Abstract
The biosynthesis of brain membrane phosphatides, e.g., phosphatidylcholine (PtdCho), may utilize three circulating compounds: choline, uridine (a precursor for UTP, CTP, and CDP-choline), and a PUFA (e.g., docosahexaenoic acid); moreover oral administration of the uridine source uridine-5′-monophosphate (UMP) can significantly increase levels of the phosphatides throughout the rodent brain. Since PtdCho can provide choline for acetylcholine (ACh) synthesis, we determined whether UMP administration also affects ACh levels in striatum and striatal extracellular fluid, in aged and young rats. Among aged animals consuming a UMP-containing diet (2.5%, w/w) for 1 or 6 weeks, baseline ACh levels in striatal dialysates rose from 73 fmol/min to 148 or 197 fmol/min (P<0.05). Consuming a lower dose (0.5%) for 1 week produced a smaller but still significant increase (from 75 to 92 fmol/min, P<0.05), and elevated striatal Ach content (by 16%; P<0.05). Dietary UMP (0.5%, 1 week) also amplified the increase in ACh caused by giving atropine (10 μM in the aCSF); atropine alone increased ACh concentrations from 81 to 386 fmol/min in control rats and from 137 to 680 fmol/min in those consuming UMP (P<0.05). Young rats eating the UMP-containing diet exhibited similar increases in basal ECF ACh (from 105 to 118 fmol/min) and in the increase produced by atropine (from 489 to 560 fmol/min; P<0.05). These data suggest that giving a uridine source may enhance some cholinergic functions, perhaps by increasing brain phosphatide levels.
Scope: Section 3 (Neurophysiology, Neuropharmacology and other forms of Intercellular Communication)
Keywords: Acetylcholine, microdialysis, uridine, phosphatidylcholine, CDP-choline, aged rat
1. Introduction
Phosphatidylcholine (PtdCho), the most abundant phosphatide, is the primary component of neuronal cell membranes. Brain PtdCho synthesis can be enhanced by its three circulating precursors (Kennedy and Weiss, 1956): choline, a pyrimidine nucleoside like uridine or cytidine, and a polyunsaturated fatty acid (PUFA) like docosahexaenoic acid (DHA) (Araki and Wurtman, 1998; Rapoport, 2001; Marszalek and Lodish, 2005); all three readily cross the blood-brain barrier (Li et al., 2001; Spector, 2001). Brain uridine can be phosphorylated by uridine kinase (Suzuki et al., 2004) to form uridine-5′-triphosphate (UTP), which can be aminated (by CTP synthetase) to cytidine-5′-triphosphate (CTP) (Genchev and Mandel, 1974). CTP subsequently forms cytidine-5′-diphosphocholine (CDP-choline) - PtdCho’s immediate precursor - which combines with diacylglycerol (DAG) molecules, including those containing PUFA, to produce PtdCho (Weiss, 1995).
Giving rodents like Mongolian gerbils a single dose of the uridine source uridine-5′-monophosphate (UMP) by gavage, increases their brain levels of CDP-choline by 50% (Cansev et al., 2005); chronic consumption of UMP via the diet (0.5%, with or without gavaged DHA) increases by 13-48% brain levels of PtdCho and other phosphatides including phosphatidylethanolamine (PtdEtn), phosphatidylserine (PtdSer), and phosphatidylinositol (PtdIns) (Wurtman et al., 2006). Daily administration of UMP and DHA for two weeks also increases dendritic spine densities in the CA1 region of adult gerbil hippocampus (Sakamoto and Wurtman, 2006) and, after 3-4 weeks, the concentrations of synaptic proteins including synapsin-I and PSD-95 (Wurtman et al., 2006).
In vitro, uridine was shown to amplify the membrane phosphatide formation and neurite outgrowth elicited by nerve growth factor (NGF) in rat pheochromocytoma (PC-12) cells. The effect involved both CTP-enhanced phosphatide synthesis (Richardson et al., 2003) and UTP activation of P2Y receptors (Pooler et al., 2005) inasmuch as it could be blocked by the antagonists suramin or Reactive Blue 2. In vivo, UMP consumption was shown to enhance striatal dopamine (DA) levels and release (Wang et al., 2005).
Since membrane PtdCho is known to be a source of choline for acetylcholine (ACh) synthesis (Ulus et al., 1989), we explored the effects of uridine consumption on brain ACh-mediated neurotransmission, using in vivo microdialysis.
2. Results
2.1. Effects of UMP dietary supplementation on baseline extracellular ACh levels in aged rat striatum
Since we previously showed that dietary consumption of UMP (2.5%) for 6 weeks can enhance brain dopamine transmission in aged rats (Wang et al., 2005), we initially fed aged rats this diet to evaluate UMP’s effects on extracellular ACh levels in rat striatum. Animals consumed the diet for 1 or 6 weeks, then dialysates were collected and assayed for ACh measurement, as described in Experimental Procedures. Microdialysis was repeated in each rat on 2 consecutive days, adding 0.5 μM neostigmine to the aCSF on one day and 1.0 μM on the other. Two-way ANOVA with repeated measures revealed significant effects of UMP treatment (F(2, 69)= 9.69, P<0.01) and of neostigmine (F(1, 69)= 54.86, P<0.01) on increasing baseline extracellular ACh levels (Fig. 1A). Dietary pretreatment with UMP was the between-subjects factor, and neostigmine was the within-subjects factor; data from animals receiving just the control diet for different times did not differ, and were pooled. When rats were perfused with aCSF containing 1.0 μM neostigmine, baseline ACh concentrations in the dialysates were increased from 73 ± 11 fmol/min in control rats to 148 ± 14 and 197 ± 35 fmol/min in UMP-pretreated rats (after 1 and 6 weeks of treatment, respectively; Fig. 1A). Using aCSF containing 0.5 μM neostigmine, baseline ACh concentrations increased from 48 ± 7 fmol/min in control rats to 70 ± 6 and 97 ± 13 fmol/min in UMP-pretreated rats (after 1 and 6 weeks of treatment, respectively; Fig. 1A).
Figure 1.
Effect of UMP on basal ACh concentrations in dialysates from aged rat striatum
(A) Aged rats were fed either control or UMP-containing (2.5%) diets for 1 or 6 weeks. Statistics were carried out using two-way ANOVA with repeated measures, and then one-way ANOVA or unpaired t-test. Animals received either 0.5 or 1.0 μM neostigmine via the probe. In subsquent experiments, all animals received 1.0 μM neostigmine. (B) Aged rats were fed either control or UMP-containing (0.5%) diets for 1 week. Statistics were carried out using unpaired t-test. Bars indicate means ± SEM. *, P < 0.05 vs. corresponding control; #, P < 0.05 vs. ACh concentration with 0.5 μM neostigmine in control rats.
We further confirmed UMP’s effect on baseline extracellular ACh level, using aged rats receiving a lower dose of UMP (0.5%; Fig. 1B) for 1 week. This dose was chosen because it significantly increases brain PtdCho levels in gerbils (Wurtman et al., 2006). Baseline ACh concentrations in the dialysates increased from 75 ± 4 fmol/min in control rats to 92 ± 2 fmol/min in rats consuming UMP (with 1.0 μM neostigmine; P < 0.05; Fig. 1B).
2.2. Effects of UMP dietary supplementation on ACh tissue content in aged rat striatum
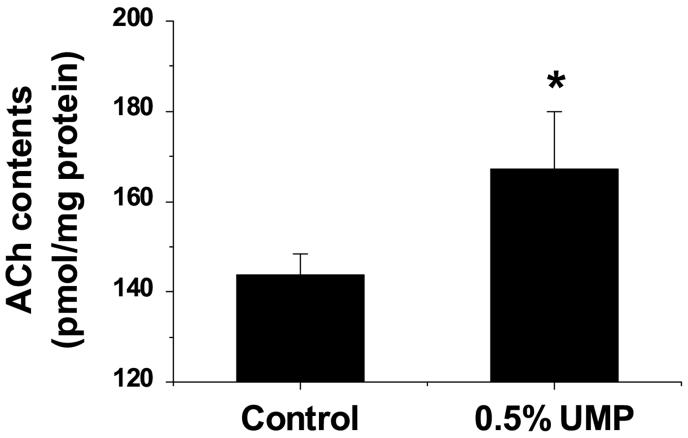
Striatal ACh tissue content was measured using a near-freezing procedure, among aged rats receiving either control or 0.5% UMP-containing diets for 1 week (but no neostigmine). Tissue collection and ACh extraction are described in detail in Experimental Procedures. ACh contents in the control rats were 144 ± 5 pmol/mg protein. UMP pretreatment significantly increased these values by 16% (P <0.05) (Fig. 2).
Figure 2.
Effect of UMP on striatal ACh tissue content
ACh tissue content was measured among aged rats fed control or UMP-containing (0.5%) diets for 1 week (n = 6). Bars indicate means ± SEM. *, P < 0.05 vs. control (unpaired t-test).
2.3. Effects of UMP dietary supplementation on ACh release enhanced by atropine in aged rats
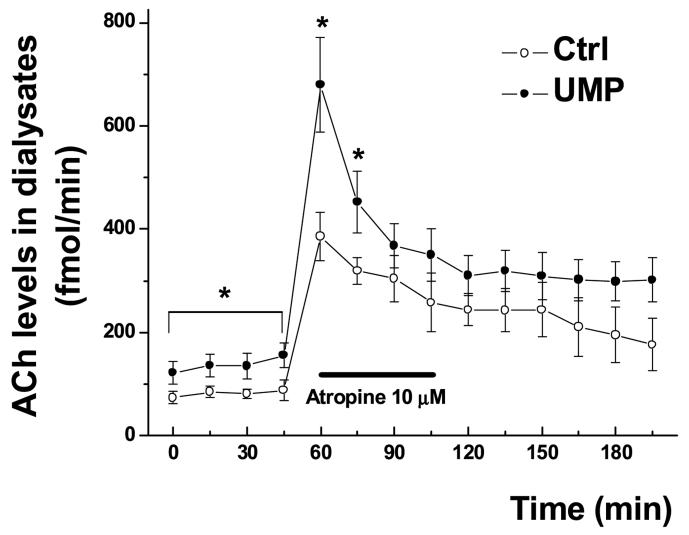
Two-way ANOVA with repeated measures revealed significant effects of UMP both on basal ACh release and on ACh release after atropine administration (10 μM the aCSF, with 1 μM neostigmine), in striatum of aged rats (Fig. 3; F(1, 33)= 14.23, P<0.01). Among rats that had consumed the 0.5% UMP-containing diet for 1 week, baseline ACh concentrations rose from 81 ± 6 fmol/min to 137 ± 12 fmol/min. Atropine significantly (P<0.01) elevated ACh release, to a maximum of 386 ± 47 fmol/min in control rats, and to 680 ± 90 fmol/min in UMP-pretreated rats.
Figure 3.
Effect of UMP on atropine-enhanced ACh release in aged rat striatum
Atropine-enhanced (10 μM) ACh release was measured in aged rats fed either control or UMP-containing (0.5%) diets for 1 week. Data are given as means ± SEM. *, P < 0.05 vs. corresponding control (two-way ANOVA with repeated measures and unpaired t-test). Baseline samples, collected for 60 minutes prior to atropine administration, exhibited a significant increase in ACh levels, as did the initial two samples collected after atropine administration.
2.4. Effects of UMP dietary supplementation on ACh release enhanced by atropine in young rats
We also examined uridine’s effect in young (3 month old) rats. Rats were pretreated as the aged ones and ACh release was enhanced by atropine. Young rats eating the UMP-containing diet exhibited an increase in basal ECF ACh (from 105 ± 10 to 118 ± 10 fmol/min; P=0.18). UMP significantly amplified atropine-enhanced ACh release: in UMP pretreated rats, average releases were 560 ± 21 fmol/min, compared with 489 ± 31 fmol/min in control rats (n=7 in each group; P<0.05).
To rule out the possibility that the increased ACh release was related to inhibition of acetylcholinesterase (AChE), the activities of this enzyme in striatum and hippocampus of young rats not undergoing dialysis were determined by a colorimetric method using acetylthiocholine substrate as described by Ellman et al., (1961). Activities of AChE were unchanged by UMP pretreatments in the striatum (150 ± 6 vs. 159 ± 5 nmol/min/mg of tissue, control vs. UMP; P>0.05) or hippocampus (9.0 ± 0.3 vs. 9.1 ± 0.5 nmol/min/mg of tissue, control vs. UMP; P>0.05).
2.5. Effects of UMP-containing diet on striatal phosphatide contents
To confirm that the UMP was increasing brain phosphatides in these animals, we measured PtdCho, PtdEtn, and PtdSer in striata of young rats consuming control or UMP diets for 6 weeks (n =6). These were 183 ± 10, 197 ± 13 and 44 ± 5 nmol/mg protein, respectively, in control rats. They were increased significantly by 17%, 27%, and 30%, respectively, in animals consuming the UMP diet (all P < 0.05, unpaired t test; Fig. 4). Synaptic levels of total phosphatides also increased significantly, from 424 ± 26 to 521 ± 24 nmol/mg protein (P < 0.05).
Figure 4.
Effects of UMP on striatal phosphatide content.
Phosphatide contents were measured in striata of 3-month-old young rats fed control or UMP-containing (2.5%) diets for 6 weeks. Bars indicate means ± SEM. *, P < 0.05 vs. control (unpaired t-test).
3. Discussion
These data show that basal ACh release (Figs. 1A, 1B), and evoked ACh release caused by atropine (Fig. 3) are both increased in striata of rats fed a choline-containing diet enriched with UMP. Moreover, striatal ACh content (Fig. 2) is significantly increased with no effects on acetylcholinesterase activity, suggesting that synthesis of ACh is elevated. Since UMP increased both the levels and apparent release of striatal ACh, we hypothesize that its primary mechanism was to increase the availability of choline (e.g., from membrane PtdCho). It could, of course, also act on M2 receptors.
That the source of the additional choline is choline bound in membrane phosphatides, mainly PtdCho, is suggested by the increased synthesis (Cansev et al., 2005) and levels (Wurtman et al., 2006; and Fig. 4) of this phosphatide after chronic pretreatment with dietary UMP. Since brain levels of PtdCho are orders of magnitude greater than those of ACh, the 17% increase in PtdCho could readily provide sufficient choline for the observed increases in ACh.
The cellular loci of the increased membrane phosphatides probably include synaptic vesicles and synaptic membranes since, as we recently observed, brain levels of various pre and post-synaptic proteins, including synapsin-1 and PSD-95, are increased among animals consuming a UMP-containing diet (Wurtman et al., 2006). Thus, uridine consumption may produce more or larger synapses or synaptic vesicles which may also contribute to increasing striatal ACh content and release.
UMP pretreatment most likely affects membrane phosphatide production as well as neurite outgrowth in PC-12 cells (Pooler et al., 2005) and hippocampal dendritic spine formation in vivo (Sakamoto and Wurtman, 2006) by two mechanisms: by increasing CTP levels, it increases the formation of CDP-choline (Cansev et al., 2005); and by increasing brain UTP, it activates metabotropic P2Y receptors (Pooler et al., 2005). Oral UMP is rapidly absorbed, appearing in the circulation as uridine (Sonoda and Tatibana, 1978). Consumption of UMP (2000 mg) by humans raises plasma uridine levels by 3 fold (from 6.0 to 21.9 μM), for 5-6 hours (Cansev, 2006). Circulating uridine crosses the blood-brain barrier via an adenosine transporter (the concentrative nucleoside transporter type 2; CNT2) (Li et al., 2001), which is unsaturated at physiologic plasma uridine concentrations (Cansev et al., 2005). Thus, dietary UMP can increase brain levels of uridine and subsequently, as shown in gerbils, brain levels of UTP, CTP and CDP-choline (Cansev et al., 2005).
Uridine also affects PtdCho synthesis and, possibly, ACh release via UTP-mediated activation of the P2Y receptor (Neary et al., 1996). A subset of the metabotropic P2Y (P2Y2, P2Y4, and P2Y6) receptors, once activated by UTP, increases intracellular levels of DAG and inositol triphosphate (IP3), and subsequently, calcium influx.
UMP consumption produces parallel increases in rat brain phosphatide levels within all regions examined, e.g. striatum, hippocampus, cortex, brain stem and cerebellum (Cansev et al., in preparation), probably reflecting the homogeneity, among regions, of the proteins that take up uridine into the brain (Cansev, 2006) and that catalyze the steps in the Kennedy cycle. Since the levels of ACh in striatal dialysates are much higher and more readily measured than those in hippocampal or cortical dialysates (Wu et al., 1988), and since the UMP-induced rise in brain phosphatides may mediate the increase in ACh, we collected ECF (dialysis) samples from striatum for these studies. Striatal neurons are involved in various types of learning in rats (e.g., stimulus-response habits as well as motor, perceptual, and cognitive skills) (Jog et al., 1999), and cholinergic interneurons modulate these neuronal circuits (Kaneko et al., 2000). Microdialysis studies have shown that when rats used response strategies rather than spatial strategies for learning, striatal ACh release increased (Chang et al., 2003). Moreover, selective ablation of striatal cholinergic neurons impairs procedural learning in mice (Kitabatake et al., 2003). Thus, the enhancement in striatal cholinergic neurotransmission, after oral UMP administration, that we observe (e.g. Figs. 1 and 3), may be associated with enhanced learning and memory in rodents.
Brain ACh-mediated neurotransmission is involved in cognitive processes in humans and other mammals (Drachman, 1977; Everitt and Robbins, 1997). The ability of oral UMP and choline to increase the levels of ACh and phosphatides in rats raises the possibility that this compound might also be useful in treating age-related memory dysfunction.
4. Experimental procedures
4.1. Animals
Young (3 months old) and aged (22 months old) male Fischer 344 rats were purchased from the National Institute on Aging (Harlan Sprague-Dawley, Indianapolis, IN). The younger animals were used to confirm the increase in brain phosphatides produced by uridine (Wurtman et al., 2006); the aged animals were used for measurement of ACh levels and release. The animals were reared in a socially restricted environment (i.e., individual housing and minimal handling), and exposed to a 12 hr light/dark cycle with food and water provided ad libitum. All experimental procedures were approved by the Massachusetts Institute of Technology Committee on Animal Care.
Animals had been fed a standard control diet (Teklad Global 16% protein rodent diet, TD.00217, which contains 0.1% choline chloride; Harlan Teklad, Madison, WI), or this diet fortified with UMP*2Na+ (TD.03398; 0.5 or 2.5%, w/w) for 1 or 6 weeks prior to microdialysis experiments. Aged rats consumed an average of 10 g of food per day and there was no difference between consumption of the control and UMP-containing diets. Thus 2.5% of UMP provided approximately 500 mg/kg/day of UMP, or 330 mg/kg/day of free uridine (100 or 66 mg/kg/day, respectively, of the 0.5% diet), and 50 mg/kg/day of choline. These diets also contained no detectable nucleosides or nucleotides as analyzed by HPLC. When the rats were sacrificed after experiments, their stomach contents were examined; residual food was present in all of the animals indicating that uridine and choline were still being absorbed.
4.2. Surgery
Stereotaxic surgery was carried out 1 day prior to a microdialysis experiment, according to the method described in detail by Osborne et al., (1991), using a CMA/11 probe (O.D. 0.24 mm, 4 mm, 6,000 Da, CMA, Sweden). The probe was permanently implanted into the right side of the striatum (AP = +0.5, ML = -3.0, DV = 7.3 mm), according to the atlas of Paxinos and Watson (2004), under ketamine and xylazine (80 and 10 mg/Kg bwt, i.p., respectively) anesthesia. Average recoveries of microdialysis probes were 12.0 ± 0.3% for ACh (n =22). Data were not corrected for probe recoveries.
4.3. Microdialysis
Microdialysis was performed for 1 or 2 days on freely moving rats, kept in a circular bowl on a rotating platform, obviating the need for a liquid swivel. Artificial cerebrospinal fluid (aCSF), containing, in mM: NaCl, 121; NaHCO3, 25; KCl, 3.5; CaCl2, 1.2; MgCl2, 1.2; NaH2PO4, 1, was bubbled with 95% O2 and 5% CO2 for 15 min, pH= 7.4. Neostigmine (0.5 or 1 μM) was added into aCSF to block enzymatic degradation of ACh. The aCSF was perfused through the probes continuously at a rate of 1.5 μl/min. Samples collected during the first hour of microdialysis were discarded; subsequent collections were for 15 min intervals.
Three individual microdialysis experiments were carried out after the following pretreatments: 1) a high UMP dose study (n = 18 rats, 4 groups) in which experimental groups received control or 2.5% UMP-containing diets for 1 or 6 weeks; 2) a low UMP dose study (n = 12 rats, 2 groups) in which experimental groups received either control or 0.5% UMP-containing diets for 1 week; and 3) a study in which ACh release was enhanced by adding atropine (10 μM) to the aCSF (n = 9 rats, 2 groups) in which experimental groups were pretreated as in experiment 2. This drug acts by blocking presynaptic M2 muscarinic receptors (Westerink et al., 1990; Buyukuysal et al., 1995; Oo et al., 1996; Moor et al., 1998). In the first 2 microdialysis experiments, four samples were collected from each rat to establish the baseline extracellular ACh levels; striatal ACh tissue content was measured among the rats of the second experiment. In the last experiment, after the 4 baseline samples, dialysis was continued with 4 samples under atropine stimulation, and thereafter, six more samples without atropine during the recovery period; this procedure yielded a total of 14 samples from each rat (4 baseline + 4 simulation + 6 recovery = 14). All experiments were carried out in a very quiet cubicle. The spontaneous activities of the rats were monitored for reference but not for data analysis. All samples were collected on crushed ice, then instantly frozen and kept at -80 °C until HPLC analysis.
4.4. Striatal ACh tissue content
Striatal ACh tissue content was measured among the animals in the second microdialysis experiment, in the contralateral, non-probe-lesioned striatum, using a near-freezing procedure (Takahashi and Aprison, 1964). Animals were sacrificed immediately after the experiment. Prior to sacrifice, black ink was pushed through the probe to stain the surrounding tissue, thus allowing for post-mortem visual confirmation of probe location. Rats were killed by immersing the head into liquid nitrogen for 5 min, followed by decapitation. Brains were sectioned using a cryostat (Leica, CM 3000) at -18 °C. The atlas of Paxinos and Watson and the ink stains in the contralateral side were used to identify major landmarks and to confirm the coordinates.
Tissue ACh was extracted according to the method described in detail by Takahashi et al., (1997). The dissected striata were homogenized on ice (50 mg of tissue with 1 ml 100 mM HClO4, 50 μM EDTA). A 10 μl aliquot was used for total protein determination (bicinchoninic acid assay, Sigma). The homogenates were then centrifuged (14,000 rpm, 15 min, 4 °C) and filtered with Ultrafree-MC centrifugal filter units (Millipore; 14,000 rpm, 1 min, 4 °C). Tissue ACh values were normalized to the amount of protein per sample.
4.5. ACh assay
ACh assay was carried out using a BAS 200A HPLC system with electrochemical detection (HPLC-ECD; BAS, West Lafayette, IN), as described by Damsma and Westerink (1991). A BAS ACh/choline analytical column cartridge and post-column enzyme reactor (MF-6150 and MF-6151) were used at 37°C to separate ACh and choline. The mobile phase (0.8 ml/min) consisted of 40 mM Na2HPO4, 0.5 mM Na2EDTA and 0.5 ml/l Kathon CG at pH 8.4. The compounds were measured using a modified working electrode (with horseradish peroxidase and a redox polymer on its surface) at a potential of +100 mV vs. Ag/AgCl. Samples were injected using an Alltech 580 autosampler, and data were collected and calculated using an AllChrom workstation (Alltech, Deerfield, IL).
4.6. Brain phosphatide assay
Striatal levels of the brain major phosphatides (PtdCho, PtdEtn, and PtdSer) were extracted according to the method of Folch et al., (1957), and measured as described previously (Lopez-Coviella et al., 1995). The corpus striatum was dissected, frozen in liquid nitrogen, sonicated in methanol, and mixed with 2 volumes of chloroform, then 2 volumes of 50% methanol/water. After centrifugation the organic phase (locus of the phosphatides) was dried under vacuum. The residue was reconstituted in chloroform/methanol (l: l), and an aliquot of the phosphatide extract was subsequently purified by TLC on silica gel G plates (Adsorbosil-Plus 1, Alltech), using a system consisting of chloroform/ethanol/triethylamine/water (30:34:30:8) as the mobile phase. Phosphatide standards were used to identify the corresponding bands under UV light after the plates were sprayed with 0.1% diphenylhexatriene in petroleum ether. The total amounts of each of the phosphatides were determined by phosphate assay (Svanborg and Svennerholm, 1961) and expressed per milligram of protein. Total protein levels were determined from methanol homogenates using the bicinchoninic acid assay.
4.7. Data analysis
Statistical analyses were carried out using SPSS 12.0. Data were represented as means ± SEMs. Unpaired t-test, one-way ANOVA and two-way ANOVA with repeated measures were used to assess the statistical significance of effects. Tukey post hoc analyses were used when appropriate. The significance level was set at p< 0.05.
Acknowledgements
The authors thank Carol Watkins, Maya Hasan, Ingrid Richardson, Mehmet Cansev and Luke Schmitt for advice and excellent assistance. UMP*2Na+ was kindly provided by Numico Research, Wageningen, the Netherlands. These studies were supported by grants from The National Institutes of Mental Health (Grant no. 2 R01 MH028783-30), and from The Center for Brain Sciences and Metabolism Charitable Trust.
Glossary
Abbreviations used:
- UMP
uridine-5′-monophosphate
- ACh
acetylcholine
- PtdCho
phosphatidylcholine
- PUFA
polyunsaturated fatty acid
- CDP-choline
cytidine-5′-diphosphocholine
- UTP
uridine-5′-triphosphate
- CTP
cytidine-5′-triphosphate
- DA
dopamine
- DAG
diacylglycerol
- HPLC-ECD
high-pressure liquid chromatography with electrochemical detection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki W, Wurtman RJ. How is membrane phospholipid biosynthesis controlled in neural tissues? J. Neurosci. Res. 1998;51:667–674. doi: 10.1002/(SICI)1097-4547(19980315)51:6<667::AID-JNR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Buyukuysal RL, Ulus IH, Aydin S, Kiran BK. 3,4-Diaminopyridine and choline increase in vivo acetylcholine release in rat striatum. Eur. J. Pharmacol. 1995;281:179–185. doi: 10.1016/0014-2999(95)00241-c. [DOI] [PubMed] [Google Scholar]
- Cansev M. Uridine and cytidine in the brain: Their transport and utilization. Brain Res. Rev. 2006;52:389–397. doi: 10.1016/j.brainresrev.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Cansev M, Watkins CJ, van der Beek EM, Wurtman RJ. Oral uridine-5'-monophosphate (UMP) increases brain CDP-choline levels in gerbils. Brain Res. 2005;1058:101–108. doi: 10.1016/j.brainres.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J. Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Westerink BHC. A microdialysis and automated on-line analysis approach to study central cholinergic transmission in vivo. In: Robinson TE, Justice JB, editors. Microdialysis in Neuroscience; Elsevier, Amsterdam: 1991. pp. 237–252. [Google Scholar]
- Drachman DA. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977;27:783–790. doi: 10.1212/wnl.27.8.783. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Genchev DD, Mandel P. CTP synthetase activity in neonatal and adult rat brain. J. Neurochem. 1974;22:1027–1030. doi: 10.1111/j.1471-4159.1974.tb04332.x. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Bio. Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc. Natl. Acad. Sci. USA. 2003;100:7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Boado RJ, Pardridge WM. Cloned blood-brain barrier adenosine transporter is identical to the rat concentrative Na+ nucleoside cotransporter CNT2. J. Cereb. Blood Flow Metab. 2001;21:929–936. doi: 10.1097/00004647-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Lopez-Coviella I, Agut J, Savci V, Ortiz JA, Wurtman RJ. Evidence that 5'-cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels. J. Neurochem. 1995;65:889–894. doi: 10.1046/j.1471-4159.1995.65020889.x. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu. Rev. Cell Dev. Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- Moor E, Schirm E, Jacso J, Westerink BH. Effects of neostigmine and atropine on basal and handling-induced acetylcholine output from ventral hippocampus. Neuroscience. 1998;82:819–825. doi: 10.1016/s0306-4522(97)00331-x. [DOI] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Oo TF, Naini A, Burke RE. Augmented pharmacologic stimulation of striatal acetylcholine release following developmental hypoxic-ischemic injury. Brain Res. 1996;706:145–150. doi: 10.1016/0006-8993(95)01240-0. [DOI] [PubMed] [Google Scholar]
- Osborne PG, O’Connor WT, Kehr J, Ungerstedt U. In vivo characterisation of extracellular dopamine, GABA and acetylcholine from the dorsolateral striatum of awake freely moving rats by chronic microdialysis. J. Neurosci. Methods. 1991;37:93–102. doi: 10.1016/0165-0270(91)90119-k. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 5th edition Elsevier; San Diego: 2004. The rat brain in stereotaxic coordinates. [Google Scholar]
- Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in NGF-differentiated PC12 cells. Neuroscience. 2005;134:207–214. doi: 10.1016/j.neuroscience.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. [DOI] [PubMed] [Google Scholar]
- Richardson UI, Watkins CJ, Pierre C, Ulus IH, Wurtman RJ. Stimulation of CDP-choline synthesis by uridine or cytidine in PC12 rat pheochromocytoma cells. Brain Res. 2003;971:161–167. doi: 10.1016/s0006-8993(03)02333-3. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Wurtman RJ. Increased dendritic spine density in adult gerbil hippocampus following oral UMP and DHA supplementation; 10th International Conference on Alzheimer’s Disease and Related Disorders; 2006; Spain: Madrid; [Google Scholar]
- Sonoda T, Tatibana M. Metabolic fate of pyrimidines and purines in dietary nucleic acids ingested by mice. Biochim. Biophys. Acta. 1978;521:55–66. doi: 10.1016/0005-2787(78)90248-4. [DOI] [PubMed] [Google Scholar]
- Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J. Mol. Neurosci. 2001;16:159–165. doi: 10.1385/JMN:16:2-3:159. [DOI] [PubMed] [Google Scholar]
- Suzuki NN, Koizumi K, Fukushima M, Matsuda A, Inagaki F. Structural basis for the specificity, catalysis, and regulation of human uridine-cytidine kinase. Structure. 2004;12:751–764. doi: 10.1016/j.str.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Svanborg A, Svennerholm L. Plasma total lipids, cholesterol, triglycerides, phospholipids and free fatty acids in a healthy Scandinavian population. Acta. Med. Scand. 1961;169:43–49. [Google Scholar]
- Takahashi A, Ishimaru H, Ikarashi Y, Kishi E, Maruyama Y. Comprehensive analysis of neurotransmitters and their metabolites including acetylcholine and choline in rat brain nuclei. Brain Res. Brain Res. Protoc. 1997;1:70–74. doi: 10.1016/s1385-299x(96)00013-x. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Aprison MH. Acetylcholine content of discrete areas of the brain obtained by a near-freezing method. J. Neurochem. 1964;11:887–898. doi: 10.1111/j.1471-4159.1964.tb06740.x. [DOI] [PubMed] [Google Scholar]
- Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–227. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Pooler AM, Albrecht MA, Wurtman RJ. Dietary uridine-5'-monophosphate supplementation increases potassium-evoked dopamine release and promotes neurite outgrowth in aged rats. J. Mol. Neurosci. 2005;27:137–145. doi: 10.1385/JMN:27:1:137. [DOI] [PubMed] [Google Scholar]
- Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56:637–660. doi: 10.1016/0024-3205(94)00427-t. [DOI] [PubMed] [Google Scholar]
- Westerink BH, De Boer P, Timmerman W, De Vries JB. In vivo evidence for the existence of autoreceptors on dopaminergic, serotonergic, and cholinergic neurons in the brain. Ann. N. Y. Acad. Sci. 1990;604:492–504. doi: 10.1111/j.1749-6632.1990.tb32015.x. [DOI] [PubMed] [Google Scholar]
- Wu CF, Bertorelli R, Sacconi M, Pepeu G, Consolo S. Decrease of brain acetylcholine release in aging freely-moving rats detected by microdialysis. Neurobiol Aging. 1988;9:357–361. doi: 10.1016/s0197-4580(88)80081-2. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Ulus I, Cansev M, Watkins C, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83–92. doi: 10.1016/j.brainres.2006.03.019. [DOI] [PubMed] [Google Scholar]