Abstract
Calcium pyrophosphate dihydrate (CPPD) crystals are commonly found in osteoarthritic joint tissues, where they predict severe disease. Unlike other types of calcium phosphate crystals, CPPD crystals form almost exclusively in the pericellular matrix of damaged articular cartilage, suggesting a key role for the extracellular matrix milieu in their development. Osteopontin is a matricellular protein found in increased quantities in the pericellular matrix of osteoarthritic cartilage. Osteopontin modulates the formation of calcium-containing crystals in many settings. We show here that osteopontin stimulates ATP-induced CPPD crystal formation by chondrocytes in vitro. This effect is augmented by osteopontin's incorporation into extracellular matrix by transglutaminase enzymes, is only modestly affected by its phosphorylation state, and is inhibited by integrin blockers. Surprisingly, osteopontin stimulates transglutaminase activity in cultured chondrocytes in a dose responsive manner. As elevated levels of transglutaminase activity promote extracellular matrix changes that permit CPPD crystal formation, this is one possible mechanism of action. We demonstrate the presence of osteopontin in the pericellular matrix of chondrocytes adjacent to CPPD deposits and near active transglutaminases. Thus, osteopontin may play an important role in facilitating CPPD crystal formation in articular cartilage.
Keywords: Osteopontin, Calcium pyrophosphate dihydrate, Transglutaminase, Osteoarthritis
Pathologic matrix mineralization is a common occurrence in joints affected by late stage osteoarthritis. Of synovial fluids sampled at the time of knee replacement, for example, 60% contain pathologic calcium-containing crystals (Derfus et al. 2002). Both calcium pyrophosphate dihydrate (CPPD) and hydroxyapatite-like basic calcium phosphate (BCP) crystals occur in osteoarthritic joints. Although the role that calcium-containing crystals play in osteoarthritis is not fully understood, there is ample evidence to suggest that these crystals are active participants in joint damage. In vitro, calcium-containing crystals induce the release of catabolic cytokines and proteases from synovial cells and chondrocytes (Cheung 2001). Clinically, their presence predicts increased severity of joint damage and more rapid progression of joint destruction (Ledingham et al. 1993; Nalbant et al. 2003).
While BCP crystals are similar in structure and composition to mineral found in normal bone and at many sites of pathologic mineralization, CPPD crystals are relatively unique to hyaline and fibro-cartilages (McCarty et al. 1966). Indeed, when found in other tissues, CPPD crystals are often seen within sites of chondroid metaplasia (Beutler et al. 1993). The smallest and presumably the earliest CPPD crystals occur in the pericellular matrix surrounding articular chondrocytes (McCarty 1972). This narrow tissue distribution suggests that the unique milieu of cartilage extracellular matrix may be necessary for CPPD crystal formation.
CPPD crystals are formed when several conditions are met. High local concentrations of extracellular inorganic pyrophosphate, the anionic component of the crystal, are clearly necessary for CPPD crystal formation (Ryan et al. 2003). Sufficient levels of extracellular calcium must also be available. Lastly, a solid matrix is crucial for CPPD crystal formation, as conditions for crystallization in solution require supra-physiologic concentrations of ions and extremes of pH and temperature (Mandel et al. 1984).
While considerable attention has been paid to other modulators of CPPD crystal formation, such as pyrophosphate production, relatively little is known about the role of extracellular matrix in this process. CPPD crystals are rarely found in areas of normal articular cartilage matrix (Bjelle 1981), suggesting that alterations in matrix composition and/or ion composition are necessary for crystallization. Ishikawa et al. demonstrated fragmented type II collagen fibers, loss of large proteoglycans, and increased levels of calcium-binding matricellular proteins in cartilage affected by CPPD crystal deposits (Ishikawa et al. 1989; Masuda et al. 1991). Matrix-modifying enzymes known as transglutaminases have also been implicated in CPPD crystal formation (Heinkel et al. 2004). Inhibition of transglutaminase activity in chondrocytes abrogates CPPD crystal formation in vitro (Heinkel et al. 2004). Transglutaminase protein levels and enzymes activities are increased in the extracellular matrix of osteoarthritic articular cartilage (Johnson et al. 2001; Summey et al. 2002), the setting in which CPPD crystals are most common. Although the mechanism through with they promote CPPD crystal formation is unclear, transglutaminases anchor their substrates to matrix and likely alter the composition, structure, and function of affected matrices (Lorand et al. 2003).
Although extracellular matrix composition plays an important role in CPPD crystal formation and growth, the cartilage matrix components that modulate mineralization remain largely unidentified. Osteopontin is a 44-66 kDa calcium-binding matricellular protein present in articular cartilage (Lorand et al 2003; Giachelli et al. 2005) . We became interested in osteopontin as a potential modulator of CPPD crystal formation in articular cartilage for the following reasons: 1). Osteopontin is present at sites of both normal and pathologic matrix mineralization and is a well-characterized modulator of mineralization (Giachelli et al. 2005). 2). Osteopontin has a pericellular distribution in cartilage (Parikh et al. 2003), and is found in increased concentrations in osteoarthritic cartilage (Pullig et al. 2000). 3). Osteopontin is an important substrate of the transglutaminase enzymes in mineralizing tissues (Beninati et al. 1994; Kaartinen et al. 1999).
Osteopontin is almost ubiquitous at sites of both normal and pathologic mineralization. However, its role in mineral formation remains poorly understood and somewhat controversial. Osteopontin typically inhibits growth of calcium-containing crystals in models of bone mineralization and nephrolithiasis (Beshensky et al. 2001; Steitz et al. 2002). Similar effects have recently been demonstrated in a gel-based model of hydroxyapatite crystal formation (Gericke et al. 2005). Fewer studies support a stimulatory role for osteopontin in mineralization (Wozniak et al. 2000). It is likely that high levels of soluble osteopontin act directly on crystal nucleation or growth. Highly anionic osteopontin may limit the size of calcium-containing crystals by binding to existing crystals and preventing further growth. Alternatively, osteopontin may act through other intermediaries. Osteopontin is present in almost every tissue, and is known to act as a signaling protein and cytokine, as well as a regulator of mineralization (Denhardt et al. 2001). Osteopontin interacts with the αvβ3 integrin (Wozniak et al. 2000) and mediates cell adhesion and differentiation, which in turn could alter cell phenotype and matrix mineralization. In osteoblasts, osteopontin production is coordinately regulated with elaboration of inorganic pyrophosphate, a potent inhibitor of hydroxyapatite crystal formation (Johnson et al. 2003; Harmey et al. 2004). Thus, osteopontin may indirectly affect mineralization by altering levels of other crystal-regulating substances.
A commonly invoked explanation for the varied effects of osteopontin both in vivo and in vitro entails variations in osteopontin's extensive post-translational modifications. Osteopontin contains sites for phosphorylation, glycosylation, transglutamination, and thrombin cleavage. Transglutaminase covalently binds osteopontin to fibronectin, to itself, and to other matrix constituents (Kaartinen et al. 1999). Functional alterations due to these post-translational modifications are well-described. Osteopontin's phosphorylation state and whether it is matrix-bound or soluble may be particularly important determinants in its effects in mineralization models (Jono et al. 2000).
The present studies were designed to determine whether osteopontin modulated CPPD crystal formation in articular cartilage. We first determined the effects of osteopontin in a well-characterized model of CPPD crystal formation by chondrocytes (Ryan et al. 1992), and were surprised to find a stimulatory effect. We then determined whether post-translational modifications altered osteopontin's ability to promote CPPD crystal formation, and explored potential mechanisms of this effect. Lastly, we confirmed the presence of osteopontin in the matrix around CPPD crystal deposits in diseased human cartilage.
METHODS
Materials
Purified milk and recombinant bovine osteopontin were from R& D Systems (Minneapolis, MN). The integrin-binding antagonist GRGDS and a control peptide GRGES were from (NeoMPS, Strasbourg, France).
Porcine chondrocyte cultures
Porcine chondrocytes were isolated from hyaline cartilage removed from the patellar and femoral surfaces of 3-5 year old pigs (Johnsonville Foods, Inc., Watertown, WI) by sequential enzymatic digestion (Rosenthal et al. 1991). Chondrocytes were plated at 4 ×105 cells/cm2 in Dulbecco's Modified Eagle's Medium (DMEM, Mediatech, Herndon, VA) with 10 % fetal calf serum in 24-well tissue culture plates. Twenty-four hours before beginning an experiment, media were replaced with serum-free media. Experiments were performed in 50 mM HEPES-buffered DMEM with 0.35 mg/ml bovine serum albumin (experimental media) within 5 days of plating. These culture conditions maintain the highly differentiated chondrocyte phenotype in short term cultures (Mitchell et al. 1992).
ATP-induced calcification (Ryan, Kurup et al. 1992)
In this model, the precipitation of 45Ca by chondrocyte monolayers in the presence of 1 mM ATP correlates with the formation of CPPD crystals, as characterized by morphology, susceptibility to digestion with pyrophosphatase and inorganic pyrophosphate content. Non-specific 45Ca binding is determined by running simultaneous controls with no added ATP. In some experiments, an additional control group with 1mM β-glycerophosphate was added to control for non-specific effects of phosphate. Chondrocytes were cultured in experimental media trace- labeled with 1 μCi/ml 45Ca with or without 1 mM ATP or β-glycerophosphate, and with or without various concentrations of osteopontin. After 48 hours, media were removed, and the cell layer was exhaustively washed with cold Hank's Balanced Salt Solution. The cell layer was treated with 0.1 N NaOH for one hour at 37°C, and radioactivity in the cell layer was quantified by liquid scintigraphy. Values were corrected for protein levels in the cell layers using the Lowry assay.
Post-translational modifications of osteopontin
De-phosphorylation of osteopontin
Purified bovine milk osteopontin was de-phosphorylated using alkaline phosphatase attached to agarose beads (Sigma Chemical Co., St Louis, MO) according to the method of Jono et al. (Jono et al. 2000). This allows for removal of the alkaline phosphatase enzyme prior to exposure to cells, and is estimated to remove 85% of phosphate residues (Goldberg et al. 1995). Protein levels were determined after alkaline phosphatase treatment, so as to correct for any protein lost during processing. To ensure that phosphate was removed from osteopontin during this procedure, inorganic phosphate levels were measured in the osteopontin solution before and after exposure to alkaline phosphate using the QuantiChrom ™ assay (Bioassay Systems, Hayward, CA). During 2 hours of exposure to alkaline phosphatase beads, inorganic phosphate levels in the osteopontin solution increased and plateaued. (Data not shown). To further demonstrate quantitative phosphate removal, we used a stain for phosphorylated proteins (GelCode Phosphoprotein Stain Reagent Set, Pierce, Rockford, IL). Identical quantities of various preparations of osteopontin were loaded onto an SDS gel, and stained according to the manufacturer's directions.
Thrombin cleavage
To determine the effect of thrombin treatment on osteopontin, osteopontin was treated with thrombin attached to agarose beads (Sigma Chemical Co.) for 15 minutes at 37° C. Beads were removed and protein levels were measured to correct for protein lost during processing. SDS-PAGE was performed to confirm quantitative cleavage.
Transglutaminase inhibition
Transglutaminase activity in chondrocytes was inhibited with 125μM cystamine or 1 mM monodansylcadaverine. We have shown previously that these concentrations of inhibitors reduce transglutaminase activity in chondrocytes by 50-90% without significant toxicity (Heinkel et al. 2004).
Osteopontin and aggrecan ELISAs
Osteopontin levels were measured in the cell layer using a commercial ELISA (Assay Designs, Ann Arbor, MI). Levels of aggrecan were measured in an identical manner using an ELISA kit (Biosource, Nivelles, Belgium). Media were removed from chondrocyte cultures. After washing, the cell layer was scraped into the assay buffer supplied with each kit. Levels of specific proteins were corrected for total cell layer protein as measured by the Lowry assay.
Determination of pyrophosphate levels
Inorganic pyrophosphate levels were measured in chondrocyte conditioned media as previously described (Rosenthal et al. 1991). This method is based on the enzymatic conversion of 14C UDPG to 14C glucose-1-phosphate and uridine triphosphate in the presence of pyrophosphate. Differential charcoal absorption of 14C UDPG before and after enzymatic conversion directly reflects pyrophosphate levels.
Identification of transglutaminase-specific crosslinks in osteopontin (Ishikawa et al. 1989; Masuda et al. 1991)
We isolated osteopontin from a guanidine extract of porcine articular cartilage using an immunoaffinity column (ImmunoPure Plus IgG Orientation Kit, Pierce, Rockford, IL) with a polyclonal osteopontin antibody (Chemicon). After exhaustive digestion, transglutaminase-specific ε –(γ glutamyl) lysine crosslinks were quantified in osteopontin by HPLC as previously described (Heinkel et al. 2004). The purity of the immunopurified protein was determined by running the sample on an SDS-PAGE gel, followed by Western blotting.
Transglutaminase activity and enzyme levels
Transglutaminase activity was measured using a standard radiometric assay based on incorporation of 3H putrescine into casein as previously described (Rosenthal et al. 1997). Factor XIIIA and type II transglutaminase protein levels were estimated by Western blotting (Rosenthal et al. 2004).
Chondrocyte viability
Chondrocyte viability under various experimental conditions described was measured with a standard assay based on leakage of LDH with cell injury (Genotech, St. Louis, MO).
Human cartilage samples
Preserved, de-identified, paraffin-embedded human knee cartilage samples were obtained from patients with CPPD deposition disease. These tissue specimens were obtained from hospitals affiliated with Iwate Medical University Hospital, Morioka, Tohoku University Hospital, Sendai or Kumamoto University Hospital, Kumamoto, Japan from 1995 to 2003. Informed consent was obtained from each patient. The study has been carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association, and has been approved by the IRBs of the hospitals involved.
Immunohistochemistry
After de-paraffinization, sections for osteopontin immunostaining were pretreated at 37° C for 10 minutes with 1mg/ml of trypsin diluted in 9 mM calcium chloride at pH 7.4. Sections for N ε-(γ-glutamyl) lysine isopeptide immunostaining were pretreated at 37° C for 30 minutes with 40 mU/ml of chondroitinase ABC (Seigagaku, Rockville, MD) diluted in 30mM sodium acetate and 10mM Tris-HCl at pH 7.4. Washed sections were treated with 0.3% (V/V) hydrogen peroxide for 30 minutes at room temperature and with 10% (V/V) normal goat serum for 40 minutes at room temperature. Sections were incubated with anti- N ε-(γ-glutamyl) lysine isopeptide mouse monoclonal antibody (1:100, CovaLab, Lyon, France) or rabbit anti-human osteopontin (1:1500, Chemicon, Temecula, CA) overnight at 4°C. After washing, sections were treated with the appropriate biotinylated anti-IgG for 30 minutes at room temperature. Antibody staining was detected with avidin-biotin-antiperoxidase complex (ABC kit; Vector Laboratories Inc., Burlingame, CA). Antigenic sites were demonstrated by reacting the sections with a 3,3′ diaminobenzidine tetrahydrochloride (DAB: Sigma Chemical Co., USA) for 7 min. Sections were counterstained with methyl green, dehydrated in ethanol, cleared in xylene and mounted. Non-immune mouse (for N ε-(γ-glutamyl) lysine isopeptide) or rabbit (for osteopontin) IgG was used in place of the primary antibody in the negative controls.
Statistics
All experiments were repeated at least 3 times. A student's T test was used to determine statistical significance between groups.
RESULTS
The effect of osteopontin on CPPD crystal formation by articular chondrocytes
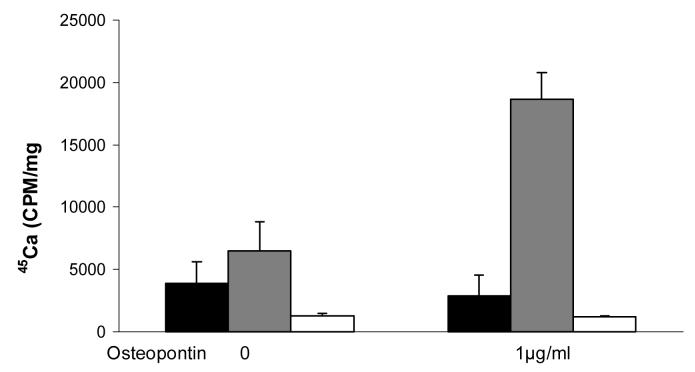
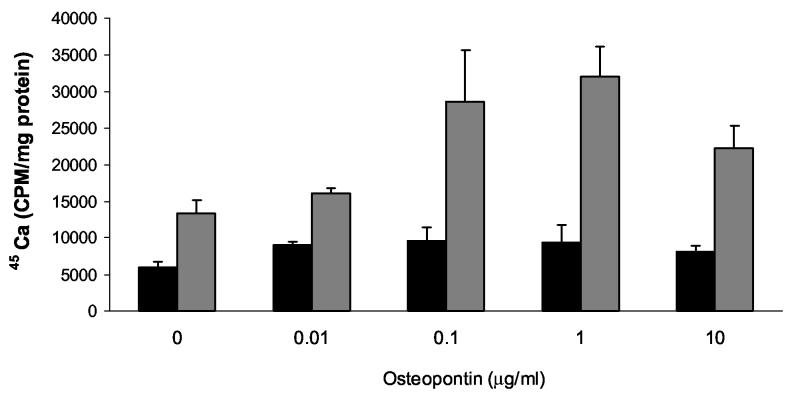
To explore the role of osteopontin in CPPD crystal formation, we employed a well characterized model based on the ATP-dependent accumulation of calcium in articular chondrocyte cultures. In this model, 45Ca precipitated in the cell layer in the presence of ATP forms CPPD crystals, as previously proven by chemical analysis, morphologic observations, and susceptibility to digestion by pyrophosphatase (Ryan et al. 1992). A significant advantage of this model is that simple calcium binding or calcium uptake by chondrocytes is controlled for by measuring 45Ca in the absence of ATP. As shown in Figure 1, the addition of 1 μg/ml recombinant osteopontin to chondrocytes increased quantities of 45Ca in the cell layer by 2.4 ± 0.04 fold in the presence of ATP compared to control values without osteopontin (n = 25, p<0.01). Osteopontin had no effect on 45Ca accumulation in the absence of ATP, or in the presence of 1 mM β glycerophosphate. These results suggest that this effect is not a non-specific effect related to osteopontin's calcium binding capacity, nor is it an effect from added phosphate. As shown in Figure 2, recombinant osteopontin increased ATP-induced 45Ca accumulation at doses as low as 0.1 μg/ml with maximal activity at 1 μg/ml. Concentrations below 0.1 μg/ml or above 10 μg/ml did not statistically significantly stimulate CPPD crystal formation. Osteopontin had no effect on the viability of chondrocytes at any of the concentrations tested. (Data not shown). The morphology of crystals formed in the presence of osteopontin and ATP is identical to that seen with ATP alone under polarizing light microscopy and demonstrates the characteristic positive birefringence of CPPD crystals (Figure 3).
Figure 1.
The effect of recombinant osteopontin in a model of CPPD crystal formation in chondrocytes. Chondrocytes were incubated with 1 μg/ml recombinant osteopontin and 45Ca , with or without 1 mM ATP or 1 mM β glycerophosphate. After 48 hours, media were removed and cell layers were washed. 45Ca levels in the cell layer were measured with liquid scintigraphy and corrected for cell protein. Black bars represent results with no ATP. Grey bars represent results with ATP. White bars represent results with β-glycerophosphate. Values are means ± standard errors (n=25). Osteopontin significantly increased 45Ca precipitation in the presence of ATP, reflecting increased CPPD crystal formation (p<0.01).
Figure 2.
The effect of various doses of recombinant osteopontin on CPPD crystal formation. Chondrocytes were incubated with varying doses of recombinant osteopontin in the presence of 45Ca with or without 1 mM ATP. After 48 hours, media were removed, and cell layers were thoroughly washed. 45Ca levels in the cell layer were measured with liquid scintigraphy and corrected for cell protein. Black bars represent results with no ATP. Grey bars represent results with ATP. Values are means ± standard deviations (n=6). Concentrations of osteopontin less than 0.1 μg/ml or greater than 10 μg/ml did not statistically significantly increase CPPD crystal formation.
Figure 3.
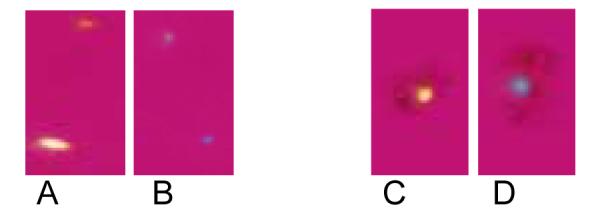
Polarizing light microscopy of crystals generated by chondrocyte monolayers. Chondrocytes were incubated with 1 mM ATP with or without recombinant osteopontin for 48 hours. The cell layers were scraped onto slides and viewed under polarizing light microscopy. CPPD crystals appear as rhomboidal positively birefringent structures. Crystals were yellow when perpendicular to the axis of the polarizer and blue when parallel. The same field was photographed in two orientations at 90 degree angles to one another. A) and B) are cell layers incubated with ATP; C) and D) are cell layers incubated with osteopontin and ATP.
The effect of de-phosphorylation and thrombin cleavage on osteopontin's ability to stimulate CPPD crystal formation
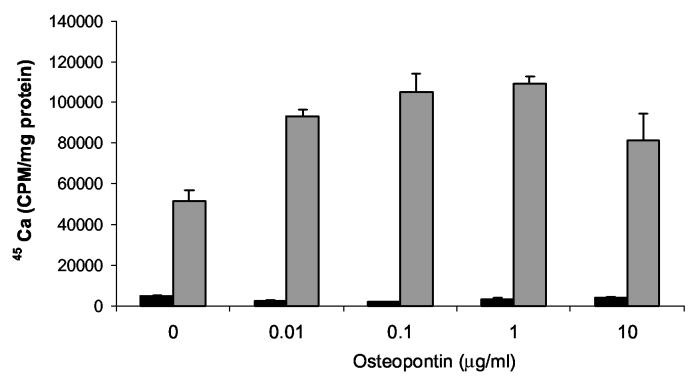
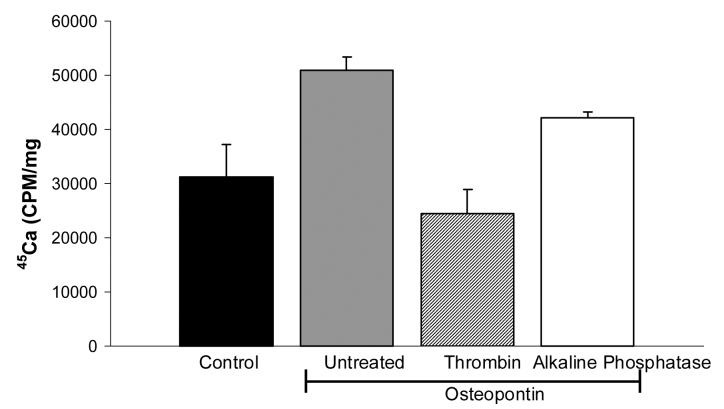
The phosphorylation state of osteopontin may have dramatic effects of on its behavior in mineralization models. For example, several studies have shown that highly phosphorylated osteopontin had opposite effects on mineral formation compared to un-phosphorylated forms (Jono et al. 2000; Gericke et al. 2005). We examined the effects of highly phosphorylated milk osteopontin on CPPD crystal formation. As seen in Figure 4, the dose response curve seen with milk osteopontin is very similar to that seen with the less phosphorylated recombinant protein. To confirm these results, we compared the effects of phosphorylated and de-phosphorylated milk osteopontin on CPPD crystal formation. After de-phosphorylation of milk osteopontin with alkaline phosphatase-coated beads, osteopontin retained its ability to stimulate CPPD crystal formation (p<.05), with a small decrease in efficacy (p<.05) (Figure 5). Using a stain for phosphorylated proteins (Figure 6), we confirmed a significant removal of phosphate moieties from milk osteopontin using alkaline phosphatase-coated beads. This stain also demonstrated fewer phosphates per milligram protein in recombinant osteopontin compared to milk osteopontin (Figure 6). Taken together, these results suggest that osteopontin's ability to stimulate CPPD crystal formation is not fully dependent on its phosphorylation state.
Figure 4.
The effect of various doses of milk osteopontin on CPPD crystal formation. Chondrocytes were incubated with varying doses of milk osteopontin in the presence of 45Ca with or without 1 mM ATP. After 48 hours, media were removed, and cell layers were thoroughly washed. 45Ca levels in the cell layer were measured with liquid scintigraphy and corrected for cell protein. Black bars represent results with no ATP. Grey bars represent results with ATP. Values are means ± standard deviations (n=6).
Figure 5.
The effect of de-phosphorylation and thrombin-cleavage on osteopontin's ability to stimulate CPPD crystal formation. Chondrocytes were incubated with no additives (Control, black bar), 1 μg/ml untreated milk osteopontin (grey bar), 1 μg/ml thrombin-cleaved osteopontin (hatched bar), or 1 μg/ml alkaline-phosphatase treated osteopontin (white bar) in the presence of 45Ca and 1 mM ATP. After 48 hours, media were removed, and the cell layers were washed. 45Ca levels were determined in the cell layer and corrected for cell protein. Results are expressed as means ± standard deviations (n=4). Thrombin-treated osteopontin is no different from control (p=0.25). Alkaline phosphatase treatment of osteopontin reduced its effects (p<.003), but levels of CPPD crystal formation remained significantly higher than control values (p<.05).
Figure 6.
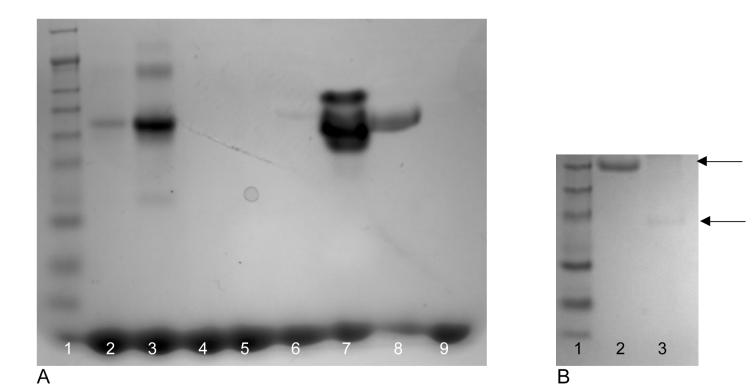
Staining for phosphorylation of osteopontin and quantitative cleavage of osteopontin by thrombin. A) Identical quantities of various preparations of osteopontin were stained for phosphorylation using GelCode phosphoprotein stain according to the manufacturer's directions. Lane1: molecular weight markers , Lane 2: 1 μg positive control, Lane 3: 10 μg positive control, Lane 4: 1 μg negative control, Lane 5: 10 μg negative control, Lane 6: 10 μg recombinant osteopontin, Lane 7: 10 μg milk osteopontin, Lane 8: 10 μg human osteopontin (Assay Designs), Lane 9: 10 μg alkaline phosphatase-treated milk osteopontin.
B) SDS Page of osteopontin before and after cleavage by thrombin. Milk osteopontin was exposed to thrombin coated beads and samples were run on an SDS PAGE gel before and after thrombin exposure. There is quantitative cleavage of osteopontin by thrombin. Lane 1: molecular weight marker, Lane 2: untreated osteopontin, Lane 3: thrombin treated osteopontin
Thrombin cleavage may also alter the behavior of osteopontin (Yamamoto et al. 2003). The thrombin cleavage site is near the integrin binding site in the osteopontin molecule, yet thrombin cleavage stimulates integrin binding in some cell types (Yokosaki et al. 2005). Exposure of milk osteopontin to thrombin-coated beads, reduced osteopontin's effect on CPPD crystal formation so as to quantitatively eliminate its stimulatory effect (p=0.25) (Figure 5). Quantitative cleavage of osteopontin by thrombin is confirmed in Figure 6. These data suggest that an intact osteopontin molecule is necessary for an optimal effect. Neither de-phosphorylation nor thrombin-cleavage altered the effect of osteopontin on 45Ca precipitation by chondrocytes in the absence of ATP. (Data not shown).
The effect of transglutaminase-inhibitors on osteopontin levels in extracellular matrix
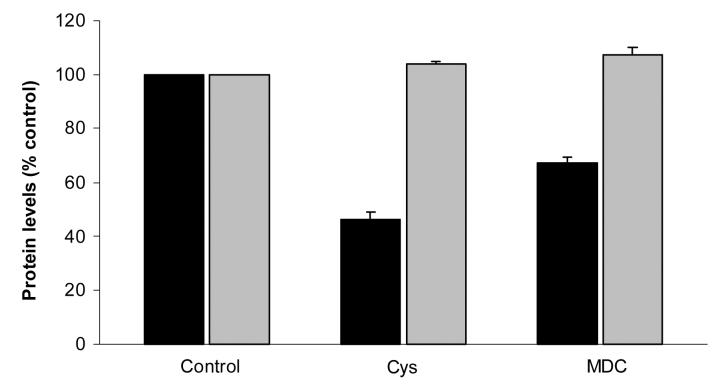
To confirm that osteopontin is a substrate for endogenous transglutaminases in articular chondrocytes, we determined the effects of transglutaminase inhibitors on osteopontin levels in chondrocyte cell layers. Transglutaminase inhibitors reduced osteopontin levels in chondrocyte cell layers (Figure 7). Cystamine decreased osteopontin levels to 46 ± 3% of control values, while monodansylcadaverine decreased osteopontin levels to 67 ± 2 % of control values (n=6, p< 0.05). In contrast, levels of aggrecan, a matrix protein not known to be a transglutaminase substrate, were unchanged by the addition of transglutaminase inhibitors. These data confirm transglutaminase-dependent extracellular matrix incorporation of osteopontin. Levels of osteopontin and aggrecan in the media were unchanged after cystamine exposure compared to control values. Aggrecan and osteopontin levels in the media significantly decreased in the presence of monodansylcadaverine in the setting of a decrease in total protein synthesis frequently caused by monodansylcadaverine (Data not shown).
Figure 7.
The effect of transglutaminase inhibitors on levels of osteopontin and aggrecan in chondrocyte cell layers. Chondrocytes were incubated with or without the transglutaminase inhibitors cystamine (Cys, 125µM) or monodansylcadaverine (MDC, 1 mM) for 24 hours. Levels of osteopontin and aggrecan were determined in the cell layer with ELISAs and corrected for protein levels in the samples. Results are normalized to the control (no inhibitor), and expressed as means ± standard deviations (n =6). The transglutaminase inhibitors reduced osteopontin levels (black bars, p<0.05), but had no effects on levels of aggrecan (grey bars). Levels of these proteins in the media were unchanged with cystamine, and moderately decreased by monodansylcadaverine.
Transglutaminase crosslinks in osteopontin from articular cartilage
Further evidence that osteopontin is a transglutaminase substrate in articular cartilage is evidenced by the direct measurement of the transglutaminase-specific dipeptide ε –(γ glutamyl) lysine in osteopontin isolated from porcine cartilage. Osteopontin was isolated from porcine cartilage using immunoprecipation. This resulted in two bands on SDS-PAGE gel. Both were immunoreactive with anti-osteopontin. (Data not shown.) Levels of crosslink were 202 ± 43 nmoles glutamyl-lysine crosslink /mg protein. This level is similar to that of osteonectin, also a known transglutaminase substrate in cartilage.
The stimulatory effect of osteopontin on CPPD crystal formation requires active transglutaminases
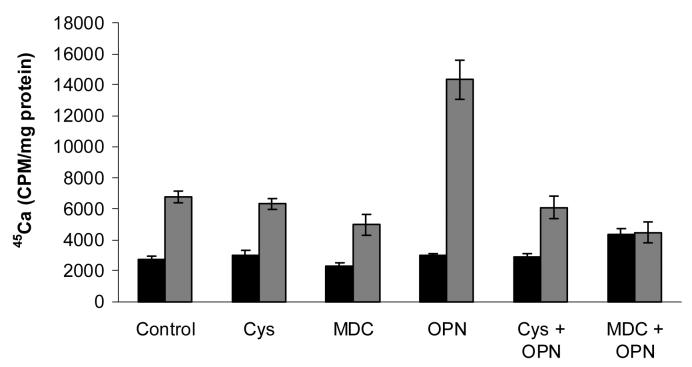
We next explored the hypothesis that transglutaminase-mediated binding of osteopontin to chondrocyte matrix affects its ability to promote CPPD crystal formation. We incubated chondrocytes with millk osteopontin in the presence of transglutaminase inhibitors and measured CPPD crystal formation. As shown in Figure 8, the transglutaminase inhibitors, cystamine and monodansylcadaverine, suppressed the stimulatory effects of osteopontin on CPPD crystal formation. Compared to osteopontin alone, 45Ca levels fell significantly with osteopontin and cystamine, and osteopontin and monodansylcadaverine, in the presence of ATP. Indeed, in the absence of active transglutaminases, osteopontin failed to stimulate CPPD crystal formation. Confirming our previous findings, transglutaminase inhibition alone decreased levels of ATP-induced 45Ca precipitation in chondrocytes (Heinkel et al. 2004). No effects were seen in the absence of ATP. These data suggest that transglutaminase-mediated incorporation of osteopontin into chondrocyte matrix promotes its stimulatory effects on CPPD crystal formation.
Figure 8.
The effect of transglutaminase inhibitors on osteopontin's ability to stimulate CPPD crystal formation. Chondrocytes were incubated with 1 μg/ml milk osteopontin (OPN) in media containing 45Ca with or without 1 mM ATP. Some chondrocyte cultures were exposed to the transglutaminase inhibitors cystamine (Cys, 125μM) or monodansylcadaverine (MDC, 1 mM). After 48 hours, media were removed, cell layers were washed, and 45Ca levels in the cell layer were quantified with liquid scintigraphy and corrected for cell protein. Black bars represent results with no ATP. Grey bars represent results with ATP. Values represent means ± standard deviations (n=16). Both cystamine and monodansylcadaverine significantly reduced the effects of osteopontin on CPPD crystal formation (p<0.001).
The effect of osteopontin on pyrophosphate and transglutaminase levels
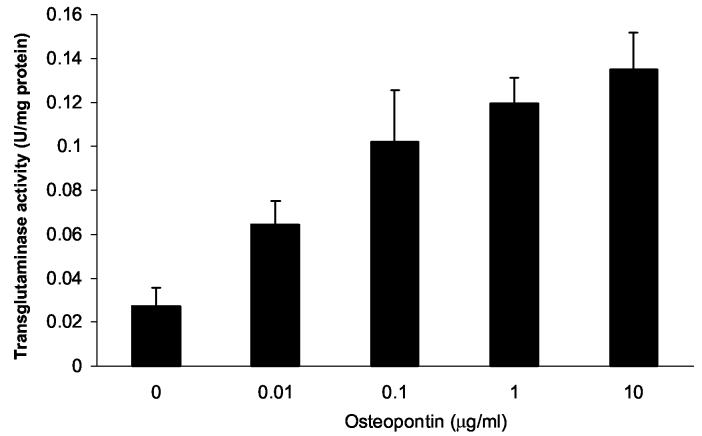
The mechanisms through which osteopontin participates in crystal formation are unclear. Osteopontin, particularly at high concentrations, directly inhibits hydroxyapatite crystal growth by coating existing crystals with highly anionic protein (Goldberg et al. 1995; Gericke et al. 2005). Lower concentrations of osteopontin may affect mineralization through other mechanisms. Pyrophosphate is an antagonist of hydroxyapatite crystal growth, but is a participant in CPPD crystal formation. It is possible that osteopontin indirectly affects CPPD crystal formation by stimulating pyrophosphate production. Alternatively, osteopontin could increase the expression or activity of the transglutaminase enzymes, and thus could contribute to CPPD crystal formation through this mechanism. To address the mechanism of the effect of osteopontin on CPPD crystal formation, we asked whether osteopontin could increase pyrophosphate elaboration or transglutaminase activity levels in cultured chondrocytes. As shown in Table 1, milk osteopontin did not significantly affect pyrophosphate levels in chondrocyte cultures. In contrast, osteopontin did increase transglutaminase activity levels in a dose-responsive manner (Figure 9). This action would further increase the matrix incorporation of osteopontin as well as other mineralization-promoting matrix components.
TABLE 1.
The effect of osteopontin on pyrophosphate levels in chondrocyte cultures.
| Osteopontin (μg/ml) | Pyrophosphate (μM)** |
|---|---|
| 0 | 2.6± 0.2 |
| 0.1 | 3.1± 0.6 |
| 1 | 3.3± 0.7 |
| 10 | 2.3± 0.1 |
| 25 | 2.3± 0.01 |
Various concentrations of milk osteopontin were added to chondrocyte cultures. After 48 hours, pyrophosphate levels were measured in conditioned media. Values represent means ± standard deviations (n=6). Osteopontin did not significantly affect pyrophosphate levels at any concentration (p= 0.1).
Figure 9.
The effect of osteopontin on chondrocyte transglutaminase activity. Chondrocytes were incubated with various doses of milk osteopontin for 48 hours. Transglutaminase activity was measured in the cell layer using a standard radiometric assay, and was corrected for protein in the sample. Osteopontin significantly increased transglutaminase activity at all concentrations tested (n= 4, p < 0.01).
Interestingly, maximally stimulatory doses of osteopontin had little effect on protein levels for the transglutaminases present in articular chondrocytes. One μg/ml of milk osteopontin did not increase protein levels of either type II transglutaminase or factor XIIIA as measured by Western blotting. (Data not shown). Thus, it is likely that osteopontin increases activity of these enzymes without altering their levels. Both enzymes are often regulated at post-translational levels (Lorand et al. 2003), and further work will be necessary to investigate the mechanism of this effect.
The role of αvβ3 integrin in osteopontin's effects
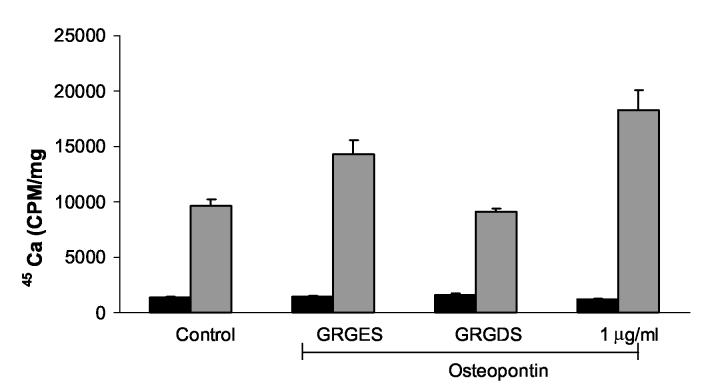
Wozniak et al. elegantly demonstrated that mineralization in bone cell cultures occurs in plaques containing large macromolecular complexes of osteopontin crosslinked by transglutaminases. They showed this early pericellular mineralization process was dependent on the αvβ3 integrin (Wozniak et al. 2000). To determine if the effects of osteopontin in our system were integrin-dependent, we blocked integrin binding with an antagonist peptide. As shown in Figure 10, the active antagonist peptide GRGDS suppressed the effect of milk osteopontin on CPPD crystal formation to about 50% of control values. The control peptide had less of a consistent effect. This experiment was repeated with multiple doses of peptides (Data not shown). While the GRGES peptide had no statistically significant effect on osteopontin-induced CPPD crystal formation at doses of 5-40 μM (142% of osteopontin alone, p =.06), the GRGDS peptide significantly reduced osteopontin-induced CPPD crystal formation at all doses tested (57% of osteopontin alone, p <. 001) Thus, it appears that osteopontin's effect on CPPD crystal formation may at least be partially mediated through integrin binding.
Figure 10.
The effect of an integrin antagonist on osteopontin-induced stimulation of CPPD crystal formation. Chondrocytes were incubated with no additives (Control), 40 μM inactive peptide control (GRGES), 40 μM integrin antagonist (GRGDS) or 1 μg/ml milk osteopontin in the presence of 45Ca and with or without 1 mM ATP. After 48 hours, media were removed, and cell layers were thoroughly washed. 45Ca levels in the cell layer were measured with liquid scintigraphy and corrected for cell protein. Black bars represent results with no ATP. Grey bars represent results with ATP. Values are means ± standard deviations (n=12). Over multiple experiments, the GRGES peptide had no statistically significant effect on osteopontin-induced CPPD crystal formation at doses of 5-40 μM (p=.06), while the GRGDS peptide significantly reduced osteopontin-induced CPPD crystal formation (p ≤.001)
Osteopontin and transglutaminase activity in human cartilage affected by CPPD crystal deposition
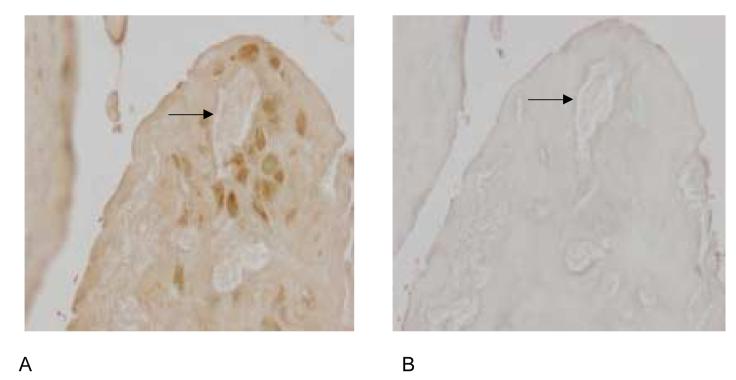
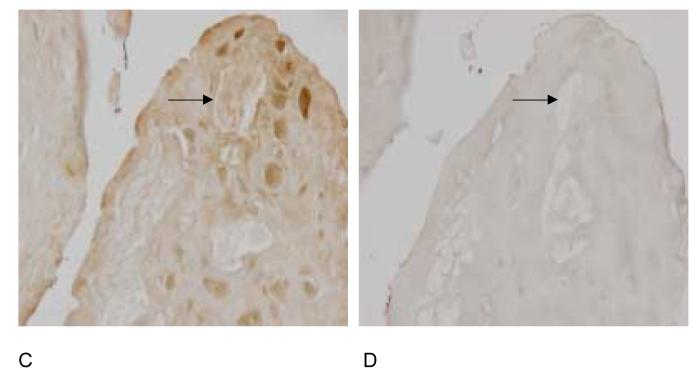
If osteopontin participates in CPPD crystal formation, it should be present in the extracellular matrix of articular cartilage from patients with CPPD deposition disease. Immunohistochemistry with osteopontin antibody demonstrated the presence of osteopontin protein in the pericellular matrix near CPPD crystal deposits (Figure 11A), while a negative control performed with non-immune serum showed little staining (Figure 11B). This pericellular distribution correlates well with the distribution of the smallest and presumably the earliest CPPD crystals. Obviously, no conclusions can be made from these studies as to the chronology of the appearance of the crystals in relation to osteopontin. We also examined the same human cartilage samples for evidence of in vivo transglutaminase activity by staining with an antibody that recognizes the ε–(γ glutamyl) lysine bond formed by transglutaminases. The pericellular pattern of distribution of transglutaminase-specific crosslinks was similar to that of osteopontin (Figure 11C), further supporting a role for transglutaminases in mediating incorporation of osteopontin into cartilage matrix. Again, no staining was seen when the primary antibody was replaced with non-immune serum (Figure 11D).
Figure 11.
Distribution of osteopontin and transglutaminase crosslinks in human CPPD-diseased articular cartilage. Immunohistochemistry was performed with anti-osteopontin antibody (Panel A), non-immune rabbit serum (Panel B), or antibody to the transglutaminase specific crosslink (Panel C), or non-immune mouse serum (Panel D) in human CPPD diseased articular cartilage. Arrows demonstrate CPPD crystals in the tissue slices. The presence of immunoreactive protein is demonstrated by brown staining.
DISCUSSION
We show here that osteopontin is present in the extracellular matrix near CPPD crystal deposits in articular cartilage and that it stimulates CPPD crystal formation by articular chondrocytes in vitro. Our model of CPPD crystal formation is based on the formation of CPPD crystals by cultured chondrocytes in the presence of the ATP (Ryan et al. 1992). Crystals formed under these conditions have previously been proven to be CPPD crystals by polarizing light microscopy, pyrophosphate content, electron beam diffraction and dissolution with pyrophosphatase. Osteopontin had no effect on 45Ca precipitation in the absence of ATP or in the presence of β-glycerophosphate. Taken together, we believe these data demonstrate that osteopontin specifically stimulates CPPD crystal formation, and that this effect is not due to a non-specific increase in calcium binding to the cell layer nor is it a response to exogenous phosphate. Thus, increased levels of osteopontin such as those that occur in osteoarthritic cartilage, could promote CPPD crystal formation.
These findings contrast with the role typically assigned to osteopontin in most mineralization models, where it often acts as an inhibitor of crystal growth and/or nucleation (Steitz et al. 2002; Johnson et al. 2003). Our work involves a different crystal type, and ample evidence suggests that factors that clearly inhibit calcium phosphate crystal formation may stimulate CPPD crystal formation. Inorganic pyrophosphate is perhaps the best characterized example of such a factor. Pyrophosphate inhibits hydroxyapatite crystal nucleation and growth, yet is absolutely required for CPPD crystal formation. Many of the studies demonstrating direct inhibitory effects of osteopontin on calcium crystal formation used fairly high osteopontin concentrations (Gericke et al. 2005), or systems in which the osteopontin remains soluble and is not incorporated into matrix (Beshensky et al. 2000). In contrast, a small number of previous studies showed that in small concentrations or with low phosphorylation states, osteopontin may stimulate calcium phosphate mineral formation (Jono et al. 2000). Taken together, these studies suggest that osteopontin has multiple and complex effects on mineralization.
A great deal of work focuses on the phosphorylation state of osteopontin in mineralization models. Tissue osteopontin is generally not as highly phosphorylated as milk osteopontin, but it is highly unlikely that completely de-phosphorylated osteopontin would be encountered by chondrocytes. We showed that highly phosphorylated forms of osteopontin were slightly more potent stimulants of CPPD crystal formation compared to less phosphorylated forms. In many mineralization models, de-phosphorylated osteopontin had opposite effects to those seen with highly phosphorylated osteopontin (Jono et al. 2000). This is not true of osteopontin in our model. Thus, it is unlikely that the effect of osteopontin in our system is highly dependent on its anionic charge or that it acts by simply coating existing crystals and altering their growth.
Osteopontin is clearly a transglutaminase substrate in articular cartilage and cultured chondrocytes. We proved this by demonstrating that transglutaminase inhibitors decrease its levels in chondrocyte matrix, that specific transglutaminase crosslinks are easily measurable in isolated cartilage osteopontin, and that active transglutaminases reside in the pericellular matrix of CPPD affected chondrocytes in a distribution similar to that of osteopontin. Transglutaminase-mediated incorporation of osteopontin into matrix augments the effect of osteopontin on CPPD crystal formation. Indeed, when transglutaminase activity is suppressed, osteopontin fails to stimulate CPPD crystal deposition. We postulate that the high levels of active transglutaminases in osteoarthritic cartilage trap osteopontin in the extracellular matrix, where it stimulates mineralization. The dose response curve for both milk and recombinant osteopontin demonstrates a bell shaped curve, with a maximum effect occurring at about 1 μg/ml. We hypothesize that chondrocyte matrix can only incorporate a certain quantity of osteopontin. At doses which exceed the capacity of the matrix to incorporate osteopontin, osteopontin remains soluble and may actually begin to inhibit CPPD crystal growth.
One could postulate several mechanisms through which osteopontin might contribute to CPPD crystal formation. Osteopontin might stimulate production of pyrophosphate, the anionic component of the CPPD crystals. We show here, however, that osteopontin has minimal effects on pyrophosphate production by chondrocytes. It is also possible that osteopontin modulates cell death, which then leads to CPPD crystal formation (Jaovisidha et al. 2002). However, osteopontin had no effect on the viability of chondrocytes at the doses employed in these studies. We also investigated the possibility that osteopontin stimulates transglutaminase activity. Surprisingly, osteopontin stimulated transglutaminase activity in chondrocytes in a dose-responsive manner. Protein levels of the chondrocyte transglutaminases, factor XIIIA and type II transglutaminase, were unaffected by osteopontin. Thus, it is likely that osteopontin activates existing transglutaminase enzymes rather than increasing their protein levels. Both type II transglutaminase and factor XIIIA are often post-translationally regulated. For example, thrombin and other serine proteases activate factor XIIIA, and type II transglutaminase activity is tightly regulated by ambient levels of calcium and GTP.
Mirroring work done in osteoblasts, we showed that the effect of osteopontin on CPPD crystal formation was partially abrogated by a peptide which blocks integrin binding. Thus, we propose that crosslinked complexes containing osteopontin acting via the αvβ3 integrin serve as foci of CPPD crystal nucleation. This is further supported by the effect of thrombin on osteopontin, as the thrombin cleavage site is near the integrin binding locus of the protein. It is likely that chondrocytes are capable of assuming a phenotype similar to the hypertrophic phenotype responsible for bone formation in the growth plate, particularly in the setting of osteoarthritis (Kirsch et al. 2000). Thus, it is not surprising that mechanisms of matrix mineralization in articular chondrocytes are similar to that of bone cells under certain conditions.
These studies are not without limitations. For example, there is not a simple way to determine if the effects of osteopontin are mediated by the protein itself, or by osteopontin-induced increases in transglutaminase activity, and a consequent increase in the quantity of cross-linked matrix. In either event, however, the actions of osteopontin clearly stimulate CPPD crystal formation, and this protein may represent a therapeutic target in this disease. In addition, non-specific effects of chemical transglutaminase inhibitors are always a concern. Monodansylcadaverine, for example, did reduce protein levels of both aggrecan and osteopontin secreted into the media. However, its effects on osteopontin levels in the matrix were quite specific, and aggrecan levels were unaffected. We have considerable experience with these inhibitors and approach their limitations by using at least two inhibitors with different mechanisms of action, by ensuring the absence of toxicity, and by comparing their effects under various conditions such as the presence and absence of ATP. Unfortunately, because there are at least two active transglutaminase enzymes in articular cartilage, the use of more specific inhibitors such as antibodies or siRNA becomes too unwieldy to be practical.
We have shown here that osteopontin promotes CPPD crystal formation in a well characterized, chondrocyte-based model. Osteopontin is present in articular cartilage affected by CPPD deposition and may be an important factor in the development of CPPD crystals in vivo. Ultimately, identifying participants involved in CPPD crystal formation may lead to the development of new interventions for CPPD crystal formation and the ensuing cartilage degeneration.
Acknowledgements
We would like to acknowledge the thoughtful assistance of Drs. Lawrence M. Ryan and Jeffrey Wesson in reviewing this manuscript. This work was supported by the National Institutes of Health AG015337 (AKR), and AR 052615 (AKR) and the Veteran's Administration Research Service (AKR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beninati S, Senger D, Cordella-Miele E, Mukherjee A, Chackalaparampil I, Shanmugam V, Singh K, Mukherjee B. Osteopontin:Its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J Biochem. 1994;115:675–82. doi: 10.1093/oxfordjournals.jbchem.a124395. [DOI] [PubMed] [Google Scholar]
- Beshensky A, Wesson J, Kleinman J, Liaw L, Steitz S, Giachelli C, et al. Renoprotective modulation of calcium oxalate studcture by osteopontin in vivo. J Am Soc Nephrol. 2000;11:558A. [Google Scholar]
- Beshensky A, Wesson J, Worcester E, Sorokina E, Snyder C, Kleinman J. Effects of urinary macomolecules on hydroxyapatite crystal formation. J Am Soc Nephrol. 2001;12:2108–16. doi: 10.1681/ASN.V12102108. [DOI] [PubMed] [Google Scholar]
- Beutler A, Rothfuss S, Clayburne G, Sieck M, Schmacher H., Jr. Calcium pyrophosphate dihydrate crystal deposition in synovium. Arthritis Rheum. 1993;36:704–15. doi: 10.1002/art.1780360520. [DOI] [PubMed] [Google Scholar]
- Bjelle A. Cartilage matrix in hereditary pyrophosphate arthropathy. J Rheumatol. 1981;8:959–964. [PubMed] [Google Scholar]
- Cheung H. Calcium crystal effects on the cells of the joint: implications for the pathogenesis of disease. Curr Opinion Rheumatology. 2001;12:223–7. doi: 10.1097/00002281-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Denhardt D, Noda M, O'Regan A, Pavlin D, Berman J. Osteopontin as a means to cope with environmental insults:regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derfus B, Kurian J, Butler J, Daft L, Carrera G, Ryan L, Rosenthal A. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–4. [PubMed] [Google Scholar]
- Gericke A, Qin C, Spevak L, Fujimoto Y, Bultler W, Sorensen E, Boskey A. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C, Speer M, Li X, Rajachar R, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–22. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- Goldberg H, Hunter G. The inhibitory activity of osteopontin on hydroxyapatite formation in vitro. Ann NY Acad Sci. 1995;760:305–8. doi: 10.1111/j.1749-6632.1995.tb44642.x. [DOI] [PubMed] [Google Scholar]
- Harmey D, Hessle L, Narisawa S, Johnson K, Terkeltaub R, Millan J. Concerted regulation of inorganic pyrophosphate and osteopontin by Akp2, Enpp1 and Ank. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinkel D, Gohr C, Uzuki M, Rosenthal A. Transglutaminase contributes to CPPD crystal formation in osteoarthritis. Frontiers in Bioscience. 2004;9:3257–61. doi: 10.2741/1477. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Masuda I, Ohira T, Yokoyama M. A histologic study of calcium pyrophosphate dihydrate crystal-deposition disease. J Bone Jt Surg. 1989;71:875–86. [PubMed] [Google Scholar]
- Jaovisidha K, Rosenthal A. Calcium crystals in osteoarthritis. Curr Opin Rheumatol. 2002;14:298–302. doi: 10.1097/00002281-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Johnson K, Goding J, Van Etten D, Sali A, Hu S-I, Farley D, Krug H, Hessle L, Millan J, Terkeltaub R. Linked deficiencies in extracellular PPi and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J Bone Miner Res. 2003;18:994–1002. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- Johnson K, Hashimoto S, Lotz M, Pritzker K, Terkeltaub R. Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am J Pathol. 2001;159:149–63. doi: 10.1016/S0002-9440(10)61682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, van Etten D, Nanda H, Grahm R, Terkeltaub R. Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J Biol Chem. 2003;278:18824–32. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- Jono S, Giachelli C. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- Kaartinen M, Pirhonen A, Linnala-Kankkunen A, Maenpaa P. Cross-linking of osteopontin by tissue transglutaminase increases its collagen binding properties. J Biol Chem. 1999;274:1729–35. doi: 10.1074/jbc.274.3.1729. [DOI] [PubMed] [Google Scholar]
- Kirsch R, Swoboda B, Nah H-D. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52:520–6. doi: 10.1136/ard.52.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L, Graham R. Transglutaminases: crosslinking enzymes with pleiotrophic functions. Nature. 2003;4:140–57. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Mandel N, Mandel G, Carroll D, Halverson P. Calcium pyrophosphate crystal deposition: An in vitro study using a gelatin matrix model. Arthritis Rheum. 1984;27:789–96. doi: 10.1002/art.1780270710. [DOI] [PubMed] [Google Scholar]
- Masuda I, Ishikawa K, Usuku G. A histologic and immunohistochemical study of calcium pyrophosphate dihydrate crystal deposition disease. Clin Orth. 1991;263:272–287. [PubMed] [Google Scholar]
- McCarty D, Hogan J, Gatter R, Grossman M. Studies on pathological calcifications in human cartilage I. Prevalence and types of crystal deposits in the menisci of two hundred fifteen cadavers. J Bone Jt Surg. 1966;48A:309–25. [PubMed] [Google Scholar]
- McCarty DJ. Pseudogout: articular chondrocalcinosis; calcium pyrophosphate crystal deposition disease. In: Hollander J, McCarty D Jr., editors. Arthritis and Allied Conditions. Eighth Edition Lea & Febiger; Philadelphia: 1972. pp. 1140–60. [Google Scholar]
- Mitchell P, Struve J, McCarthy G, Cheung H. Basic calcium phosphate crystals stimulate cell proliferation and collagenase message accumulation in cultured adult articular chondrocytes. Arthritis Rheum. 1992;35:343–350. doi: 10.1002/art.1780350314. [DOI] [PubMed] [Google Scholar]
- Nalbant S, Martinez J, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher H., Jr Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cart. 2003;11:50–4. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- Parikh A, Lee G, Tchivilev I, Graff R. A neocartilage ideal for extracellular matrix macromolecule immunolocalization. Histochem Cell Biol. 2003;120:427–34. doi: 10.1007/s00418-003-0580-x. [DOI] [PubMed] [Google Scholar]
- Pullig O, Weseloh G, Gauer S, Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biology. 2000;19:245–55. doi: 10.1016/s0945-053x(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Cheung H, Ryan L. Transforming growth factor beta 1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum. 1991;34:904–911. doi: 10.1002/art.1780340717. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Mosesson M, Gohr C, Masuda I, Heinkel D, Siebenlist K. Regulation of transglutaminase activity in articular chondrocytes through thrombin receptor-mediated factor XIII synthesis. Thromb Haemost. 2004;91:558–68. doi: 10.1160/TH03-07-0462. [DOI] [PubMed] [Google Scholar]
- Rosenthal AK, Derfus BA, Henry LA. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum. 1997;40(5):966–970. doi: 10.1002/art.1780400526. [DOI] [PubMed] [Google Scholar]
- Ryan L, Kurup I, Derfus B, Kushnaryov V. ATP-induced chondrocalcinosis. Arthritis Rheum. 1992;35:1520–4. doi: 10.1002/art.1780351216. [DOI] [PubMed] [Google Scholar]
- Ryan L, Rosenthal A. Metabolism of extracellular pyrophosphate. Curr Opin Rheum. 2003;15:311–4. doi: 10.1097/00002281-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Steitz S, Speer M, McKee M, Liaw L, Almeida M, Yang H, Giachelli C. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–46. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summey BJ, Graff R, Lai T, Greenberg C, Lee G. Tissue transglutaminase localization and activity regulation in the extracellular matrix of articular cartilage. J Orth Res. 2002;20:76–82. doi: 10.1016/S0736-0266(01)00064-X. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Fausto A, Carron C, Meyer D, Hruska K. Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of αv β3-integrin expression. J Bone Min Res. 2000;15:1731–40. doi: 10.1359/jbmr.2000.15.9.1731. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, Okazaki I, Seki N, Fujii T, Uede T. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112:181–8. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alphavbeta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005;24:418–27. doi: 10.1016/j.matbio.2005.05.005. [DOI] [PubMed] [Google Scholar]