Abstract
Objectives
To examine whether cochlear implantation increases the risk of meningitis in the absence of other risk factors, and to understand the pathogenesis of pneumococcal meningitis post cochlear implantation.
Study Design and Setting
A quantitative threshold model in rats was established to examine how the presence of a cochlear implant and surgical insertion trauma alter the risk of pneumococcal meningitis post implantation from all potential routes of infection from the upper respiratory mucosa to the meninges.
Results
The presence of a cochlear implant reduced the threshold of bacteria required to cause pneumococcal meningitis from all routes of infection (hematogenous, the middle ear and inner ear) in healthy animals. Mild electrode insertion trauma did not alter the threshold of infection.
Conclusion
Our animal model has demonstrated that cochlear implantation is an independent risk factor for pneumococcal meningitis. The presence of a foreign body such as a cochlear implant increases the risk of pneumococcal meningitis regardless of the routes of bacterial infection.
Significance
Early detection and treatment of pneumococcal infection such as otitis media may be required in implant recipients as the experimental data suggest that the cochlear implantation leads to a reduction of threshold for meningitis.
Introduction
The use of cochlear implants in patients with a severe-profound hearing impairment has been considered to be very safe. Although meningitis is a potential central nervous system (CNS) complication post cochlear implantation, it was considered to be very rare1. During 2002, however, a sudden increase in reported cases, both in Europe and in North America, sparked international concern about the possible association between meningitis and cochlear implantation2
In the reported cases of meningitis, Streptococcus pneumoniae is the most common organism isolated2–4. The incidence of pneumococcal meningitis in cochlear implant recipients was found to be greater than that of an age matched cohort in the general population3. However, it is unclear if cochlear implants increase the risk of post implant pneumococcal meningitis, as many of the reported cases also have pre-existing risk factors for meningitis. Risk factors identified from the clinical records of patients with cochlear implants included: an implant with a positioner; inner-ear malformations with and without cerebral spinal fluid (CSF) leak; the presence of a CSF leak after cochlear implantation; a history of ventriculoperitoneal-shunt placement; and a history of otitis media3,4. Many cochlear implant recipients also had previous episodes of meningitis prior to their implantation. In order to determine if a cochlear implant independently increases the risk of meningitis following a subsequent pneumococcal infection, it is important to eliminate these other confounding factors when conducting an experimental study. This is best achieved in a controlled laboratory environment where the confounding factors can be easily eliminated.
A pneumococcal meningitis model in healthy implanted rats was established previously5. The bacteria can reach the meninges from the upper respiratory mucosa in implanted rats, either via hematogenous routes or directly via the middle and inner ear. It has been shown that in healthy non-implanted rats, a threshold of bacteria is required to induce pneumococcal meningitis and this threshold varies depending upon the route of infection6. The present study examines if the presence of a cochlear implant alters the threshold of bacteria required for meningitis.
Material and Method
Source of the animals
All the experimental animals were bred and housed in the animal house within the Department of Otolaryngology, University of Melbourne. All procedures and animal handling were conducted in accordance with guidelines set by the Animal Research & Ethics Committee of the Royal Victorian Eye and Ear Hospital and “The Australian code of practice for the care and use of animals for scientific purposes” from the National Health and Medical Research Council 2004.
In total, 54 otologically normal adult Hooded Wistar rats (10 to 16 weeks old) weighing between 100 and 450 g, were used in the study. One cohort of 18 rats received a cochlear implantation to the left ear 4 weeks prior to inoculation with bacteria. The second control cohort of 18 animals had a cochleostomy 4 weeks prior to inoculation. The third cohort of 18 (surgical insertion of implant only) had a cochleostomy, followed by immediate insertion and removal of the cochlear implant 4 weeks prior to inoculation. The third cohort was designed to investigate if standard electrode array insertion technique, which might induce a certain degree of inner cochlear trauma, altered the risk of CNS infection.
Eighteen rats were allocated to each of the three different routes of bacterial inoculation (middle ear, inner ear and intra-peritoneal (IP); Table 1). The S. pneumoniae count for each route of inoculation was chosen based on a previously established threshold model to ensure a subthreshold level of infection in healthy rats with a cochleostomy6.
Table 1.
Experimental Design (summarizing the number of rats used in each cohort)
| Routes of inoculation and the size of the S. pneumoniae inoculum in colony- forming units (CFU) | Cochleostomy only (control) | Cochleostomy and cochlear implantation | Cochleostomy and acute cochlear implantation |
|---|---|---|---|
| Intraperitoneal: 4 x 106 CFU | 6 | 6 | 6 |
| Middle Ear: 3 x 104 CFU | 6 | 6 | 6 |
| Inner Ear: 1 x 103 CFU | 6 | 6 | 6 |
The number of animals used and the size of the inoculum for each route were based on previous work.
The bacterial counts for each inoculation route has shown not to induce meningitis in 3 groups of 6 healthy rats with cochleotomy over 5 days of monitored infection period.6
Surgical anesthesia
Detail anesthesia and post operative care have been described in detail previously5,6. In brief, rats were anesthetized with an IP injection of a mixture of 8 mg/kg xylazine (Ilium Xylazil-20®, Troy Laboratories Pty. Ltd. NSW, Australia) and 75 mg/kg ketamine hydrochloride (Ketamine®, Parnell Laboratories, NSW, Australia). A local anesthetic agent (0.1 ml of lignocaine hydrochloride with 0.0182 mg/ml of adrenalin tartrate, Troy Laboratories, NSW, Australia) was injected subcutaneously (SC) around the surgical incision. The animals were given 0.03–0.05 mg/kg SC buprenorphine (Temgesic®, Reckitt Benckiser, NSW, Australia) for analgesia immediately after surgery.
Animal surgery - Scala tympani electrode array design and cochlear implant surgery
The dummy scala tympani electrode consisted of a 5 mm of polyimide tubing (Cole-Parmer®, Illinois, USA) with an outer diameter of 0.10 mm, coated with a layer of silicone (Medical Grade Elastomer MDX4-4210, Factor II, AZ, USA) to a diameter of 0.15 mm5. The dummy electrodes were cleaned with absolute alcohol in an ultrasonic cleaner then rinsed with MilliQ water 3 times and bathed in MilliQ water for 10 minutes before drying, packaging and sterilizing using H2O2 sterilization (STERRAD® 100S).
Eighteen adult Wistar rats were implanted in the left cochlea with a dummy electrode. Using sterile techniques, a post auricular skin incision was made to expose the bulla which was open up to the round window membrane (RWM). The bulla cavity was inspected for any abnormality of the middle ear mucosa. The stapedial artery, which is located just below the round window niche, was cauterized. A cochleostomy (approximately 0.2 mm in diameter) was made just below the round window niche (RWN) and at the location of previously cauterized stapedial artery5,7. The dummy electrode was inserted 2–3mm into the scala tympani via the cochleostomy which was sealed with temporalis fascia. The extra cochlear portion of the implant remained within the bulla cavity after the insertion.
Surgical procedures to control rats
Eighteen adult rats underwent a cochleostomy to the left ear as described above. A dummy electrode was not inserted into the inner ear. The cochleostomy site was covered with temporalis fascia.
Surgical procedures to rats with acute scala tympani electrode insertion
Eighteen adult rats underwent a cochleostomy to the left ear. A dummy scala tympani electrode was inserted into the inner ear via the cochleostomy and then removed. The cochleostomy site was covered with temporalis fascia.
All animals were given two doses of prophylactic antibiotics, enrofloxacin (Baytril 50®, Bayer Australia Lt.d., NSW, Australia) 10mg/kg SC diluted 1:1 with saline. One dose was given immediately after surgery and the second dose 12 hours later.
Inoculation of S. pneumoniae
Four weeks after surgery, all animals were inoculated with Streptococcus pneumoniae 447A which carries type 2 capsular antigen and was selected for the study based on our previous experience with this specific serotype in a feline otitis media model8,9 and a rat meningitis model5,6. This organism was a clinical isolate from the CSF of a child who had been diagnosed with meningitis. The detailed preparation of the bacterial inocula has been described in our previous studies5,6. The number of bacteria for each route of inoculation is given in Table 1 and was derived from the previously established threshold model6. Retrospective viable counts of the inoculum confirmed that each rat received the desired amount of bacteria.
Three methods of bacterial inoculation (intraperitoneal (IP), middle ear, inner ear) were carried out to study the effect of different routes of infection on the risk of meningitis in rats implanted with a dummy electrode and in rats with acute electrode insertion alone. Bacteremia, as a result of IP inoculation, was introduced to study hematogenous spread of infection without the possible confounding effect of direct invasion of the meninges from the middle ear infection. The direct inoculation of the bacteria into the inner ear was introduced to study direct route of infection from the middle ear to the meninges via inner ear without the bacteremia that may accompany middle ear infection. Note that no antibiotics were given after S. pneumoniae infection.
Intraperitoneal inoculation
Eighteen rats (6 from each cohort) were anesthetized using a mixture of isoflurane and oxygen and 4 x 106 colony-forming units (CFU) of the bacteria in 1 ml of PBS was directly injected into the peritoneal cavity using a sterile 20 Gauge needle and 1ml syringe.
Middle ear inoculation
Under general anesthesia, the left bulla of 18 rats (6 from each cohort) was surgically exposed and inoculated with 3x104 CFU in 10 μl of PBS. To retain the micro-organisms in the bulla, the cavity was first filled with Gelfoam®(Pharmacia & Upjohn, Michigan, USA). After the inoculation of the bacteria, the opening of the bulla was covered with temporalis fascia and the wound was sutured in 2 layers.
Inner ear inoculation
Under general anesthesia, the left bulla of 18 rats (6 from each cohort) was surgically exposed and a cochleostomy next to the previous surgical site to access the scala tympani was performed with a straight Kirschner wire. Two microliters of perilymph were removed and 1 μl of bacterial inoculum containing 1 x 103 CFU was inoculated into the scala tympani over 1 minute using an infusion catheter, 5 μl micro-syringe (ILS, Stützerbach, Germany), and a micro-syringe pump controller (World Precision Instruments Inc, Sarasota, FL, USA; Table 1). After inoculation, the cochleostomy was covered with temporalis fascia. The opening of the bulla was covered with temporalis fascia and the wound was sutured in 2 layers.
Post infection monitoring
Following the inoculation, each animal was examined, at minimum, twice daily for clinical signs of meningitis over 5 days. A clinical assessment was recorded in a 12 point scored monitoring sheet5. Animals were euthanized if one of the following conditions was met: a score of 10 or above; a weight loss of greater than 25%; or a score of 5–10 with rectal temperature of greater than 41ºC. Animals without clinical evidence of meningitis were euthanized at the end of the fifth day.
Microbiological specimen collection and tissue preparation
The outcome of the study was to detect the presence of meningitis. The meninges and brains were harvested for histological analysis and were used to confirm the diagnosis of meningitis. CSF, blood culture and middle ear cultures were collected as adjunct to the brain histology for the detection of bacteria to ensure that the strain causing the disease was the same as that used for the inoculum.
Once the rats developed early signs of meningitis (see below), isoflurane and oxygen were used to deeply anesthetize them, to allow collection of CSF, middle ear fluid and blood for microscopy and culture5,6. The method of specimen collection has been described previously10–14.
After specimen collection, the animals were then given a lethal dose of pentobarbitone sodium (120 mg/kg intramuscularly, Lethabarb®, Virbac Pty. Ltd. NSW, Australia) and were transcardially perfused with 0.9% saline, and then 10% neutral buffered formalin (NBF) pH 7.4 at 4°C. The brain, meninges and the cochleae were harvested and placed in 10% NBF for further processing.
All fifty-four brains with meninges were harvested and stored in 10% NBF for 48 hours then embedded in paraffin. The specimens were sectioned 10 μm thick, stained with both Haematoxylin and Eosin (H &E) and Gram stain and examined under light microscopy for presence of inflammation and Gram-positive cocci.
Nine pairs of randomly selected cochleae were harvested from the temporal bones and fixed in 10% NBF. They were decalcified in a solution of 10% ethylene diamine tetra-acetic acid in 0.1M phosphate buffer (pH 7.4) and orientated in the mid-modiolar position then embedded in Spurr’s resin. Two sets of 21 2-μm sections were collected at 126-μm intervals throughout the cochlea. One set of 21 sections was stained with H &E and the other set was stained with Gram stain.
Histological analysis
The sections of histological specimens were examined under a light microscope. The brain and meninges were examined for the presence of inflammatory cells within the subarachnoid space and brain tissue, thickening and hyperplasia of the meningeal cells, Gram-positive cocci within the subarachnoid space and brain tissue. The cochleae were examined for the presence of bacteria and inflammatory cells.
Statistical analysis
The effects of cochlear implantation and standard surgical insertion technique on the threshold of infection for the three different routes of inoculation was evaluated statistically using Fisher’s exact test (one- tailed)6.
Results
When rats developed signs of meningitis, they appeared to lethargic, were unresponsive to stimulation by sound or light, had a hunched posture, exhibited poor grooming, weight loss and a rectal temperature above 38°C. When these signs developed, CNS histology consistently showed evidence of meningitis with inflammatory cells and Gram-positive diplococci within the subarachnoid space. The correlation between the clinical signs of meningitis and the histopathological evidence of meningitis was established in our previous work5,6.
Eighteen rats with the cochlear implant developed both clinical and histological evidence of meningitis regardless of the route of inoculation. There was some variation in the time required for the implanted rats to develop meningitis. Three of 6 IP inoculated rats developed meningitis within 32 hours whereas the other three in this group showed signs of meningitis after approximately 102–120 hours. Three of the implanted rats with middle ear inoculation developed meningitis within 48 to 52 hours and the other three implanted rats inoculated by this route developed meningitis around 96–120 hours after inoculation. Likewise, three of the implanted rats with inner ear inoculation developed meningitis within 50 hours whereas the other three rats developed meningitis from 96 to 102 hours. In contrast, none of the eighteen rats with cochleostomy-only (control cohort) developed meningitis when inoculated with the same number of bacteria. The effect of cochlear implantation on the attack rate of meningitis was statistically highly significant (p=0.001 Fisher exact test, one-tailed; Table 2).
Table 2.
Attack rate of meningitis based on clinical and histological evidence of meningitis
| Routes of inoculation and the size of the S. pneumoniae in colony-forming units (CFU) | Cochleostomy only (per 6 rats) (Control) | Cochleostomy and cochlear implantation (per 6 rats) | Cochleostomy and acute cochlear implantation (per 6 rats) |
|---|---|---|---|
| Intra-peritoneal: 4 x 106 CFU | 0 | 6 * | 0 N.S. |
| Middle ear : 3 x 104 CFU | 0 | 6 * | 2 N.S. |
| Inner ear : 1 x 103 CFU | 0 | 6 * | 0 N.S. |
The effect of a cochlear implant on the risk of meningitis is statistically significant (p=0.001, one-tailed Fisher’s exact test)
N.S. The effect of acute electrode insertion only is not statistically significant p> 0.2, one-tailed Fisher’s exact test)
Two of the 18 rats that had undergone acute electrode insertion only developed meningitis when the bacteria were inoculated into the middle ear cavity. Both rats developed meningitis 110–112 hours post inoculation. The effect of acute insertion of the implant on the attack rate of meningitis was not statistically significant. (p>0.2 for middle ear, inner ear and IP inoculation, Table 2).
Microbiology
The results of bacterial cultures of CSF, blood and middle ear swab are summarised in Table 3. Typing of bacteria isolated from the rats showed them to be the same serotype as the inoculum.
Table 3.
Microbiology results
| Cohorts of rats: Three groups of 6 rats were used for each route of inoculation | Positive blood culture (number of rats)* | Positive CSF culture (number of rats)* | Positive Middle ear swab (number of rats)* | Meningitis: confirmed with histology (number of rats)* |
|---|---|---|---|---|
| Intraperitoneal : | ||||
| Cochleostomy only | 2 | 0 | 0 | 0 |
| Cochlear implantation | 5 | 4 | 0 | 6 |
| Acute implantation | 2 | 0 | 0 | 0 |
| Middle ear : | ||||
| Cochleostomy only | 2 | 0 | 6 | 0 |
| Cochlear implantation | 6 | 4 | 6 | 6 |
| Acute implantation | 5 | 3 | 5 | 2 |
| Inner ear : | ||||
| Cochleostomy only | 1 | 0 | 3 | 0 |
| Cochlear implantation | 6 | 6 | 5 | 6 |
| Acute implantation | 0 | 0 | 0 | 0 |
Positive culture indicates that S. pneumoniae type 2 was isolated from the specimens when rats were euthanized.
Cochlear histology
The pattern and the distribution of bacteria and inflammatory cells within the cochleae of rats with clinical and histological evidence of meningitis were consistent with our previous studies5,6. In brief, the amount and distribution of inflammatory cells were symmetrical in both cochleae of rats with an IP inoculation (Figure 1). However, the inflammatory changes within the cochleae were asymmetrical in meningitic animals following middle and inner ear inoculations (Figure 2 and 3). In these cases a more severe labyrinthitis was observed in the cochlea ipsilateral to the inoculation. Inflammatory cells and bacteria were also found to infiltrate the fibrous connective tissue seal surrounding the intra-cochlear portion of the dummy electrode array in rats receiving middle and inner ear inoculations (Figure 4). In rats without meningitis, the histological appearance of the cochleae was normal for IP and middle ear inoculations; rats receiving inner ear inoculation typically exhibited small numbers of inflammatory cells and serofibrinous exudate in the basal turn of the cochleae (Figure 5).
Figure 1.
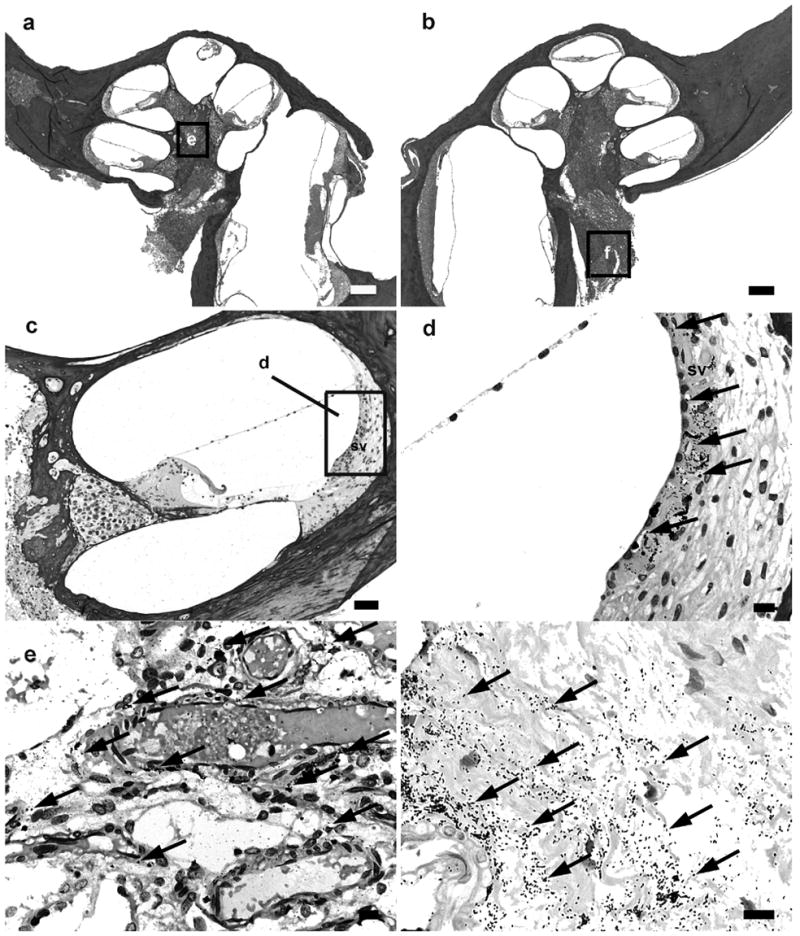
Lower power H & E photomicrographs illustrating the implanted (a) and contralateral control (b) cochleae of a rat 32 hours following IP inoculation of 4 x 106 CFU S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. However the scalae of both cochleae were devoid of gross infection. Higher power photomicrograph of Gram stain from basal turn of the contralateral cochlea (c), the lateral wall of the scala media of the contralateral cochlea (d), the modiolus of the ipsilateral cochlea (e) and the internal acoustic meatus of the contralateral cochlea (f), illustrates the presence of bacteria (arrows). The approximate location of the higher power micrographs (e,f) are illustrated in (a) and (b). sv: stria vascularis. Scale bar: (a) & (b) 200 μm; (c) 100 μm; (d-f) 10 μm.
Figure 2.
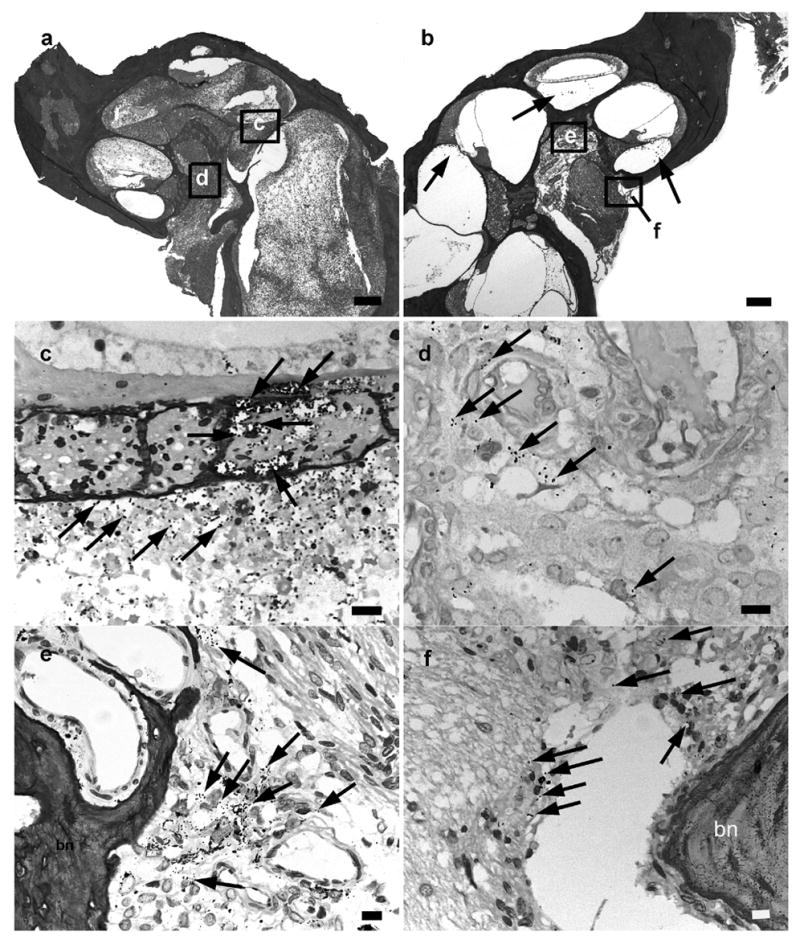
Lower power H&E photographs illustrating the implanted (a) and contralateral control (b) cochleae of a rat 52 hours following middle ear inoculation of 3 x 104 CFU S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. In this example there is severe labyrinthitis throughout the implanted ear (a) while the contralateral cochlea exhibits evidence of infection mainly localized to the scala tympani of the basal turn (b). Higher power micrograph of Gram stain of the OSL (c) and modiolus of the implanted cochlea (d), the modiolous (e) and the internal acoustic meatus (f) of the contralateral cochlea, illustrates the presence of bacteria (arrows). The approximate location of the higher power micrographs (c-f) are illustrated in (a) and (b). bn: bone. Scale bar: (a) & (b) 200 μm; (c-f) 10 μm.
Figure 3.
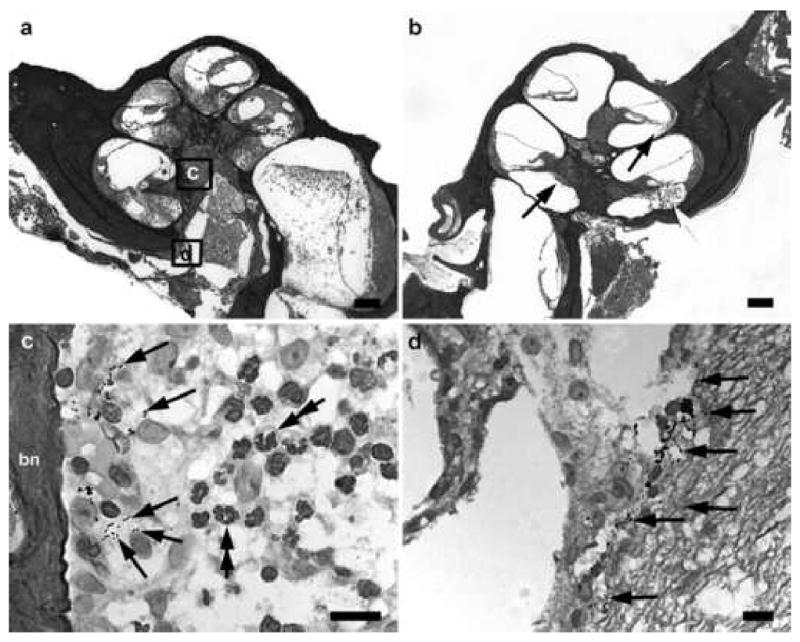
Lower power H & E photomicrographs illustrating the implanted (a) and contralateral control (b) cochleae of a rat 48 hours following inner ear inoculation of 1 x 103 CFU S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. Extensive labyrinthitis of the inoculated left ear involved all three scalae. In contrast, the contralateral cochlea exhibited a less severe labyrinthitis with infection predominantly localized to the scala tympani. Higher power photomicrograph of Gram stain from the modiolus (c) and the internal acoustic meatus (d) implanted left cochlea illustrates the presence of bacteria (arrows). The approximate location of the higher power micrographs (c,d) are illustrated in (a) and (b). bn: bone. Scale bar: (a) & (b) 200 μm; (c) & (d) 10 μm.
Figure 4.
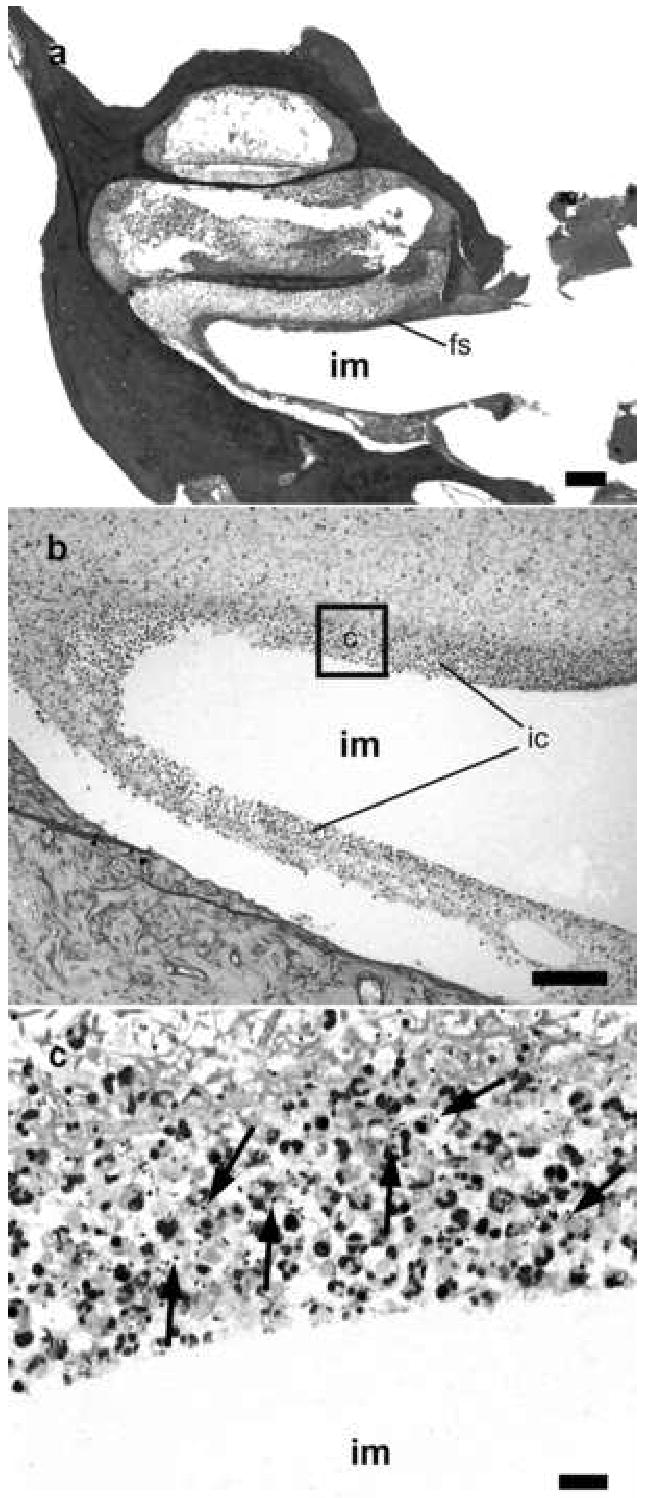
Lower power H & E photomicrograph taken at the level of the round window niche of the implanted left (a) cochlea of a rat 96 hours following middle ear inoculation of 3 x 104 CFU S. pneumoniae. This animal exhibited clinical and histological (CNS) evidence of meningitis. Higher power photomicrograph of Gram stain of the scala tympani at the location of the scala tympani electrode array (b & c) illustrates the presence of bacteria (arrows) and inflammatory cells within and surround the peri-implant fibrous seal. The approximate location of the higher power micrograph (c) is illustrated in (b). fs: peri-implant fibrous seal; im: scala tympani electrode array; ic inflammatory cells. Scale bar: (a) 200 μm; (b) 100μm; (c) 10μm.
Figure 5.
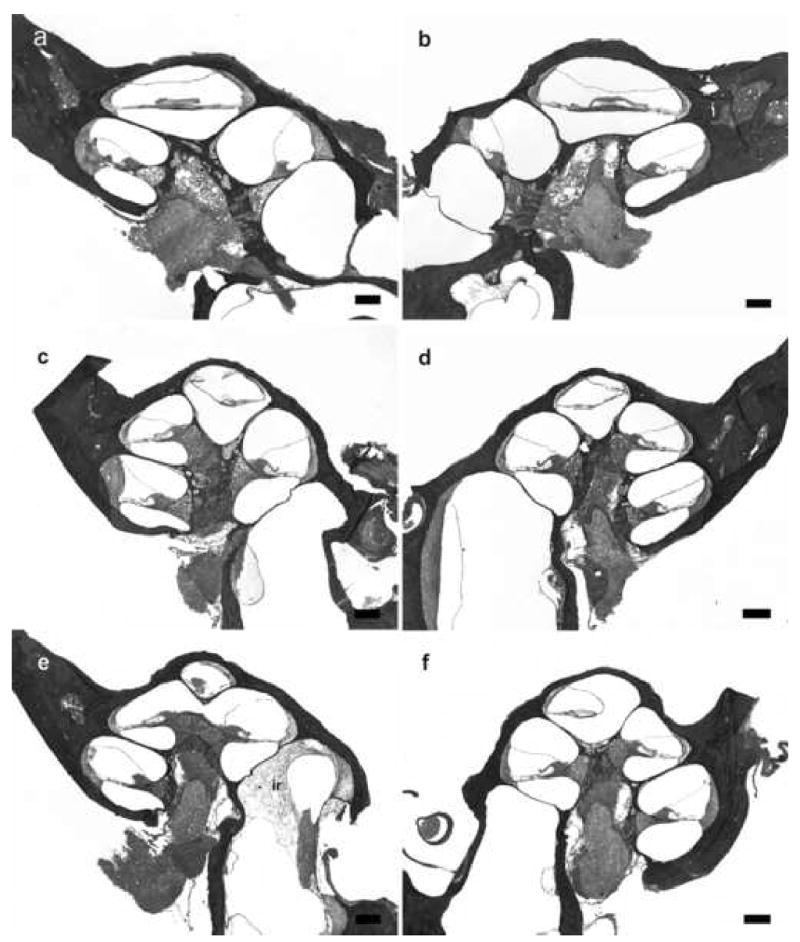
Lower power H & E photomicrographs illustrating representative cochlear histology in acutely implanted non-meningitic animals. Left (a) and right (b) cochleae 120 hours after following IP inoculation of 4 x 106 CFU S. pneumoniae. No inflammatory cells and bacteria were evident within both cochleae. Left (c) and right (d) cochleae 120 hours after middle ear inoculation of 3 x 104 CFU S. pneumoniae. Similarly, no inflammatory cells and bacteria were evident within the cochleae. Left (e) and right (f) cochleae 120 hours following inner ear inoculation of 1 x 103 CFU S. pneumoniae. Inflammatory cells response (ir) was evident in the basal turn of the ipsilateral cochlea. There were no inflammatory cells and bacteria in the other parts of cochlea or in the contralateral cochlea. There were no evidence of trauma to the OSL and the modiolus from acute insertion of the scala tympani electrode array. Reissiner’s membrane defects in some cochlear turns as seen in micrograph (e), were most likely to be artifacts from the histology preparation. An absence of organ of Corti was observed in the basal turn in the ipsilateral acutely implanted cochleae (a) and (e). The loss of organ of Corti did not alter the outcome of infection in these two animals. Scale bar: (a-f) 200 μm.
Following acute electrode insertion only, there was no trauma to the OSL or the modiolus. The normal architecture of the organ of Corti was present in the basal turn of all but 2 animals. In these 2 animals, there was no recognizable organ of Corti in the basal turn. The basilar membrane in this region was completely flat (Figure 5). Neither of these 2 animals developed meningitis.
Microscopic examination of the ipsilateral middle ear mucosa of the round window niche revealed evidence of middle ear inflammation in rats with direct middle ear inoculation. The contralateral control bullae showed no evidence of middle ear inflammation. There was no microscopic evidence of inflammatory changes within the middle ear mucosa of rats with IP or direct inner ear inoculation.
Discussion
This study demonstrates, for the first time, that the presence of a cochlear implant in healthy rats reduces the threshold of bacteria required to induce pneumococcal meningitis for all three routes of inoculation; (i) general hematogenous route via IP inoculation; (ii) inoculation of the middle ear; (iii) direct inoculation of the cochlea. Our previous work has illustrated that a minimal threshold of bacteria is required to induce pneumococcal meningitis in healthy rats and the threshold is different for three different routes of infection6. Moreover a cochleostomy without cochlear implantation, did not alter the threshold level of infection6, and this result was reconfirmed in this present study. There was no evidence of meningitis when the quantities of bacteria below the threshold level (subthreshold) were inoculated into rats that had a cochleostomy. However, with the same bacterial counts, all 18 implanted rats acquired meningitis via the three different routes of inoculation. The significance of this finding is that the presence of a foreign body, such as the cochlear implant, can be considered as an independent risk factor for pneumococcal meningitis. The exact mechanism(s) of how cochlear implant contributes to the risk of acquiring meningitis is unclear based on previous clinical data2–4. However, this study illustrates that one of the possible mechanisms is by reducing the threshold of bacteria required to induce meningitis. The presence of a foreign body such as a cochlea implant may reduce the ability of the rats' immune system to fight pneumococcal infection. Therefore, fewer bacteria are required to overwhelm the immune system compared to non-implanted rats. Previous studies have illustrated that the presence of a rigid, perforated, polytetrafluoroethylene tube in the subcutaneous tissue of animals increased the apoptotic activity of polymorphonuclear leukocytes and impaired their ability to phagocytose bacteria15–17. It is likely that a foreign body in the inner ear can also reduce the ability of the immune cells to eliminate S. pneumoniae, although the detailed molecular mechanism(s) of how the foreign body impairs the function of immune cells is still unknown. However, with an impaired local immunity around the implant, a lesser quantity of bacteria was required to induce meningitis in the implanted rats. Therefore the threshold of pneumococcal infection has been lowered in the presence of a foreign body, probably as a result of an enhanced capacity of the bacteria to proliferate around the implant. Similarly, in a study that involved the injection of Staphylococcus aureus subcutaneously into sites with and without implanted foreign bodies, significantly smaller numbers of bacteria were required to induce infection in the presence of tissue cages16. In that particular study, an infection rate of 100% was observed in the implant site following the inoculation of 1 x 103 CFU of S. aureus. However, in the absence of a subcutaneous foreign body, an inoculation of 1 x 108 CFU of S. aureus did not induce skin infection16. Even though this study used a different bacterium, a different type of foreign body and a different implant location, the findings are consistent with our work.
The possible mechanism(s) of pneumococcal meningitis in implanted patients can be further explored by examining the effects of cochlear implantation on the routes by which S. pneumoniae reach the meninges. In the presence of the cochlear implant, the otogenic route of spread of infection (infection spread from middle ear to the inner ear then to the meninges) has been considered to be the main pathway for post implant meningitis18,19. However, our work has demonstrated that both hematogenous and direct otogenic routes of infection can induce meningitis in implanted rats5. Furthermore, only 40% of subjects with post implant meningitis were found to have concurrent acute otitis media3. Therefore, there may be differences in the pathogenesis of pneumococcal meningitis among subjects with a cochlear implant depending upon the route of bacterial infection. The presence of the implant may reduce the local inner ear immunity and allow a direct invasion of CNS when bacteria are inoculated into the middle or the inner ear. On the other hand, the implant can also reduce the global CNS immunity to allow bacterial invasion of the blood-brain barrier from the systemic circulation.
The significance of hematogenous spread of infection, from the upper respiratory tract mucosa to the meninges, in precipitating meningitis in implanted human is unclear. However, the present study demonstrates that implanted rats were at risk of pneumococcal meningitis when the bacteria were introduced IP. The exact mechanism of how the presence of an implant can alter the threshold of bacteria via a hematogenous route remains unclear. It is possible that the bacteria seeded around the implant from the systemic circulation and then move to the central nervous system from the inner ear. However, the histology of cochleae of implanted rats with meningitis did not show any evidence of seeding of the bacteria within the scala tympani around the implant site. This might be due to the removal of bacteria from cochlea when the implant was removed during the histology processing. If this is true, one would still expect to see a more severe degree of labyrinthitis in the implanted cochlea compared to the contralateral site. Interestingly, the severity of labyrinthtis was similar when comparing implanted to non-implanted cochleae following IP inoculation; the bacteria were predominately located in the internal acoustic meatus and modiolus in both cochleae. This observation is consistent with our previous work5 and implies that the presence of a foreign body in the inner ear reduces the global immune surveillance of CNS to allow bacteria in the circulation to invade the blood-brain barrier. A previous study in an animal model showed that any breach of the dura reduces the threshold of bacteremia required to produce meningitis20. This suggests that any direct or indirect injury to the dura may reduce CNS immunity and result in a reduction in the number of bacteria required to induce meningitis. Due to the close anatomical relationship of inner ear to the CNS, the presence of an implant in the inner ear may also reduce the overall immune defense of the CNS in a way that is not clear. Interestingly, As demonstrated in our previous work6, the presence of a cochleostomy in the inner ear did not alter the threshold for meningitis via the hematogenous route. The local breach of the bony capsule of the basal turn (cochleostomy) did not appear to affect the integrity of the global immune surveillance of the CNS. This further strengthens the notion that it was the presence of the foreign body in the inner ear and not the cochleostomy surgery per se that reduced the threshold for meningitis via the hematogenous route.
Inner ear trauma as a result of cochlear implantation has been considered to be a possible risk factor for post implant meningitis18. Our earlier study suggested that trauma to the osseous spiral lamina of the basal turn may provide easier access for the bacteria to access peri-neural and peri-vascular spaces within the modiolus and the internal acoustic meatus5. However, in the present study, the standard surgical insertion technique for cochlear implantation in healthy rats did not shift the threshold required for infection. Moreover, minimal or no trauma to the bony and membranous labyrinth was observed in the cochleae of rats that received acute insertion of electrode array further strengthening the notion that a cochlear implant is an independent risk factor for post-implant pneumococcal meningitis.
The histological appearance of the cochleae in implanted rats with meningitis was consistent with our previous study, and dependent upon the route of infection5. A symmetrical distribution of Gram-positive bacteria and inflammatory cells within the internal acoustic meatus and modiolus was found in both cochleae of rats with IP inoculation. Although few isolated bacteria were seen within the stria vascularis, no bacteria or inflammatory cells were seen within the scala tympani and vestibuli and there was no evidence to suggest that bacteria traversed blood vessels to enter the scala media. An asymmetrical distribution of the bacteria and inflammatory cells was seen in meningitic rats with both middle and inner ear inoculations: the labyrinthitis of the ipsilateral inoculated ear, involving all three scalae, was more extensive than that observed in the contralateral cochlea. The difference in the severity of labyrinthitis between the ipsilateral and the contralateral cochlea, viewed in the context of a reduced threshold for CNS infection, suggested a reduction of the inner ear immunity at the local level due to the presence of the dummy electrode array. In rats with middle ear infection and meningitis, bacteria infiltrated the fibrous tissue seal around the implant to reach the scala tympani. This also provides support for the notion that meningitis might have been caused by direct spread of infection from the middle ear into scala tympani and then to the CNS. As bacteremia was also identified in these rats, however, it is difficult to completely exclude the possibility of hematogenous spread to the meninges following middle ear infection. However, one would have expected a more symmetrical distribution of the bacteria and inflammatory cells within their cochleae if this was the dominant route.
Although the benefits of cochlear implants in human subjects far outweigh the small risk of meningitis post cochlear implantation, every effort should be made to ensure long-term patient safety. Acute otitis media has been diagnosed concurrently with meningitis in some implanted patients. If there is a reduction in the threshold of bacteria required to induce meningitis in implanted recipients, as suggested by this animal study, then aggressive antibiotic treatment of patients with acute otitis media is necessary to prevent subsequent meningitis. Furthermore, the presence of a cochlear implant can reduce the threshold of bacteremia required for subsequent pneumococcal meningitis. Therefore, in implanted subjects with febrile illness of unknown focus and possible pneumococcal bacteremia, full microbiological investigation and early treatment with broad spectrum antibiotics would be advisable. Pneumococcal vaccination is also highly recommended for all future and current cochlear implant recipients to reduce the risk of meningitis following pneumococcal infection2. The animal model established in this study can also be used to examine the effectiveness of pneumococcal vaccination, to improve the prosthesis design and/or modify surgical procedure in order to raise the threshold of bacteria required to cause meningitis.
The findings from the present study also have a wider implication than cochlear implant surgery and pneumococcal infection. The presence of foreign body in the CNS, such as a CSF shunt, has shown to increase the risk of meningitis2 and this is likely to be related to the reduction of the threshold of bacteria required to cause meningitis. It is also possible that any surgical intervention associated with a breach of the dura can also reduce the threshold of bacteria required to produce meningitis. The model described in this study can be further developed or modified to examine the effects of other neurosurgical prostheses or neurosurgical interventions on the subsequent risk of bacterial meningitis.
Conclusion
The cochlear implant increases the risk of pneumococcal meningitis in healthy rats by reducing the threshold of bacteria required to cause the disease. The threshold of infection is reduced for all potential routes of spread of infection, including the bloodstream, the upper respiratory tract, and the inner ear to the meninges. Early diagnosis and treatment of otitis media and systemic febrile illness may reduce the risk of pneumococcal meningitis in cochlear implanted recipients by preventing bacterial counts from reaching the threshold required for infection. Pneumococcal vaccination is also highly recommended for all future and current cochlear implant recipients to reduce the risk of meningitis. There is no evidence to suggest that the standard surgical insertion technique of cochlear implantation increases the risk of pneumococcal meningitis.
Acknowledgments
We would like to thank staff from the Departments of Otolaryngology, and Microbiology and Immunology, University of Melbourne and The Bionic Ear Institute for their support and help in the research project. We are grateful to Dimitra Stathopoulos for editorial comments; Prue Nielsen and Maria Clarke for histology; Dr. Sue Pierce for veterinary support and Elisa Borg for animal maintenance (Department of Otolaryngology); and Kristy Azzopardi for preparation of the bacteria (Department of Microbiology and Immunology).
Footnotes
Awards and Presentation: This manuscript received the 2006 AAO-HNSF/ARO Resident Research Award in the Basic Science Category. This work is to be presented by the primary author at the 2006 AAO-HNSF Annual Meeting in Toronto, Ontario, Canada.
Source of financial support: The Garnett Passe and Rodney Williams Memorial Foundation Scholarship in Otolaryngology Head and Neck Surgery; The Wagstaff Fellowship, Royal Victorian Eye & Ear Hospital; NIH-NIDCD-N01-DC-3-1005; the Bionic Ear Institute and the Department of Otolaryngology, University of Melbourne.
References
- 1.Roland JT., Jr . Complications of cochlear implant surgery. New York: Thieme; 2000. pp. 171–175. [Google Scholar]
- 2.FDA. [Accessed July 09, 2006.];Public health web notification: risk of bacterial meningitis in children with cochlear implants. 2006 Available at www.fda.gov/cdrh/safety/cochlear.html.
- 3.Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:435–445. doi: 10.1056/NEJMoa031101. [DOI] [PubMed] [Google Scholar]
- 4.Cohen NL, Roland JT, Jr, Marrinan M. Meningitis in cochlear implant recipients : the north american experience. Otol Neurotol. 2004;25:275–281. doi: 10.1097/00129492-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Wei BPC, Shepherd RK, Robins-Browne R, Clark G, O'Leary SJ. Pneumococcal meningitis: development of a new animal model. Otol Neurotol. 2006 doi: 10.1097/01.mao.0000231603.25961.f1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei BPC, Shepherd RK, Robins-Browne R, Clark G, O'Leary SJ. Pneumococcal meningitis threshold model: a potential tool to assess infectious risk of new or existing inner ear surgical interventions. Otol Neurotol. 2006 doi: 10.1097/01.mao.0000227898.80656.54. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu W, Xu J, Shepherd RK. Cochlear implantation in rats: a new surgical approach. Hear Res. 2005;205:115–122. doi: 10.1016/j.heares.2005.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahm MC, Clark GM, Franz BK, Shepherd RK, Burton MJ, Robins-Browne R. Cochlear implantation in children: labyrinthitis following pneumococcal otitis media in unimplanted and implanted cat cochleas. Acta Otolaryngol. 1994;114:620–625. doi: 10.3109/00016489409126115. [DOI] [PubMed] [Google Scholar]
- 9.Berkowitz RG, Franz B, Shepherd RK, Clark GM, Bloom D. Cochlear implant and otitis media: a pilot study to assess the feasibility of Pseudomonas aeruginosa and Streptococcus pneumoniae infection in the cat. J Otolaryngol Soc Aust. 1985;5:297–299. [Google Scholar]
- 10.Tunkel AR, Wispelwey B, Quagliarello VJ, et al. Pathophysiology of blood-brain barrier alterations during experimental Haemophilus influenzae meningitis. J Infect Dis. 1992;165 (Suppl 1):S119–120. doi: 10.1093/infdis/165-supplement_1-s119. [DOI] [PubMed] [Google Scholar]
- 11.Townsend GC, Scheld WM. Adult rat model of meningitis. In: Zak O, Sande MA, editors. Handbook of animal models of infection : experimental models in antimicrobial chemotherapy. London: Academic Press; 1999. pp. 627–629. [Google Scholar]
- 12.Vogel U, Frosch M. Infant rat model of acute meningitis. In: Zak O, Sande MA, editors. Handbook of animal models of infection : experimental models in antimicrobial chemotherapy. London: Academic Press; 1999. pp. 619–626. [Google Scholar]
- 13.Quagliarello VJ, Long WJ, Scheld WM. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat. Temporal sequence and role of encapsulation. J Clin Invest. 1986;77:1084–1095. doi: 10.1172/JCI112407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988;82:1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984;73:1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 17.Fabre T, Belloc F, Dupuy B, et al. Polymorphonuclear cell apoptosis in exudates generated by polymers. J Biomed Mater Res. 1999;44:429–435. doi: 10.1002/(sici)1097-4636(19990315)44:4<429::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Arnold W, Bredberg G, Gstottner W, et al. Meningitis following cochlear implantation: pathomechanisms, clinical symptoms, conservative and surgical treatments. ORL J Otorhinolaryngol Relat Spec. 2002;64:382–389. doi: 10.1159/000067579. [DOI] [PubMed] [Google Scholar]
- 19.Bluestone CD. Bacterial meningitis in children with cochlear implants. N Engl J Med. 2003;349:1772–1773. [PubMed] [Google Scholar]
- 20.Weed LH, Wegeforth P, Ayer JB, Felton LD. The production of meningitis by release of celebrospinal fluid. JAMA. 1919;72:190–193. [Google Scholar]


