the left superficial cervical LN. At post-inoculation day (PID) 60, calves were euthanatized with an overdose of barbiturate, and the left superficial cervical LN was aseptically removed and transported to the laboratory in cold complete medium.
On the day of excision, lymph nodes were processed to obtain LN cells for co-culture with blood-derived macrophages (described below). After ex vivo T cell expansion and monocyte maturation, macrophages were cultured with or without T cells for 48 hours, and supernatants were withdrawn to measure nitrite levels and IFN-γ production. Cell lysates were then plated onto Middlebrook 7H10 agar to count CFU recovered for determination of bacterial killing efficacy.
At PID 57, each calf was tested for a DTH response to M. paratuberculosis PPD. Each calf received an intradermal injection of 0.1 mL PPD in the skin over the right side of the neck (the side opposite that which was inoculated with M. paratuberculosis), and skin at the site of PPD injection was measured before and 72 hours after injection.
2.5. Isolation of PBMC, monocytes, and macrophages, and in vitro infection with M. paratuberculosis
PBMC were isolated by hypotonic lysis of venous blood and plated overnight in 75 cm2 tissue culture flasks in complete medium to allow plastic adherence of monocytes. Nonadherent cells were washed from the flasks with warm PBS. Cultures were incubated for 7 days in complete medium to allow differentiation into macrophages. Preliminary experiments demonstrated that 82±3% of live cells after 7 days of culture were macrophages based upon forward and side scatter properties; macrophages within the live gate were uniformly positive for CD14 surface expression (data not shown). Macrophages were infected with opsonized M. paratuberculosis at a ratio of 10 bacilli per macrophage. After 4 hours, macrophages were washed 3 times in warmed RPMI medium without antibiotics, scraped from the flasks with a rubber cell scraper, and counted. Macrophages were plated in 96-well flat-bottomed tissue culture plates at 1 x 105 macrophages per well. Uninfected macrophage cultures were plated in parallel.
2.6. Harvest of LN-derived T cells
To obtain a single-cell suspension of LN cells, superficial cervical LNs were thinly sectioned on a sterile plate, finely minced with a sterile scalpel blade, and washed from the plate with fresh complete medium through a 70-μm nylon filter (Fisher Scientific, Pittsburg, PA). Resulting suspensions were centrifuged over Histopaque (Sigma, St. Louis, MO) to separate mononuclear cells from cell debris. Mononuclear cells were washed in PBS, counted using trypan blue, and resuspended in complete medium at a concentration of 1 x 107 cells/mL. Aliquots containing 100 μL of this suspension (1 x 106 cells) were plated into 96-well round-bottomed plates. Parallel cultures containing 2x105 cells per well were plated to test in vitro responses by measuring IFN-γ within culture supernatants (see below).
2.7. Expansion of lymphocytes by M. paratuberculosis antigens
Unstimulated cells were cultured with an additional 100 μL of complete medium. Antigen-stimulated cultures received an additional 100 μL of complete medium containing 20 μg/mL PPD (final concentration, 10 μg/mL). After 7 days, cultures were harvested and sorted to obtain purified γδ T cells and CD4+ T cells.
2.8. Purification of γδ T cell and CD4+ T cell populations
Unstimulated and PPD-stimulated LN cells were sorted by positive selection to obtain γδ T cells and CD4+ T cells after 7 days of culture. Wells from each treatment were pooled and resulting suspensions (containing 2 x 107 cells) were washed in PBS. Cells were labeled with anti-γδ TCR or anti-CD4 mAbs and rat anti-mouse microbeads (Miltenyi Biotec, Auburn, CA) and sorted with an AutoMACS cell sorter (Miltenyi Biotec). Following labeling with microbeads, cells were stained with phycoerythrin-conjugated rat anti-mouse secondary antibody. After positive selection, sort purity was checked with a FACScan flow cytometer (Becton-Dickinson, San Jose, CA). For most samples, one cycle of purification was sufficient to obtain >95% pure γδ T cells or CD4+ T cells. When purity was less than 95%, a second cycle of purification was performed. T cells were resuspended in complete medium at a concentration of 5 x 106/mL and rested for 4 hours prior to use in assays.
2.9. Measurement of nitrites and IFN-γ
Rested autologous T cells (5x105 cells) were added to cultured macrophages (1x105 infected or uninfected macrophages), resulting in a ratio of 5 T cells to 1 macrophage in 200 μL of medium. Macrophage:T cell co-cultures were incubated at 37° C and 5% CO2. Preliminary experiments revealed that infected macrophages lifted from the plates by 96 hours but were still adherent at 48 hours post-T cell transfer (data not shown); therefore, cultures were evaluated at 48 hours. Plates were centrifuged and the supernatants removed (175 μL per well). Nitrites were measured in culture supernatants using the Griess reaction; the limit of detection for this assay was 0.78 μM. IFN-γ within supernatants was measured using a commercial ELISA for bovine IFN-γ (BOVIGAM, Biocor Animal Health, Omaha, NE). A standard curve was constructed using recombinant bovine IFN-γ (PBP001, Serotec, Raleigh, NC). The limit of detection for this ELISA was 78 pg/mL. All assays were performed in duplicate.
2.10. Bacterial killing by CFU determination
After removal of the culture supernatants, macrophage:T cell co-cultures were lysed with 0.1% deoxycholate for 2 minutes to release intracellular bacteria. Resulting bacterial suspensions were suspended in sterile saline. Ten-fold dilutions of bacterial suspensions were plated onto Middlebrook 7H10 agar, and colonies were counted using a stereomicroscope after 4 weeks of incubation.
2.11. In vitro stimulation of macrophages with rIFN-γ and LPS
Peripheral blood mononuclear cells were isolated from two paratuberculosis-free animals by density centrifugation over a Histopaque gradient. PBMC were cultured in complete medium at 1x106 cells/mL for 24 hours to allow plastic adherence of monocytes to culture flasks. Nonadherent cells were discarded, fresh complete medium was added to cover the adherent cells, and cultures were incubated for 7 days at 37°C and 5% CO2 in complete medium to allow differentiation into macrophages. Macrophages were scraped from the flasks with a rubber cell scraper, counted, and plated onto 96 well plates at a density of 1 x 105 cells/mL. Preliminary experiments in our lab indicated that recombinant bovine IFN-γ (PBP001), used as a standard in our ELISA assays to quantify IFN-γ in culture supernatants, did not have functional activity in bovine macrophage cultures (data not shown). Preliminary studies in our lab (not shown) as well as by others (Charley et al., 1988) have shown that recombinant porcine IFN-γ (PPP001, Serotec, Raleigh, NC) is biologically active in bovine cells; therefore, PPP001 was used in the following experiments. Twenty-four hours prior to infection, macrophages were incubated in medium alone or with varying concentrations or recombinant porcine IFN-γ, with or without 1 μg/mL LPS; these concentrations were maintained throughout the infection period. Macrophages were infected with serum-opsonized M. paratuberculosis at a ratio of 10 bacilli per macrophage. Cultures were washed after 4 hours, covered with fresh medium without antibiotics, and incubated for 48 hours prior to determination of nitrite concentrations (using the Greiss reaction) and bacterial viability (by flow cytometry, see below).
2.12. Assessment of bacterial viability by flow cytometry
After 48 hours of incubation, culture supernatants were removed for measurement of nitrite concentrations as described above, and macrophages were lysed with deoxycholate and resuspended in a solution of fluorescein diacetate (200 ng/mL) for immediate analysis using a FACScan flow cytometer. Using CellQuest software (Becton-Dickinson), an electronic gate was drawn around bacteria (based upon forward vs. side scatter properties), and the percent of fluorescein-bright bacteria was recorded as the live population.
2.13. Statistical analysis
Data for nitrite concentrations, IFN-γ production, and bacterial survival were compared between macrophage cultures with added T cells and macrophage cultures without added T cells and between cultures containing PPD-stimulated T cells and those containing unstimulated T cells. If the data was not normally distributed, log transformation was performed to normalize the distribution. One-way analysis of variance (ANOVA) was performed on log transformed data, and group means were compared with Student’s t-test. If log transformation failed to normalize the distribution, groups were compared with a Wilcoxon/Kruskal-Wallis rank sums test. Data are expressed as means±standard error of the mean (SEM). Significance was set at P<0.05.
3. Results
3.1 Response to challenge
To determine the in vivo response to challenge of calves with M. paratuberculosis strain 19698, all calves were tested for DTH responses using PPD prepared to this strain (Steadham et al., 2002). Skin at the DTH test site was measured prior to and 72 hours after intradermal injection of PPD. Compared to pre-test thickness of 2.1±0.1 mm (mean±SEM), post-test skin thickness at the DTH site increased to 4.0±0.7 mm (P<0.05). The increased skin thickness at the DTH site was due to the accumulation of mononuclear cells around dermal blood vessels as well as separation of dermal collagen fibers by edema fluid and fibrin (Simutis et al., 2005), characteristic for a type IV hypersensitivity reaction.
In vitro responses to challenge were confirmed in these calves by measuring antigen-specific IFN-γ responses to M. paratuberculosis PPD. Mononuclear cells from the left superficial cervical lymph node (the node draining the site of subcutaneous inoculation with M. paratuberculosis) as well as mononuclear cells from peripheral blood were cultured after harvest at PID 60 in the presence or absence of PPD for 7 days, at which time IFN-γ was measured in the culture supernatants by a commercially-available ELISA. Consistent with the results of DTH testing, PPD-stimulated lymphocytes from each source (LN or peripheral blood) produced significantly greater IFN-γ than unstimulated lymphocytes derived from the same source (Fig. 1). We have shown previously that there is no difference in IFN-γ production between PPD-stimulated or unstimulated lymphocytes from control (saline-inoculated) calves using this subcutaneous challenge system (Simutis et al., 2005); thus, antigen-specific IFN-γ production by lymphocytes in the present study is consistent with an immune response to inoculation with M. paratuberulcosis.
Fig 1.
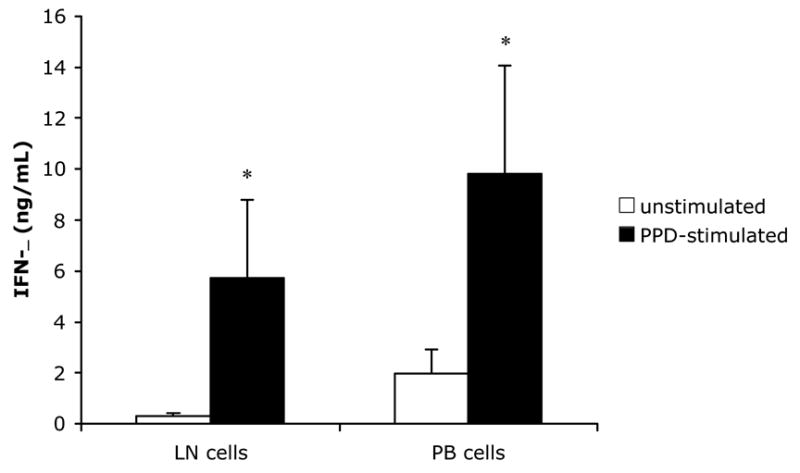
IFN-γ production by mononuclear cell cultures from calves inoculated subcutaneously with 100 mg (wet weight) of M. paratuberculosis. Lymph node (LN) and peripheral blood (PB) cells were harvested at PID 60 and cultured for 7 days in the presence (PPD-stimulated) or absence (unstimulated) of M. paratuberculosis antigen. IFN-γ was measured in culture supernatants with a commercially-available ELISA for bovine IFN-γ as described in Materials and Methods. Bars represent the mean±SEM from 8 animals. For both LN and PB cells, PPD-stimulated lymphocytes produce significantly greater amounts of IFN-γ than unstimulated cells from the same source (P<0.05).
3.2. IFN-γ production by purified lymphocyte subsets co-cultured with M. paratuberculosis-infected macrophages
The ability of M. paratuberculosis-infected macrophages to stimulate IFN-γ production by γδ T cells and CD4+ T cells was determined in co-culture assays containing infected macrophages and T cells at PID 0 and at PID 60. Because of the low recovery of LN cells from preinoculation LNs (i.e., PID 0), only unstimulated T cell subsets were tested. Uninfected macrophage cultures (with or without added T cells) did not produce detectable amounts of IFN-γ (Fig. 2A). When infected in vitro with M. paratuberculosis, cultures released increased amounts of soluble IFN-γ into culture supernatants. Because of the variability between animals, cultures containing added γδ T cells or added CD4+ T cells did not produce significantly greater amounts of IFN-γ than infected cultures without added T cells (Fig. 2A).
Fig. 2.
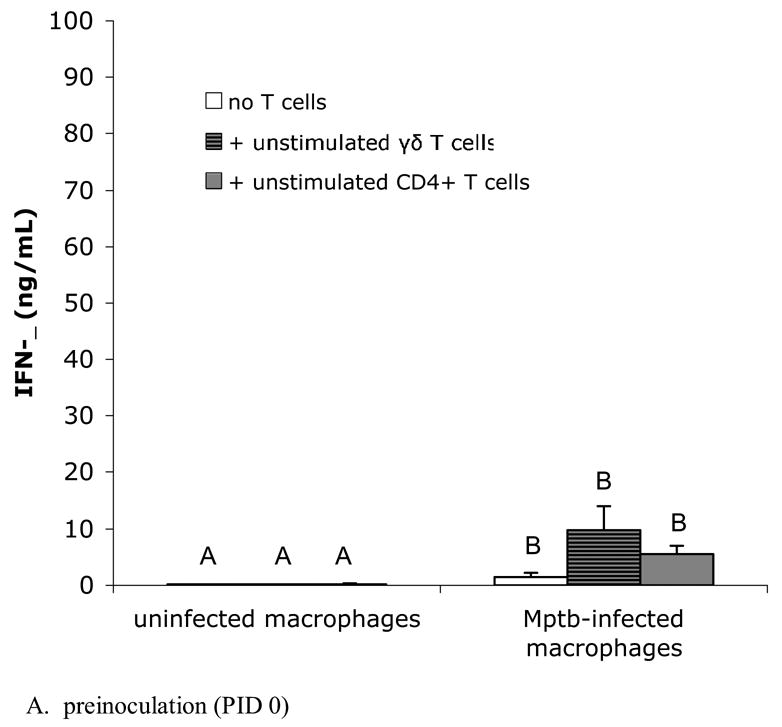
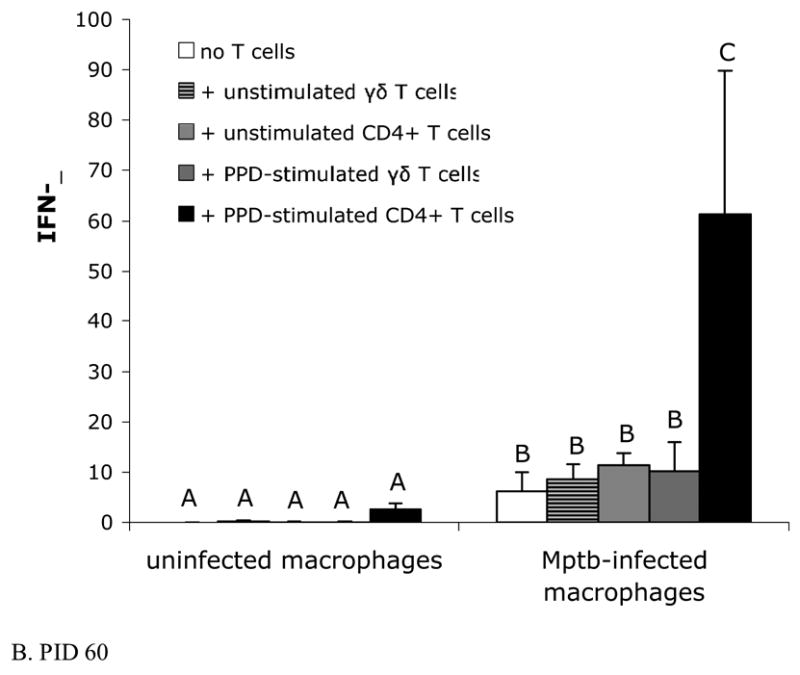
Purified, PPD-stimulated CD4+ T cells from M. paratuberculosis-inoculated animals produce significant IFN-γ when co-cultured with M. paratuberculosis-infected macrophages. IFN-γ was measured in culture supernatants with a commercially-available ELISA for bovine IFN-γ as described in Materials and Methods. Bars represent the mean±SEM from 8 animals. (A) Blood-derived macrophages and lymph node derived T cells from preinoculation (PID 0). In vitro infected macrophage cultures produce increased amounts of IFN-γ compared to uninfected cultures, with or without added T cells, but addition of γδ T cells or CD4+ T cells to infected cultures does not further increase IFN-γ production. Columns not sharing a letter are significantly different (P<0.05). Mptb = M. paratuberculosis. (B) Blood-derived macrophages and T cells from lymph nodes draining sites of in vivo M. paratuberculosis inoculation (PID 60). Co-cultures of in vitro-infected, blood-derived macrophages with PPD-stimulated CD4+ T cells from the draining lymph node produce significantly greater amounts of IFN-γ compared to infected cultures with added unstimulated CD4+ T cells, uninfected macrophage cultures with added CD4+ T cells, or infected macrophage cultures without added T cells. Columns not sharing a letter are significantly different (P<0.05). PPD = purified protein derivative.
At PID 60, macrophages were co-cultured with autologous PPD-stimulated or unstimulated γδ T cells, or with PPD-stimulated or unstimulated CD4+ T cells. As expected, uninfected cultures produced negligible amounts of IFN-γ, and addition of unstimulated or PPD-stimulated T cells to these uninfected macrophages did not increase IFN-γ production by these cultures (Fig. 2B). Compared to uninfected macrophage cultures, macrophage cultures infected in vitro with M. paratuberculosis produced significantly increased amounts of soluble IFN-γ. When PPD-stimulated CD4+ T cells from calves at PID 60 were added to infected autologous macrophage cultures, significantly greater IFN-γ (61.2±28.5 ng/mL) was produced compared to uninfected cultures containing PPD-stimulated CD4+ T cells (2.59±1.2 ng/mL) or M. paratuberculosis-infected macrophage cultures without added T cells (6.1±3.8 ng/mL). This response by autologous PPD-stimulated CD4+ T cells was considered antigen-specific, as the amount of IFN-γ produced by infected cultures containing unstimulated CD4+ T cells was similar to that produced by infected macrophages without added T cells (Fig. 2B). Purified T cells cultured in the absence of infected macrophages did not produce IFN-γ (data not shown).
3.3. Nitrite production by purified lymphocyte subsets co-cultured with M. paratuberculosis-infected macrophages
NO production was assessed by measuring nitrites in culture supernatants using the Griess reaction. As shown in Fig. 3, macrophages at both PID 0 and PID 60 produced increased amounts of nitrites when infected with M. paratuberculosis compared to uninfected macrophages (for PID 60, P<0.05). Infected macrophage cultures at PID 60 (Fig 3B) produced greater amounts of nitrites than infected macrophage cultures at PID 0 (Fig 3A). Addition of T cells, either PPD-stimulated or unstimulated and either γδ T cells or CD4+ T cells, did not cause further increases in nitrite production compared to infected macrophage cultures without added T cells. Uninfected macrophage cultures with and without added T cells produced low or undetectable levels of nitrites. Note that despite substantial IFN-γ production by co-cultures containing PPD-stimulated CD4+ T cells (Fig. 2B), nitrites were not increased in these cultures compared to other culture conditions (Fig 3B). In preliminary experiments, nitrite production by T cells cultured in the absence of macrophages was below the limit of detection (data not shown).
Fig. 3.
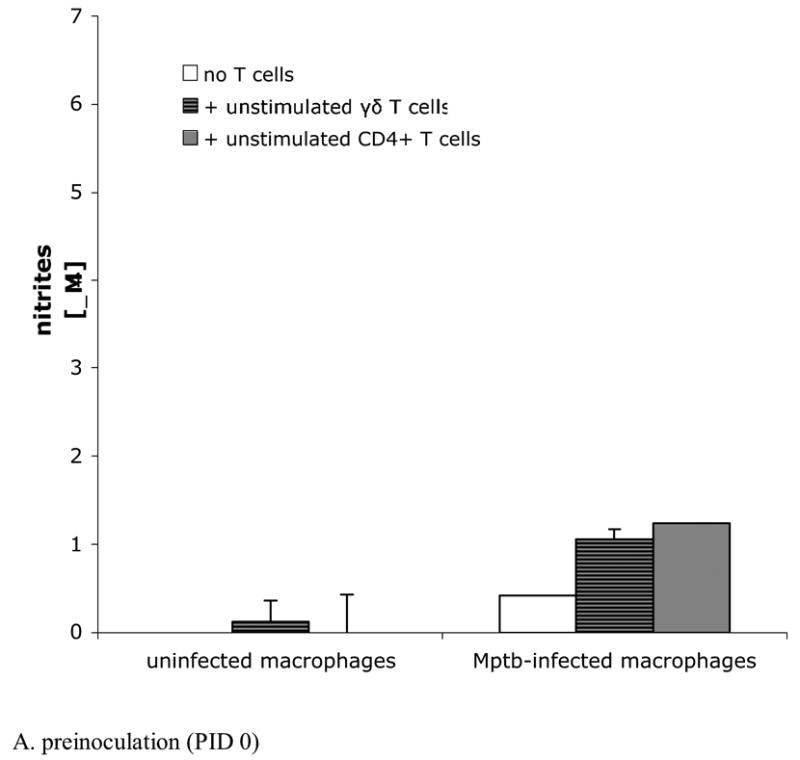
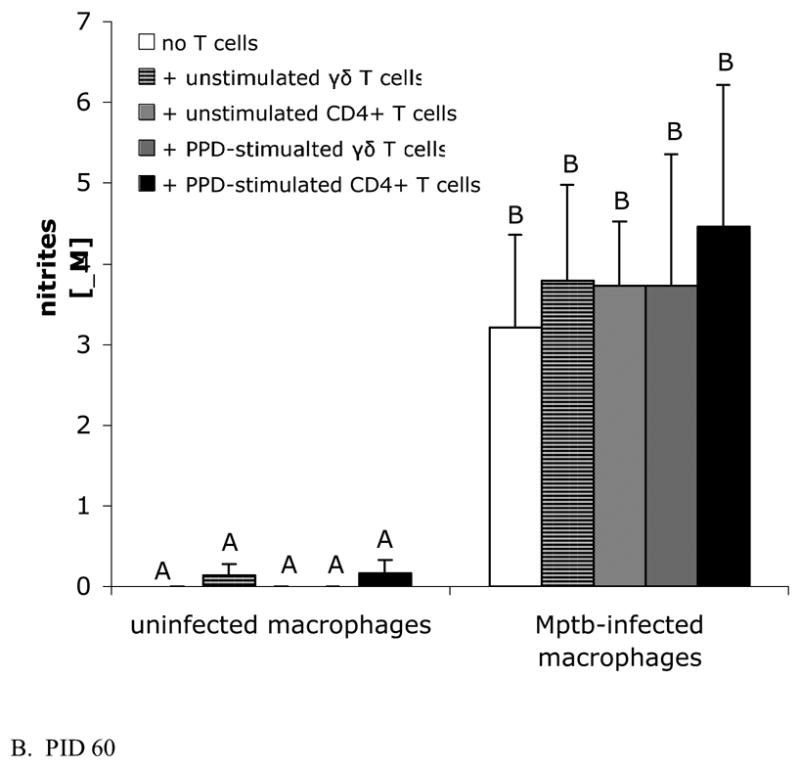
M. paratuberculosis induces nitrite production by infected macrophages, but addition of autologous T cells does not further increase nitrite production. Nitrites were measured in culture supernatants using the Griess reaction. Bars represent the mean±SEM from 8 animals. (A) Cells obtained from calves prior to inoculation with M. paratuberculosis. Blood-derived macrophages infected in vitro with M. paratuberculosis with and without added T cells produce similar levels of nitrites as infected cultures containing autologous unstimulated γδ T cells or unstimulated CD4+ T cells. Mptb = M. paratuberculosis. (B) Blood-derived macrophages and sorted T cells from lymph nodes draining sites of M. paratuberculosis inoculation at PID 60. Infected macrophages, cultured with or without autologous unstimulated or PPD-stimulated γδ T cells or CD4+ T cells, produce similar levels of nitrites. Note that nitrites in cultures containing PPD-stimulated CD4+ T cells are not increased compared to cultures without added T cells, despite the substantial IFN-γ produced by CD4+ T cell-containing cultures (Fig. 2B). Cultures containing uninfected macrophages (with or without added T cells) produce low to undetectable amounts of nitrites. PPD = purified protein derivative.
3.4. Intracellular bacterial viability following culture of macrophages with purified T cells
To assess bacterial killing, cultures were lysed with 0.1% deoxycholate and the resulting bacterial suspensions were plated on 7H10 agar to determine CFU recovered. Because of the low recovery of LN cells from preinoculation LNs (i.e., PID 0), only unstimulated T cell subsets were tested. Mean numbers of CFU recovered from lysed cultures at PID 0 were similar between infected macrophage cultures alone and those with added unstimulated γδ T cells or CD4+ T cells (Fig 4A).
Fig. 4.
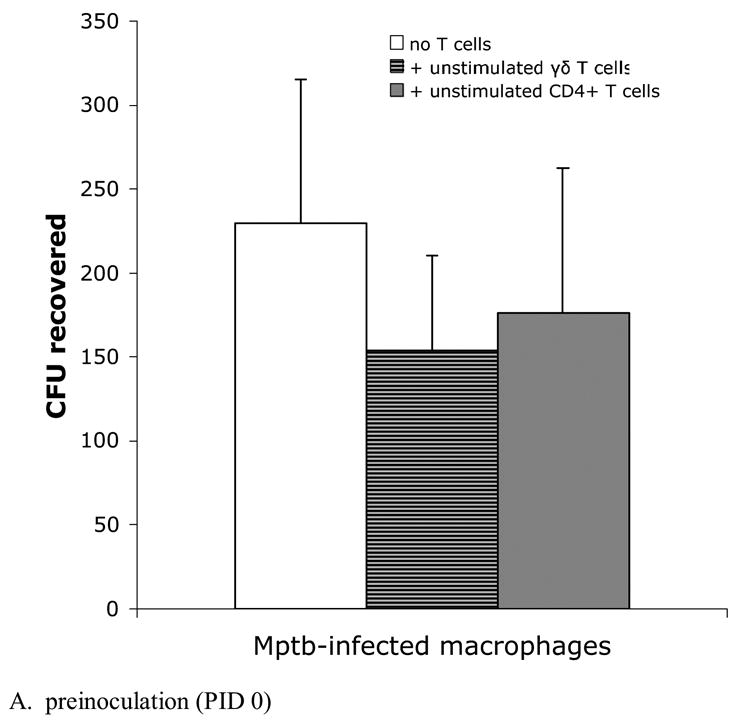
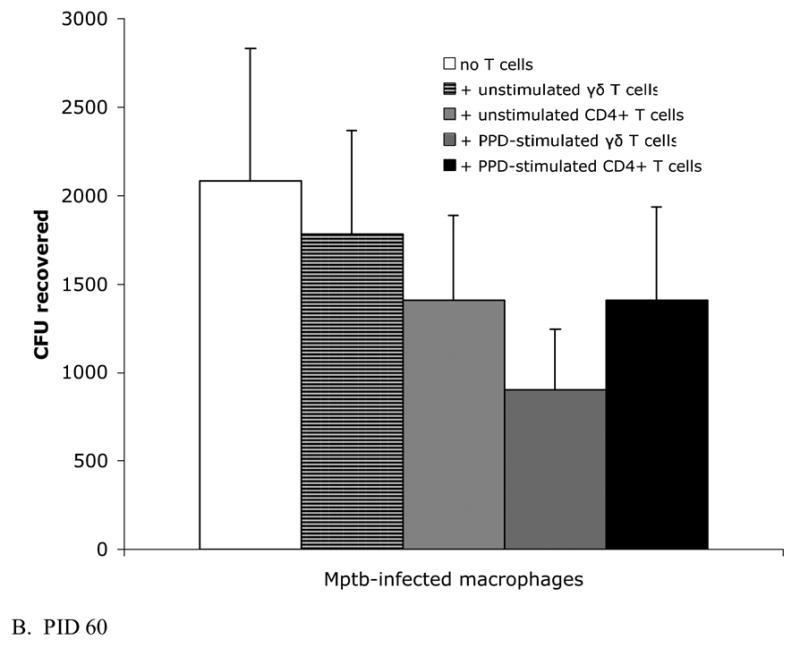
Efficiency of bacterial killing is similar among macrophage cultures incubated with γδ T cells and CD4+ T cells. Infected macrophage cultures described in Fig 2 were lysed with 0.1% deoxycholate and dilutions of bacterial suspensions were plated onto 7H10 agar. Bars represent the mean±SEM from 8 animals. (A) Blood-derived infected macrophages and preinoculation lymph node T cells. The survival of bacteria from macrophage cultures containing either unstimulated γδ T cells or unstimulated CD4+ T cells is similar. (B) Blood-derived infected macrophages and T cells from lymph nodes draining sites of M. paratuberculosis inoculation at PID 60. Despite significant production of IFN-γ by autologous PPD-stimulated CD4+ T cells (Fig 2B), killing is not increased compared to other culture conditions. PPD = purified protein derivative.
At PID 60, both unstimulated and PPD-stimulated T cells were added to infected macrophage cultures to asses bacterial killing. Although there was a trend toward decreased recovery of CFU from macrophage cultures containing added PPD-stimulated γδ T cells (P=0.14), there were no statistically significant differences in CFU recovery between infected cultures with or without added T cells (Fig. 4B).
To test whether IFN-γ concentration or nitrite production correlated with reduction in bacterial viability in vitro, we performed regression analysis on IFN-γ and nitrite production and CFU recovered. IFN-γ concentration correlated poorly with CFU recovered (r2=0.0171). Similarly, nitrite production and bacteria recovered appeared to act independently (r2=0.2285).
3.4 Production of NO from uninfected and M. paratuberculosis-infected bovine macrophage cultures with recombinant IFN-γ and LPS
Interestingly, the substantial IFN-γ response elicited by the co-culture of PPD-stimulated CD4+ T cells and M. paratuberculosis-infected macrophages did not result in increased production of nitrites compared to the amount elicited by M. paratuberculosis-infected macrophages without added T cells. To determine if infection of macrophages with M. paratuberculosis restricts NO production by activated macrophages, we infected blood-derived macrophages from naïve animals with M. paratuberculosis and subsequently activated the macrophages with recombinant IFN-γ and E. coli LPS. Fig. 5 shows the results of three separate experiments from one representative naïve animal. Uninfected macrophages treated with 100 U/mL rIFN-γ and 1 μg/mL LPS produce significantly greater amounts of nitrites compared to untreated, uninfected macrophage cultures (dashed horizontal line indicates the mean nitrite concentration from parallel uninfected macrophage cultures). In contrast, M. paratuberculosis-infected macrophages similarly treated with 100 U/mL rIFN-γ and 1 μg/mL LPS produced similar levels of nitrites as untreated, uninfected macrophage cultures, suggesting that IFN-γ concentrations capable of activating uninfected macrophages (in concert with LPS) are insufficient at inducing significant NO production by M. paratuberculosis-infected macrophages. Consistent with our co-culture experiments, treatment of M. paratuberculosis-infected macrophages with rIFN-γ and LPS does not significantly alter macrophage mycobactericidal function (Fig. 5B).
Fig. 5.
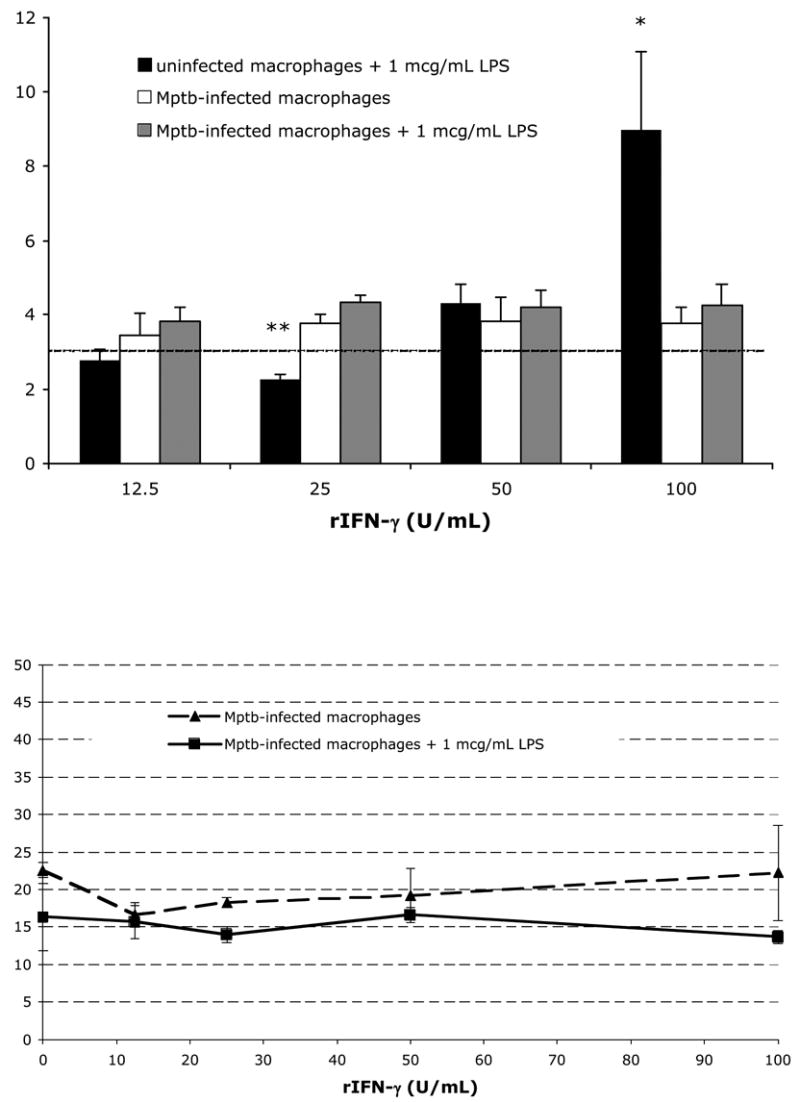
Treatment of M. paratuberculosis-infected bovine macrophages with recombinant IFN-γ does not increase nitrite production or increase mycobacterial killing. (A) Data from one representative naïve animal; bars represent the mean±SEM from 3 experiments. Blood-derived macrophages (either uninfected or infected in vitro with M. paratuberculosis) were incubated with varying concentrations of recombinant rIFN-γ alone or in combination with 1 μg/mL E. coli LPS. Dashed horizontal line indicates the mean nitrite concentration from parallel uninfected macrophage cultures (n=3). * P<0.05 compared to infected cultures receiving 100 U/mL IFN-γ. **P<0.001 compared to infected cultures receiving 25 U/mL IFN-γ. (B) Live bacteria recovered from macrophage cultures 48 hours after in vitro infection. Infected cultures received varying concentrations of IFN-γ alone or in combination with 1 μg/mL E. coli LPS. Percent live bacteria indicates the percent of fluorescein-positive bacteria among total bacterial cells recovered from lysed infected macrophage cultures using the fluorescein diacetate procedure as described in Materials and Methods. Each point represents the mean±SEM from 2 naïve animals.
4. Discussion
In the present study, we evaluated lymphocyte function after primary restimulation with M. paratuberculosis PPD followed by a secondary restimulation with M. paratuberculosis-infected macrophages. Primary restimulation was performed to expand M. paratuberculosis antigen-specific lymphocytes within the draining LN cell population, and from these expanded lymphocyte populations, we positively selected γδ T cells and CD4+ T cells, which would include activated, antigen-specific effector/memory lymphocytes. Upon secondary restimulation by infected macrophages, the expanded, antigen-specific T cells would be capable of expressing full effector function.
Compared to infected macrophage cultures without added T cells, M. paratuberculosis-infected cultures containing added autologous γδ T cells do not produce increased amounts of nitrites and do not have significant reductions in mycobacterial viability, suggesting that γδ T cells do not directly activate macrophages during M. paratuberculosis infection. When added to autologous infected macrophages, γδ T cells (whether obtained prior to or after in vivo infection and whether rested or antigen-stimulated) do not produce significant amounts of IFN-γ.
Rather than directly activating infected macrophages, γδ T cells may play a role in inflammatory cell trafficking, and several studies using γδ TCR KO mice have suggested a role for γδ T cells in the formation and organization of granulomas during mycobacterial infection (D'Souza et al., 1997; Saunders et al., 1998; Tanaka et al., 2000). γδ TCR KO mice infected with M. tuberculosis had larger granulomas with fewer lymphocytes and less organization than wild-type mice (D'Souza et al., 1997). Compared to the lesions in wild-type mice, which were composed of epithelioid macrophages and multinucleated giant cells intermixed with lymphocytes, the inflammation in γδ TCR KO mice was composed of larger coalescing areas of pyogranulomatous inflammation containing abundant neutrophils. In contrast, γδ TCR KO mice infected with strain 724 of M. avium developed granulomas with fewer neutrophils and increased numbers of lymphocytes and lacked the prominent central areas of necrosis seen in wild type mice (Saunders et al., 1998). γδ TCR KO mice inoculated with a high dose of M. paratuberculosis had decreased size and numbers of hepatic granulomas which lacked the epithelioid macrophages and multinucleated giant cells seen in wild-type mice (Tanaka et al., 2000).
Co-culture of infected macrophages with autologous CD4+ T cells producing large amounts of IFN-γ similarly does not promote the killing of intracellular M. paratuberculosis. Although in vitro infection of macrophages with M. paratuberculosis resulted in increased production of nitrites compared to uninfected macrophages, the substantial amount of IFN-γ produced by purified, PPD-stimulated CD4+ T cells co-cultured with M. paratuberculosis-infected macrophages failed to further elicit elevations in nitrite production compared to co-cultures without added T cells. Using regressions analysis, we found no correlation between IFN-γ concentration within the culture supernatant and killing of intracellular bacteria, a finding previously noted when using CD4+ T cells and macrophages from naturally-infected cattle (Hostetter et al., 2006). This suggests that M. paratuberculosis infection may inhibit the ability of macrophages to mount a significant NO response, even in the presence of IFN-γ. To test this possibility, we activated infected and uninfected macrophages in vitro by pretreating cultures with IFN-γ and LPS and maintaining the concentration of these substances throughout the infection period. Although LPS in combination with high concentrations of IFN-γ induced significant production of nitrites by uninfected macrophage cultures, similar amounts of LPS and IFN-γ were unable to elicit nitrite production from M. paratuberculosis-infected cultures above that produced by infected cultures without activation. Nitrites produced by infected macrophages were extremely low and were consistent with the results of other investigators using bovine cells (Zhao et al., 1997). Assessment of nitrite production is traditionally used to determine activation of macrophages, as in the present study, and increased production of nitrites was used as a solely as a marker of macrophage activation rather than to determine a potential mechanism of bacterial killing. Indeed, NO may not play a role in killing of M. paratuberculosis in vivo, as it has been estimated that the amount of nitric oxide required to kill M. paratuberculosis in vitro (approximately 270 mM) is not physiologically attainable (Zhao et al., 1997).
Because we did not block major histocompatability complex (MHC): T cell receptor (TCR) interactions in this study, we cannot determine if the increased IFN-γ produced by cultures containing infected macrophages and PPD-stimulated CD4+ T cells resulted solely from antigen presentation to primed T cells or simply as a response to high concentrations of IL-12 produced by the infected macrophages. That both antigen presentation and high levels of IL-12 are involved in increased IFN-γ production is suggested from studies by Collins, et al. (1999), which showed that antigen substantially enhances the IFN-γ response by sensitized bovine PBMC to IL-12 stimulation (Collins et al., 1999). Whereas unstimulated PBMC cultured in the presence of IL-12 for 48 hours produced only small amounts of IFN-γ (5–10 ng/mL), IFN-γ concentrations of 25-100 ng/mL were observed when PBMC from sensitized animals were cultured in the presence of antigen plus IL-12 (Collins et al., 1999). Other have shown that IL-12 mRNA expression is increased in M. paratuberculosis-infected macrophages in vitro within 6 hours of infection; this expression remains high through 24 hours but decreases to control levels by 72 hours after infection (Weiss et al., 2002), suggesting that M. paratuberculosis-infected macrophages rapidly produce IL-12 to enhance the developing T cell response.
We and others have previously demonstrated that the commercial paratuberculosis vaccine, which induces strong antigen-specific IFN-γ production by draining lymph node CD4+ T cells (Simutis et al., 2005), does not cause a decrease in the number of bacteria recovered from infected tissues in comparison to non-vaccinated animals (Uzonna et al., 2003). CD4+ T cells and IFN-γ have been shown to be essential in clearance of M. tuberculosis, as mice deficient in MHC class II molecules or experimentally depleted of CD4+ T cells by monoclonal antibodies or mice lacking the IFN-γ gene rapidly succumb to aerosol infection (Mogues et al., 2001). Despite the apparent importance of IFN-γ in controlling mycobacterial infection, addition of IFN-γ to M. paratuberculosis-infected macrophage cultures does not cause significant increases in bacterial killing (Weiss et al., 2002; Zhao et al., 1997; Zurbrick et al., 1988). M. paratuberculosis-antigen-specific production of IFN-γ is elevated in animals with the subclinical form of the disease (Stabel, 1996), yet these animals ultimately progress to clinical disease with uncontrolled infection. The IFN-γ-secretion pathway by antigen-specific CD4+ T cells may be intact in infected animals, at least through the subclinical phase of infection. As M. paratuberculosis-infected macrophages appear unresponsive to exogenous IFN-γ in vitro, the failure of bacterial killing may lie in a defect in macrophage function.
Acknowledgments
The authors thank Weiwei Zhang for expert technical assistance. This work was funded by the National Institute of Allergy and Infectious Diseases K08 AI 50647, the Healthy Livestock Initiative at Iowa State University, and by the United States Department of Agriculture Veterinary Services Notice No. 03-10 Cooperative Agreements in the Study of Johne’s Disease.
Abbreviations
- M. paratuberculosis, Mptb
Mycobacterium avium subsp. paratuberculosis
- PPD
Purified protein derivative of M. paratuberculosis
- PID
Post-inoculation day
- LN
Lymph node
- NO
Nitric oxide
- CFU
Colony-forming units
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boom WH, Balaji KN, Nayak R, Tsukaguchi K, Chervenak KA. Characterization of a 10- to 14-kilodalton protease-sensitive Mycobacterium tuberculosis H37Ra antigen that stimulates human gamma delta T cells. Infect Immun. 1994;62:5511–5518. doi: 10.1128/iai.62.12.5511-5518.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, Brennan P, O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990;249:67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Charley B, McCullough K, Martinod S. Antiviral and antigenic properties of recombinant porcine interferon gamma. Vet Immunol Immunopathol. 1988;19:95–103. doi: 10.1016/0165-2427(88)90001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheville NF, Jensen AE, Halling SM, Tatum FM, Morfitt DC, Hennager SG, Frerichs WM, Schurig G. Bacterial survival, lymph node changes, and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am J Vet Res. 1992;53:1881–1888. [PubMed] [Google Scholar]
- Chiodini RJ. Immunology: resistance to paratuberculosis. Vet Clin North Am Food Anim Pract. 1996;12:313–343. doi: 10.1016/s0749-0720(15)30409-6. [DOI] [PubMed] [Google Scholar]
- Chiodini RJ, Buergelt CD. Susceptibility of Balb/c, C57/B6 and C57/B10 mice to infection with Mycobacterium paratuberculosis.PG - 309-19. J Comp Pathol. 1993:109. doi: 10.1016/s0021-9975(08)80295-2. [DOI] [PubMed] [Google Scholar]
- Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- Collins RA, Howard CJ, Duggan SE, Werling D. Bovine interleukin-12 and modulation of IFNgamma production. Vet Immunol Immunopathol. 1999;68:193–207. doi: 10.1016/s0165-2427(99)00020-3. [DOI] [PubMed] [Google Scholar]
- D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- Garcia VE, Sieling PA, Gong J, Barnes PF, Uyemura K, Tanaka Y, Bloom BR, Morita CT, Modlin RL. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- Hostetter J, Zhang W, Simutis F. Mycobacterium avium subspecies paratuberculosis infection of cattle does not diminish peripheral blood-derived macrophage mycobactericidal activity. Immunol Lett. 2006;107:76–79. doi: 10.1016/j.imlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Jones-Carson J, Vazquez-Torres A, van der Heyde HC, Warner T, Wagner RD, Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–557. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- Kennedy HE, Welsh MD, Bryson DG, Cassidy JP, Forster FI, Howard CJ, Collins RA, Pollock JM. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1(+) gamma delta T cells. Infect Immun. 2002;70:1488–1500. doi: 10.1128/IAI.70.3.1488-1500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koets A, Rutten V, Hoek A, van Mil F, Muller K, Bakker D, Gruys E, van Eden W. Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function. Infect Immun. 2002;70:3856–3864. doi: 10.1128/IAI.70.7.3856-3864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HC, Park YH, Hamilton MJ, Barrington GM, Davies CJ, Kim JB, Dahl JL, Waters WR, Davis WC. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect Immun. 2004;72:6870–6883. doi: 10.1128/IAI.72.12.6870-6883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Emoto M, Hiromatsu K, Yamamoto S, Matsuura K, Gomi H, Ikeda T, Itohara S, Yoshikai Y. The role of gamma delta T cells in priming macrophages to produce tumor necrosis factor-alpha. Eur J Immunol. 1995;25:1465–1468. doi: 10.1002/eji.1830250551. [DOI] [PubMed] [Google Scholar]
- Rhodes SG, Hewinson RG, Vordermeier HM. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J Immunol. 2001;166:5604–5610. doi: 10.4049/jimmunol.166.9.5604. [DOI] [PubMed] [Google Scholar]
- Rojas RE, Torres M, Fournie JJ, Harding CV, Boom WH. Phosphoantigen presentation by macrophages to mycobacterium tuberculosis--reactive Vgamma9Vdelta2+ T cells: modulation by chloroquine. Infect Immun. 2002;70:4019–4027. doi: 10.1128/IAI.70.8.4019-4027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BM, Frank AA, Cooper AM, Orme IM. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508–5514. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simutis FJ, Cheville NF, Jones DE. Investigation of antigen-specific T-cell responses and subcutaneous granuloma development during experimental sensitization of calves with Mycobacterium avium subsp paratuberculosis. Am J Vet Res. 2005;66:474–482. doi: 10.2460/ajvr.2005.66.474. [DOI] [PubMed] [Google Scholar]
- Stabel JR. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J Vet Diagn Invest. 1996;8:345–350. doi: 10.1177/104063879600800311. [DOI] [PubMed] [Google Scholar]
- Steadham EM, Martin BM, Thoen CO. Production of a Mycobacterium avium ssp. paratuberculosis purified protein derivative (PPD) and evaluation of potency in guinea pigs. Biologicals. 2002;30:93–95. doi: 10.1006/biol.2002.0321. [DOI] [PubMed] [Google Scholar]
- Sweeney RW, Jones DE, Habecker P, Scott P. Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am J Vet Res. 1998;59:842–847. [PubMed] [Google Scholar]
- Tanaka S, Itohara S, Sato M, Taniguchi T, Yokomizo Y. Reduced formation of granulomata in gamma(delta) T cell knockout BALB/c mice inoculated with Mycobacterium avium subsp. paratuberculosis. Vet Pathol. 2000;37:415–421. doi: 10.1354/vp.37-5-415. [DOI] [PubMed] [Google Scholar]
- Uzonna JE, Chilton P, Whitlock RH, Habecker PL, Scott P, Sweeney RW. Efficacy of commercial and field-strain Mycobacterium paratuberculosis vaccinations with recombinant IL-12 in a bovine experimental infection model. Vaccine. 2003;21:3101–3109. doi: 10.1016/s0264-410x(03)00261-5. [DOI] [PubMed] [Google Scholar]
- Vesosky B, Turner OC, Turner J, Orme IM. Gamma interferon production by bovine gamma delta T cells following stimulation with mycobacterial mycolylarabinogalactan peptidoglycan. Infect Immun. 2004;72:4612–4618. doi: 10.1128/IAI.72.8.4612-4618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, Nam KH, Morita CT. Conservation of nonpeptide antigen recognition by rhesus monkey V gamma 2V delta 2 T cells. J Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- Waters WR, Miller JM, Palmer MV, Stabel JR, Jones DE, Koistinen KA, Steadham EM, Hamilton MJ, Davis WC, Bannantine JP. Early Induction of Humoral and Cellular Immune Responses during Experimental Mycobacterium avium subsp. paratuberculosis Infection of Calves. Infect Immun. 2003;71:5130–5138. doi: 10.1128/IAI.71.9.5130-5138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Evanson OA, Moritz A, Deng MQ, Abrahamsen MS. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect Immun. 2002;70:5556–5561. doi: 10.1128/IAI.70.10.5556-5561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock RH, Buergelt C. Preclinical and clinical manifestations of paratuberculosis (including pathology) Vet Clin North Am Food Anim Pract. 1996;12:345–356. doi: 10.1016/s0749-0720(15)30410-2. [DOI] [PubMed] [Google Scholar]
- Yin Z, Zhang DH, Welte T, Bahtiyar G, Jung S, Liu L, Fu XY, Ray A, Craft J. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-gamma: failure to down-regulate IL-12 receptor beta 2-chain expression. J Immunol. 2000;164:3056–3064. doi: 10.4049/jimmunol.164.6.3056. [DOI] [PubMed] [Google Scholar]
- Zhao B, Collins MT, Czuprynski CJ. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect Immun. 1997;65:1761–1766. doi: 10.1128/iai.65.5.1761-1766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbrick BG, Follett DM, Czuprynski CJ. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect Immun. 1988;56:1692–1697. doi: 10.1128/iai.56.7.1692-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


