Abstract
Animal and epidemiological studies suggest that green tea catechins may reduce the risk of cardiovascular diseases (CHD). The health benefit of green tea has been attributed to its antioxidant and anti-inflammatory properties; however, considerable evidence suggests that green tea and its catechins may reduce the risk of CHD by lowering the plasma levels of cholesterol and triglyceride. Although the mechanism underlying such effect of green tea is yet to be determined, it is evident from in vitro and in vivo studies that green tea or catechins inhibit the intestinal absorption of dietary lipids. Studies in vitro indicate that green tea catechins, particularly EGCG, interfere with the emulsification, digestion, and micellar solubilization of lipids, critical steps involved in the intestinal absorption of dietary fat, cholesterol, and other lipids. Based on the observations, it is likely that green tea or its catechins lower the absorption and tissue accumulation of other lipophilic organic compounds. The available information strongly suggests that green tea or its catechins may be used as safe and effective lipid-lowering therapeutic agents.
Keywords: intestinal absorption, lipids, green tea, (−)-epigallocatechin gallate
1. Introduction
Green tea is a popular beverage, derived from the tea plant, Camellia sinensis. Its peculiar green color results from the inactivation of polyphenol oxidase by treating fresh tea leaves with hot steam and air [1]. The major polyphenols in green tea are catechins constituting about one third of its total dry weight. The major catechins present in green tea (Figure 1) are (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)- epigallocatechin (EGC) and (−)-epicatechin (EC).
Fig. 1.
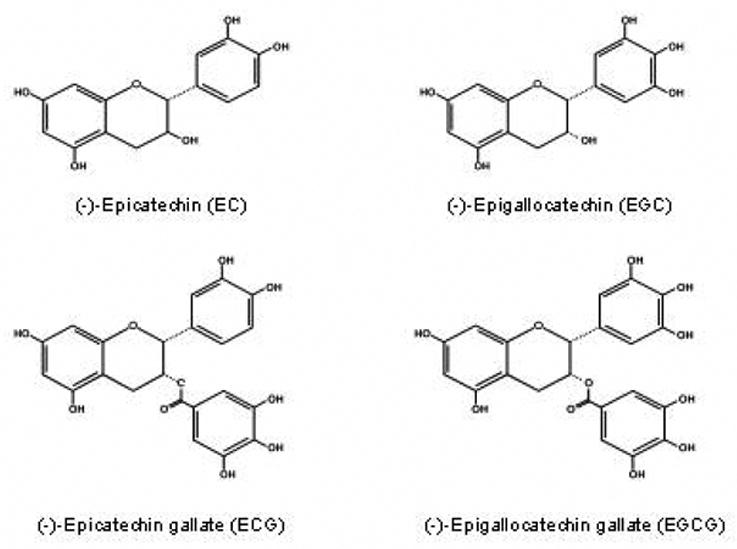
Structures of major green tea catechins
Evidence from animal studies indicates that green tea and its catechins retard the development or progression of atherosclerosis in apoE-deficient mice [2,3] and hypercholesterolemic hamsters [4,5]. Epidemiological studies have shown an inverse association between coronary heart disease (CHD) risk and green tea consumption in humans [6–11].
Studies have shown that catechins possess antioxidant activities and effectively inhibit low-density lipoprotein (LDL) oxidation and lipid peroxidation in vitro [12–17]. At present, it remains debatable whether the reduction in CHD risk in humans associated with green tea consumption is attributable to the prevention of LDL oxidation or to the antioxidant potential of green tea or its catechins [18,19]; however, evidence from animal studies clearly indicates that green tea or its catechins lower the blood levels of cholesterol in cholesterol-fed rats [20,21], mice [22], and hamsters [23], as well as the plasma levels of triglyceride in hamsters fed a high fat diet [23] and in rats fed a high-fructose diet [24].
Green tea catechins - particularly the principal green tea catechin, EGCG - are not readily absorbed, with small percentages of orally ingested catechins appearing in the blood in rats [25] and humans [26,27]. Due to rather poor absorption and greater availability of green tea catechins in the intestinal lumen, it is likely that the lipid-lowering effect of green tea and catechins is mediated largely via their influence on the intestinal processes involved in digestion and absorption of lipids [28–30]. Available information suggests that green tea and its catechins interfere with or inhibit the luminal emulsification, hydrolysis, and micellar solubilization of lipids. The possibility also exists that green tea or catechins may influence the uptake and intracellular processing of lipids and assembly and secretion of chylomicrons.
2. Inhibition of Intestinal Lipid Absorption by Green Tea and Catechins
Using ovariectomized rats with mesenteric lymph-duct cannula, Löest et al. [28] showed that fresh green tea extract, intraduodenally infused at the doses equivalent to 1–2 cups of tea, significantly lowered the lymphatic absorption of cholesterol in a dosedependent manner in rats with mesenteric lymph-duct cannula. Similarly, green tea extracts profoundly inhibited the absorption of α-tocopherol, another lipid of extreme hydrophobicity; however, the absorption of fat (fatty acids) was altered in a biphasic fashion, with a significant increase at a low dose [of ECGC?] and a moderate decrease at a higher dose. Ikeda et al. [29] demonstrated that mixtures of catechins extracted from Japanese green tea lowered the absorption of cholesterol and triglyceride in rats with thoracic lymph-duct cannula. The investigators observed that a mixture of EGCG and ECG were more effective than a mixture of EC and EGC in lowering the absorption of cholesterol, suggesting that the gallate esters of green tea catechins were more potent inhibitors of cholesterol absorption. In another study [30], Ikeda et al. observed that heat-treated catechins high in gallocatechin gallate and catechin gallate were more effective in inhibiting cholesterol absorption than a catechin mixture high in EGCG and ECG. It appears that tea catechins are less effective in inhibiting fat absorption. USing the fecal isotope ratio method, Raederstorff et al. [31] found that EGCG lowered the absorption of cholesterol in a dose-dependent manner in rats, whereas it decreased fat absorption only moderately even at a high dose. This finding is consistent with the observation of Ikeda et al. [29] that the inhibition of fat absorption by catechins was both moderate and dependent on the types of fat incorporated into lipid emulsions.
3. Inhibition of Luminal Lipid Hydrolysis by Green Tea and Catechins
Studies in vitro have shown that green tea and catechins inhibit pancreatic lipase activity. Juhel et al. [32] first reported that a green tea extract significantly inhibited gastric and pancreatic lipase activities, as determined by using a relatively high level of catechins under gastric and duodenal conditions in vitro. The addition of the green tea extract at 60 mg/g triolein prevented the emulsification of fat in the presence of bile acids. Similarly, Ikeda [33] demonstrated that a mixture of catechins high in EGCG and ECG dose-dependently inhibited pancreatic lipase in vitro and suppressed the postprandial rise in serum triglyceride.
A recent study by Shishikura et al. [34] examined the effect of green tea catechins on lipid emulsification using a model emulsion consisting of olive oil, phosphatidylcholine (PC), and bile salt. Green tea catechins, particularly EGCG, at the levels achievable by typical daily intake, markedly altered the physicochemical properties of a lipid emulsion by increasing its particle size and reducing the surface area [34]. Such changes likely slow the rate of hydrolysis of fat, as pancreatic lipase activity decreases with increasing emulsion droplet size and decreasing surface area [35]. Of particular interest is the finding that among the green tea catechins, EGCG was the main compound present on the lipid phase of the emulsion, indicating that EGCG is the principal catechin responsible for the changes in emulsion properties. This finding is consistent with the observation that EGCG is more effective than other catechins in lowering intestinal lipid absorption [29, 36]. The investigators [34] proposed that the hydroxyl moieties of EGCG interact with the hydrophilic head group of PC at the exterior of a lipid emulsion by forming hydrogen bonds. Such interactions may lead to formation of cross links followed by coalescence of the emulsion droplets.
Consistent with the above findings, our recent study [36] showed that green tea catechins also inhibit pancreatic phospholipase A2 (PLA2), as determined under in vitro conditions. Among the major catechins, EGCG was most effective in inhibiting PLA2 activity. The degree of PLA2 inhibition by catechins, at 0.6 μmol, increased in the order of EC, EGC, ECG and EGCG. When labeled PC was infused intraduodenally along with EGCG in rats with mesenteric lymph cannula, a significant amount of PC remained unhydrolyzed in the small intestinal lumen and the cecum, with a marked decrease in the lymphatic output of the labeled tracer. Our findings from this study provide strong evidence that the decreased absorption of lipids by green tea catechins, particularly EGCG, is partly attributable to the inhibition of PLA2 activity. As proposed by Shishikura et al. [34], it is possible that EGCG may form complexes with the surface PC of a lipid emulsion, hindering access to the substrate by PLA2 or directly with the enzyme protein altering its conformation and catalytic activity [1,37,38].
The potent inhibitory effect of EGCG on pancreatic PLA2 activity may be largely responsible for the decreased absorption of lipids because luminal PC hydrolysis is critical to facilitating intestinal lipid digestion and absorption as evidenced from studies in vitro [39–43] and in vivo [44]. Many studies in vitro demonstrated that if PC present on the exterior of a lipid emulsion remains intact, it interferes with the hydrolysis of the core triglyceride by pancreatic lipase. Pancreatic lipase/colipase was shown to be ineffective in hydrolyzing triglyceride incorporated into PC-containing lipid emulsions and the initial hydrolysis of the surface PC by pancreatic PLA2 significantly increased the hydrolysis of triglyceride by pancreatic lipase/colipase [39,42,43,45]. In addition, a study with intestinal cells [44] showed that a minimal hydrolysis of triglyceride was required for stimulation of the cell uptake of other extremely hydrophobic lipids such as cholesterol. Thus, the initial action of pancreatic PLA2 is critical to the hydrolysis of triglyceride by lipase, formation of mixed micelles and subsequent transfer of micellar lipids to the enterocyte through the unstirred water layer [46,47].
In our study [36], α-tocopherol was included in a lipid emulsion as another marker of extremely hydrophobic lipids and retinol as a relatively less hydrophobic lipid to determine whether EGCG differentially inhibits the absorption of lipids differing in hydrophobicity in rats. Data showed that EGCG lowered the lymphatic output of α- tocopherol to 46% of the controls, whereas it did not affect the lymphatic absorption of retinol and lowered fat (fatty acid) absorption only moderately (by less than 9%). These findings are in keeping with those of Homan and Hamelehle [48] that the presence of PC in bile salt micelles markedly reduced the uptake of cholesterol, whereas it did not interfere with the cell uptake of less hydrophobic lipids such as retinol, oleic acid, and monoacylglycerol. Thus, the inhibition of luminal PC hydrolysis by EGCG may explain the rather marked inhibition of the lymphatic absorption of cholesterol and α-tocopherol of extreme hydrophobicity and the moderate or no effect of EGCG on less hydrophobic compounds such as retinol and fatty acid [36].
In view of the above findings, it would be of interest to determine whether EGCG interferes with the absorption of non-nutrient lipophilic compounds, including persistent organic pollutants (POPs). Previously, it has been shown that green tea profoundly lowered the absorption of POPs, including polychlorinated biphenyls (PCBs), thus decreasing the tissue burden of the POPs [49]. Attention should be directed to determining whether green tea or catechins can be used as an effective dietary means of reducing the absorption and tissue accumulation of certain environmental lipophilic POPs.
4. Influence of Green Tea and Catechins on the Intestinal Uptake and Intracellular Processing of Lipids
A critical step for the uptake and absorption of lipids by the enterocyte is the micellar solubilization of hydrolyzed lipids, which facilitates the transfer of lipids via the unstirred water layer to the enterocyte for uptake. Studies have shown that EGCG is more effective than other green tea catechins in precipitating cholesterol from bile salt micelles [29,30] but that it does not significantly affect the micellar solubility of fatty acids and monoacylglycerol, products of triglyceride hydrolysis by pancreatic lipase. The observations are in keeping with the findings that EGCG is a potent inhibitor of cholesterol absorption but has little or moderate inhibitory effect on fatty acid (fat) absorption [29,31].
Increasing evidence suggests that uptake of lipids by the enterocyte is partly mediated by specific transporters on the brush border membrane (BBM). The possibility exists that green tea catechins may interact with proteins implicated in the uptake and efflux of lipids. For example, the transport of cholesterol across the BBM is modulated by proteins such as multidrug resistance P-glycoprotein 1 (MDR1) [50], ATP-binding cassette (ABC) proteins [51,52], B type-1 scavenger receptors [53,54], and Niemann Pick C1-like 1 protein [55]. As stated above, ingested catechins may form complexes with BBM proteins through hydrophobic interactions and hydrogen bonding. This possibility is supported by the findings that certain flavonoids modulate the activities of MDR glycoproteins by interacting with the ATP-binding site and steroid-interacting region [56,57]. Thus, it is probable that green tea catechins may influence the uptake of cholesterol and other lipids by the enterocyte through interaction with transporters, particularly those exposed to the intestinal lumen. At present, it remains unknown whether green tea or its constituents influence their expression in the enterocyte.
After lipids are taken up by the entrocyte, green tea may alter the intracellular processing and packaging of lipids including their reacylation or resynthesis of lipids. In our previous study [28], we observed a transient but significant decrease in the relative amount of esterified cholesterol appearing in lymph when a lipid emulsion was luminally infused with green tea extract, suggesting that green tea may inhibit intestinal acyl CoA:cholesterol acyltransferase in the enterocyte. Evidence indicates that flavonoids such as quercetin and naringenin inhibit the activity of diacylglycerol acyltransferase and the lipidation of ApoB-containing lipoproteins by microsomal triglyceride transfer protein in Caco-2 cells [58] and HepG2 cells [59]. Thus, it is possible that green tea catechins may influence critical steps involved in the assembly and secretion of chylomicrons from the enterocyte into the lymphatics. Further studies are warranted to determine whether green tea or its constituents alter the expression of genes involved in regulating these processes.
5. Summary and Conclusion
Based on the information available thus far, it is evident that green tea and its catechins effectively lower the intestinal absorption of lipids. Among the green tea catechins, EGCG is the most potent inhibitor of lipid absorption. The potent inhibitory effect of EGCG appears to be associated with its ability to form complexes with lipids and lipolytic enzymes, thereby interfering with the luminal processes of emulsification, hydrolysis, micellar solubilization, and subsequent uptake of lipids. EGCG appears to be more effective in lowering the absorption of lipids of extreme hydrophobicity, such as cholesterol and α-tocopherol, with little or a moderate effect on less hydrophobic lipids such as retinol and fatty acid. It is probable that green tea or it constituents lower the absorption of other lipophilic compounds such as POPs. Further studies are warranted to define the mechanisms underlying the inhibition of lipid absorption by green tea and its catechins.
Footnotes
Supported in part by NIH R21AT001363-01A2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harbowy ME, Balentine D. Tea Chemistry, Critical Rev Plant Sci. 1997;16:415–80. [Google Scholar]
- 2.Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, Elis A, Aviram M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- 3.Chyu KY, Babbidge SM, Zhao X, Dandillaya R, Rietveld AG, Yano J, Dimayuga P, Cercek B, Shah PK. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;109:2448–53. doi: 10.1161/01.CIR.0000128034.70732.C2. [DOI] [PubMed] [Google Scholar]
- 4.Yang TTC, Koo MWL. Hypocholesterolemic effects of Chinese tea. Pharmacol Res. 1997;35:505–12. doi: 10.1006/phrs.1997.0176. [DOI] [PubMed] [Google Scholar]
- 5.Vinson JA, Teufel K, Wu N. Green and black teas inhibit atherosclerosis by lipid, antioxidant, and fibrinolytic mechanisms. J Agric Food Chem. 2004;52:3661–5. doi: 10.1021/jf035255l. [DOI] [PubMed] [Google Scholar]
- 6.Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–11. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous Dietary flavonoids and risk of coronary heart disease. Nutr Rev. 1994;52:59–61. [PubMed] [Google Scholar]
- 8.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women [published erratum appears in Am J Epidemiol 1999;150:432] Am J Epidemiol. 1999;149:943–9. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 9.Stensvold I, Tverdal A, Solvoll K, Foss OP. Tea consumption. relationship to cholesterol, blood pressure, and coronary and total mortality. Prev Med. 1992;21:546–53. doi: 10.1016/0091-7435(92)90062-m. [DOI] [PubMed] [Google Scholar]
- 10.Kono S, Shinchi K, Wakabayashi K, Honjo S, Todoroki I, Sakurai Y, Imanishi K, Nishikawa H, Ogawa S, Katsurada M. Relation of green tea consumption to serum lipids and lipoproteins in Japanese men. J Epidemiol. 1996;6:128–33. doi: 10.2188/jea.6.128. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaga S, White IR, Frost C, Tanaka K, Kono S, Tokudome S, Akamatsu T, Moriyama T, Zakouji H. Green tea consumption and serum lipids and lipoproteins in a population of healthy workers in Japan. Ann Epidemiol. 2002;12:157–65. doi: 10.1016/s1047-2797(01)00307-6. [DOI] [PubMed] [Google Scholar]
- 12.Nanjo F, Honda M, Okushio K, Matsumoto N, Ishigaki F, Ishigami T, Hara Y. Effects of dietary tea catechins on α-tocopherol levels, lipid peroxidation, and erythrocyte deformability in rats fed on high palm oil and perilla oil diets. Biol Pharm Bull. 1993;16:1156–9. doi: 10.1248/bpb.16.1156. [DOI] [PubMed] [Google Scholar]
- 13.Miura S, Watanabe J, Tomita T, Sano M, Tomita I. The inhibitory effects of tea polyphenols (flavan-3-ol derivatives) on Cu2+ mediated oxidative modification of low-density lipoprotein. Biol Pharm Bull. 1994;17:1567–72. doi: 10.1248/bpb.17.1567. [DOI] [PubMed] [Google Scholar]
- 14.Miura S, Watanabe J, Sano M, Tomita T, Osawa T, Hara Y, Tomita I. Effects of various natural antioxidants on the Cu2+-mediated oxidative modification of lowdensity lipoprotein. Biol Pharm Bull. 1995;18:1–4. doi: 10.1248/bpb.18.1. [DOI] [PubMed] [Google Scholar]
- 15.Da Silva EL, Piskula M, Terao J. Enhancement of antioxidative ability of rat plasma by oral administration of (−)-epicatechin. Free Radic Biol Med. 1998;24:1209–16. doi: 10.1016/s0891-5849(97)00438-3. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa T, Suzukawa M, Ito T, Yoshida H, Ayaori M, Nishwaki M, Yonemura A, Hara Y, Nakamura H. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am J Clin Nutr. 1997;66:261–6. doi: 10.1093/ajcn/66.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Vinson JA, Jang J, Yang J, Dabbagh Y, Liang X, Serry M, Proch J, Cai S. Vitamins and especially flavonoids in common beverages are powerful in vitro antioxidants which enrich lower density lipoproteins and increase their oxidative resistance after ex vivo spiking in human plasma. J Agric Food Chem. 1999;47:2502–4. doi: 10.1021/jf9902393. [DOI] [PubMed] [Google Scholar]
- 18.van het Hof KH, Wiseman SA, Yang CS, Tijburg LBM. Plasma and lipoprotein levels of tea catechins following repeated tea consumption. Proc Soc Exp Biol Med. 1999;220:203–9. doi: 10.1046/j.1525-1373.1999.d01-34.x. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson JM, Croft KD, Mori TA, Burke V, Beilin LJ, Puddey IB. Regular ingestion of tea does not inhibit in vivo lipid peroxidation in humans. J Nutr. 2002;132:55–8. doi: 10.1093/jn/132.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol. 1986;32:613–22. doi: 10.3177/jnsv.32.613. [DOI] [PubMed] [Google Scholar]
- 21.Yang TTC, Koo MWL. Hypocholesterolemic effects of Chinese tea. Pharmacol Res. 1997;35:505–12. doi: 10.1006/phrs.1997.0176. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Ishigaki A, Hara Y. Long-term effect of a trace amount of tea catechins with perilla oil on the plasma lipids in mice. Int J Vitam Nutr Res. 1998;68:272–4. [PubMed] [Google Scholar]
- 23.Chan PT, Fong WP, Cheung YL, Huang Y, Ho WKK, Chen ZY. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999;129:1094–1101. doi: 10.1093/jn/129.6.1094. [DOI] [PubMed] [Google Scholar]
- 24.Yang MH, Wang CH, Chen HL. Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem. 2001;12:14–20. doi: 10.1016/s0955-2863(00)00140-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Lee M-J, Li H, Yang CS. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–50. [PubMed] [Google Scholar]
- 26.Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131:1731–7. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- 27.Lee M-J, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)- epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Caner Epidemiol Biomark Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 28.Löest HB, Noh SK, Koo SI. Green tea extract inhibits the lymphatic absorption of cholesterol and α-tocopherol in ovariectomized rats. J Nutr. 2002;132:1282–8. doi: 10.1093/jn/132.6.1282. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda I, Imasato Y, Sasaki E, Nakayama M, Nagao H, Takeo T, Yayabe F, Sugano M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim Biophys Acta. 1992;1127:141–6. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda I, Kobayashi M, Hamada T, Tsuda K, Goto H, Iamizumi K, Nozawa A, Sugimoto A, Kakuda T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J Agric Food Chem. 2003;51:7303–7. doi: 10.1021/jf034728l. [DOI] [PubMed] [Google Scholar]
- 31.Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14:326–32. doi: 10.1016/s0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 32.Juhel C, Armand M, Pafumi Y, Rosier C, Vandermander J, Lairon D. Green tea extract (AR25®) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J Nutr Biochem. 2000;11:45–51. doi: 10.1016/s0955-2863(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda I, Tsuda K, Suzuki Y, Kobayashi M, Unno T, Tomoyori H, Goto H, Kawata Y, Imaizumi K, Nozawa A, Kakuda T. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J Nutr. 2005;135:155–9. doi: 10.1093/jn/135.2.155. [DOI] [PubMed] [Google Scholar]
- 34.Shishikura Y, Khokhar S, Murray BS. Effect of tea polyphenols on emulsification of olive oil in a small intestine model system. J Agric Food Chem. 2006;54:1906–13. doi: 10.1021/jf051988p. [DOI] [PubMed] [Google Scholar]
- 35.Armand M, Pasquier B, André M, Borel P, Senft M, Peyrot J, Salducci J, Portugal H, Jaussan V, Lairon D. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am J Clin Nutr. 1999;70:1096–1106. doi: 10.1093/ajcn/70.6.1096. [DOI] [PubMed] [Google Scholar]
- 36.Shu W, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A2 and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006 doi: 10.1016/j.jnutbio.2006.03.004. (in press). [DOI] [PubMed] [Google Scholar]
- 37.Hollman PC, Tijburg LB, Yang CS. Bioavailability of flavonoids from tea. Crit Rev Food Sci Nutr. 1997;37:719–38. doi: 10.1080/10408399709527799. [DOI] [PubMed] [Google Scholar]
- 38.Guharay J, Sengupta B, Sengupta PK. Protein-flavonol interaction: fluorescence spectroscopic study. Proteins. 2001;43:75–81. doi: 10.1002/1097-0134(20010501)43:2<75::aid-prot1019>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Borgström B. Importance of phospholipids, pancreatic phospholipase A2, and fatty acid for the digestion of dietary fat: in vitro experiments with the porcine enzymes. Gastroenterology. 1980;78:954–62. [PubMed] [Google Scholar]
- 40.Thomson AB, Cleland L. Intestinal cholesterol uptake from phospholipid vesicles and from simple and mixed micelles. Lipids. 1981;16:881–7. doi: 10.1007/BF02534992. [DOI] [PubMed] [Google Scholar]
- 41.Reynier MO, Lafont H, Crotte C, Sauve P, Gerolami A. Intestinal cholesterol uptake: comparison between mixed micelles containing lecithin or lysolecithin. Lipids. 1985;20:145–50. doi: 10.1007/BF02534246. [DOI] [PubMed] [Google Scholar]
- 42.Blackberg L, Hernell O, Olivecrona T. Hydrolysis of human milk fat globules by pancreatic lipase: Role of colipase, phospholipase A2, and bile salts. J Clin Invest. 1981;67:1748–52. doi: 10.1172/JCI110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton JS, Carey MC. Inhibition of human pancreatic lipase–colipase activity by mixed bile salt-phospholipid micelles. Am J Physiol. 1981;241:G328–36. doi: 10.1152/ajpgi.1981.241.4.G328. [DOI] [PubMed] [Google Scholar]
- 44.Koo SI, Noh SK. Phosphatidylcholine inhibits and lysophosphatidylcholine enhances the lymphatic absorption of α-tocopherol in adult rats. J Nutr. 2001;131:717–22. doi: 10.1093/jn/131.3.717. [DOI] [PubMed] [Google Scholar]
- 45.Young SC, Hui D. Pancreatic lipase-mediated triacylglycerol hydrolysis is required for cholesterol transport from lipid emulsions to intestinal cells. Biochem J. 1999;339:615–20. [PMC free article] [PubMed] [Google Scholar]
- 46.Thomson AB, Cleland L. Intestinal cholesterol uptake from phospholipid vesicles and from simple and mixed micelles. Lipids. 1981;16:881–7. doi: 10.1007/BF02534992. [DOI] [PubMed] [Google Scholar]
- 47.Reynier MO, Lafont H, Crotte C, Sauve P, Gerolami A. Intestinal cholesterol uptake: comparison between mixed micelles containing lecithin or lysolecithin. Lipids. 1985;20:145–50. doi: 10.1007/BF02534246. [DOI] [PubMed] [Google Scholar]
- 48.Homan R, Hamelehle KL. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J Lipid Res. 1998;39:1197–1209. [PubMed] [Google Scholar]
- 49.Morita K, Matsueda T, Iida T. Effect of green tea (matcha) on gastrointestinal tract absorption of polychlorinated biphenyls, polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxins in rats. Fukuoka Igaku Zasshi. 1997;88:162–8. [PubMed] [Google Scholar]
- 50.Tessner TG, Stenson WF. Overexpression of MDR1 in an intestinal cell line results in increased cholesterol uptake from micelles. Biochem Biophys Res Commun. 2000;267:565–71. doi: 10.1006/bbrc.1999.1996. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz G, Kaminski WE. ABC transporters and cholesterol metabolism. Front Biosci. 2001;6:D505–D514. doi: 10.2741/schmitz. [DOI] [PubMed] [Google Scholar]
- 52.Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res. 2001;42:1513–20. [PubMed] [Google Scholar]
- 53.Thurnhofer H, Hauser H. Uptake of cholesterol by small intestinal brush border membrane is protein-mediated. Biochemistry. 1990;29:2142–48. doi: 10.1021/bi00460a026. [DOI] [PubMed] [Google Scholar]
- 54.Cai SF, Kirby RJ, Howles PN, Hui DY. Differentiation-dependent expression and localization of the class B type 1 scavenger receptor in intestine. J Lipid Res. 2001;42:902–9. [PubMed] [Google Scholar]
- 55.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 56.Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA. 1998;95:9831–6. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and ATPase activities by interaction with dietary flavonoids. Mol Pharmacol. 2001;59:1171–80. doi: 10.1124/mol.59.5.1171. [DOI] [PubMed] [Google Scholar]
- 58.Casaschi A, Wang Q, Dang K, Richards A, Theriault A. Intestinal apolipoprotein B secretion is inhibited by the flavonoid quercetin: potential role of microsomal triglyceride transfer protein and diacylglycerol acyltransferase. Lipids. 2002;37:647–52. doi: 10.1007/s11745-002-0945-8. [DOI] [PubMed] [Google Scholar]
- 59.Borradaile NM, de Dreu LE, Barrett PH, Behrsin CD, Huff MW. Hepatocyte apoBcontaining lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 2003;42:1283–91. doi: 10.1021/bi026731o. [DOI] [PubMed] [Google Scholar]


