Abstract
Previous work has shown that latent respiratory motor pathways known as crossed phrenic pathways are inhibited via a spinal inhibitory process; however, the underlying mechanisms remain unknown. The present study investigated whether spinal GABA-A and/or glycine receptors are involved in the inhibition of the crossed phrenic pathways after a C2 spinal cord hemisection injury. Under ketamine/xylazine anesthesia, adult, female, Sprague Dawley rats were hemisected at the C2 spinal cord level. Following 1 week post injury, rats were anesthetized with urethane, vagotomized, paralyzed and ventilated. GABA-A receptor (bicuculline and Gabazine) and glycine receptor (strychnine) antagonists were applied directly to the cervical spinal cord (C3–C7) while bilateral phrenic nerve motor output was recorded. GABA-A receptor antagonists significantly increased peak phrenic amplitude bilaterally and induced crossed phrenic activity in spinal-injured rats. Muscimol, a specific GABA-A receptor agonist, blocked these effects. Glycine receptor antagonists applied directly to the spinal cord had no significant effect on phrenic motor output. These results indicate that phrenic motor neurons are inhibited via a GABA-A mediated receptor mechanism located within the spinal cord to inhibit the expression of crossed phrenic pathways.
Introduction
Hemisection of the upper cervical spinal cord interrupts descending respiratory premotor pathways. Thus, phrenic motor neurons become quiescent below the site of injury resulting in paralysis of the ipsilateral hemidiaphragm (Porter 1895). Paralysis can be partially restored if respiratory drive is increased (Fuller et al. 2003; Golder et al. 2005; Goshgarian 2003). The underlying mechanism of this restored motor function includes the activation of latent crossed phrenic motor pathways. The crossed phrenic pathway consists of premotor axons which arise bilaterally in the medulla, descend down the spinal cord in the lateral and ventral funiculi (Dobbins and Feldman 1994), and cross the midline of the spinal cord at the level of the phrenic motor nucleus to innervate contralateral phrenic motor neurons (Moreno et al. 1992).
Many studies have shown that increased respiratory drive or various pharmacological agents that result in increased ventilation can activate crossed phrenic pathways (Fuller et al. 2003; Golder et al. 2005; Ling et al. 1994; Nantwi and Goshgarian 2001). Recovered respiratory-related activity in the phrenic nerve ipsilateral to hemisection under these conditions has been referred to as, ‘crossed phrenic activity’ (Goshgarian 2003). More recently, however, activation of serotonin 1A receptors in the dorsal horn elicited crossed phrenic activity (Zimmer and Goshgarian, 2006). Since activation of serotonin 1A receptors results in cellular hyperpolarization (Barnes and Sharp 1999), it was suggested that these receptors are found on non-respiratory modulated dorsal horn sensory neurons which chronically inhibit phrenic motor neurons after spinal cord injury. Thus, it was hypothesized that serotonin 1A receptor agonists disinhibit phrenic motor neurons; however, the mechanisms underlying this disinhibition are unknown. Similarly, cervical dorsal root rhizotomy activates crossed phrenic pathways (Goshgarian 1981; Fuller et al. 2002) suggesting that primary afferent fibers in the dorsal roots excite the dorsal horn sensory neurons which, in turn, inhibit phrenic motor neurons. It is well established that after spinal cord injury, sensory reflex activity is increased dramatically and dorsal horn neurons become hyperexcitable (Hains et al. 2003). Therefore, we hypothesize that this excitability may lead to the active inhibition of phrenic motor neurons through local spinal networks.
The primary neurotransmitters underlying fast synaptic inhibition are γ–aminobutyric acid (GABA) and glycine, and both are involved in respiratory motor control (Hayashi and Lipski 1992; McCrimmon et al. 1995, 1997; Funk et al. 2000). It is well known that GABA actively inhibits phrenic motor neurons during the expiratory phase of respiration by activating GABA-A receptors (Fedorko et al., 1987; Merrill and Fedorko, 1984). GABA-A receptors are also involved in the inhibition of phrenic motor neurons during the inspiratory phase (Chitravanshi and Sapru, 1999; Funk et al., 2000: Parkis et al. 1999). Glycine also inhibits phrenic motor neurons. Stimulation of muscle afferents inhibits phrenic motor neurons by glycine, not GABA (Eldridge et al., 1981, 1987) and Renshaw inhibition utilizes a large glycinergic component (González-Forero and Alvarez, 2005; Schneider and Fyffe, 1992). Therefore, the present study was designed to determine whether GABA and/or glycine receptors are involved in the active inhibition of phrenic motor neurons that has been observed after spinal cord injury.
Methods
All procedures were approved by the Wayne State University Animal Investigation Committee prior to experimentation. The surgical procedures have been published previously in detail (Zimmer and Goshgarian, 2006). Briefly, female Sprague Dawley rats (Harlan, retired breeders, approximately 1 year of age) were anesthetized with ketamine (70 mg/kg) and xylazine (40 mg/kg) and the C2 dorsal vertebra removed to expose the spinal cord. The spinal cord was hemisected at C2, the muscles sutured (3.0 Vicryl) and the skin closed with wound clips. All rats received saline (10mls, s.c.) to prevent dehydration and buprenorphine (0.01mg/kg s.c.), an analgesic for pain management. The rats were warmed on a heating pad until they aroused from anesthesia and placed individually in cages. Rats recovered from surgery for 1 week before experimentation. Sham control rats were treated exactly as above, including cutting the dura, except that the spinal cord was not cut.
Experimental protocol
One week post surgery, hemisected (n=21) or control rats (n=20) were anesthetized with urethane (1.6 mg/kg IP) and the femoral artery and vein cannulated to measure blood pressure and administer fluids, respectively. To minimize mechanoreceptor feedback, a bilateral vagotomy in the midcervical region was performed and the rat paralyzed with pancuronium (0.5mg/kg). Rats were tracheotomized and placed on a rodent ventilator that was supplied with an oxygen/air mixture to obtain an FIO2 of approximately 50% to minimize chemoreceptor feedback. A dorsal laminectomy and duratomy was performed to expose the dorsal surface of the cervical spinal cord from C3–C7. The animal was placed in a stereotaxic frame in the prone position and the right and left phrenic nerves were isolated from a dorsal approach and placed on bipolar electrodes. Phrenic nerve recordings were filtered and amplified using Tektronix TM502 amplifiers (gain 10K, HP 3kHz, LP 1kHz). Body temperature was maintained at 37°C with a rectal thermometer and a heating pad.
End-tidal CO2 (ETCO2) measurements (Novametrix, Model 1265, Wallingford, CT) were recorded. The apneic threshold was determined by slowly increasing the ventilator rate and volume until apnea occurred, after which, the ventilator rate and volume was slowly decreased until respiration returned. The apneic threshold was considered to be 1 mmHg below the value at which respiration returned and the rat was subsequently ventilated ~3–5 mmHg above the apneic threshold. The rat was allowed to stabilize for approximately 20 minutes before measurements began. Baseline phrenic recordings were taken for 20 minutes followed by the application of drugs (see below) to the dorsal surface of the spinal cord (C3–C7). The drugs (10 μl volumes) were pipetted along the surface of the dorsal cervical spinal cord starting at C3 and ending at C7. Since the animal was in a stereotaxic frame, the head of the rat was elevated above the neck and prevented the drugs from reaching the medulla.
After the experiment, the spinal cord was removed and the C2 region of the spinal cord was fixed in 10% neutral buffered formalin. After cryoprotection (30% sucrose in 0.01M PBS), the tissue was frozen, and sections were cut on a cryostat (20μM). Sections were placed on coated slides, air-dried and stained using hemotoxylin and eosin. Slides were examined to determine the extent and completeness of the spinal cord hemisection. Animals that were either not complete or showed some sparing of phrenic nerve activity ipsilateral to the hemisection were omitted from the study (n=6)
Drugs
GABA-A antagonists, bicuculline methiodide (10 μl of 100 μM solution; Sigma, n=22) and Gabazine (SR-95531, 10 μl of 100 μM solution; Sigma, n=4), were applied to the cervical dorsal spinal cord of both sham control (n=12) and hemisected (n=14) rats. In order to verify that bicuculline was acting via the GABA-A receptor, muscimol, a specific GABA-A agonist (Sigma; n=4), was applied approximately 5 min after bicuculline to block the effect of the antagonist. In other cases (n=8), the cervical spinal cord at the C7/C8 level was transected to determine if the drug was affecting neurons within the thoracic cord which could potentially feedback to excite phrenic motor neurons.
The glycine receptor antagonist, strychnine hydrochloride (Sigma) was applied to the dorsal spinal cord of sham control (n=6) and hemisected (n=6) rats; the doses started in a range that others found acceptable to induce effects in the medulla (50 μM, Pierrefiche et al., 1998) and then approximately every 10 min, higher doses were applied to the cervical spinal cord in increasing concentrations (100 μM, 1mM, 10mM). In some cases (n=4), a high dose of strychnine was injected systemically (0.2 mls of 1–10mM solution, i.v.) to ensure that the drug was active.
Data analysis
Right and left phrenic neurograms were full wave rectified and integrated using Spike2 data analysis software (v 4.13, CED Cambridge, UK). The integrated trace was assessed for respiratory frequency, peak burst amplitude, burst duration and burst area. Peak burst amplitude and burst area were presented as a percentage of control conditions. Data was analyzed for approximately 60sec before drugs were applied to the dorsal spinal cord and then again after the drugs were applied and the peak response observed. In cases where convulsive bursting was observed, we attempted to record just prior to large bursting episodes or tried to eliminate the large bursts from our analysis. In hemisected rats, since the left nerve showed no phrenic activity before drug application, the data was expressed as a percentage of the right phrenic motor output.
Statistics
Statistics were performed using Systat software (Version 10.2, Richmond, CA). Univariate and multivariate ANOVAs were used to determine the effect of surgery and drug on phrenic motor output, and to determine if spinal cord injury had any effect on body weight, blood pressure, apneic threshold or ETCO2. All values are presented as means ± SE and p<0.05 was considered significant.
Results
Body weight was significantly reduced one week after hemisection surgery (p=0.00005), but was not altered after sham surgery (Table 1). Spinal cord injury did not result in a change in the apneic threshold (p=0.623), and therefore, no difference was observed between the ETCO2 in sham controls and spinal cord injured rats (p=0.784) (Table 1). Similarly, there were no significant differences in the resting blood pressure (p=0.078) between sham control and hemisected rats (Table 1).
Table 1.
Body weight (BW before, grams) at the time of hemisection surgery or at the time of experimentation (BW after, grams), blood pressure (BP, mmHg), apneic threshold (mmHg), and end-tidal CO2 (mmHg) in sham control and C2 hemisected rats (1 wk post injury).
| BW before | BW after | BP | Apneic threshold | ETCO2 | |
|---|---|---|---|---|---|
| Control | 312 ± 7 | 310 ± 5 | 84.6 ± 4.5 | 34 ± 2 | 38 ± 1 |
| Hemisected | 312 ± 6 | 288 ± 4* | 72.8 ± 4.6 | 33 ± 1 | 37 ± 1 |
An asterisk (*) denotes a significant change from control value.
Completeness of the spinal cord hemisection was assessed using two methods; histology to verify the extent of the lesion (Fig. 1) and assessment of phrenic neural activity. Two methods were necessary since the spinal cord was occasionally damaged during dissection due to the buildup of scar tissue between neural and muscle tissues. Phrenic neurograms on the side ipsilateral to hemisection showed no activity prior to drug application indicating a functionally complete lesion. While some studies indicate that spontaneous crossed phrenic activity can occur early after injury in male rats (Golder and Mitchell, 2005; Doperalski, et al., 2006), we have consistently observed that spontaneous crossed phrenic activity does not occur in female rats until at least 6 weeks after injury or in most cases, later (Nantwi et al., 1999; unpublished observations, M.B. Zimmer).
Figure 1.
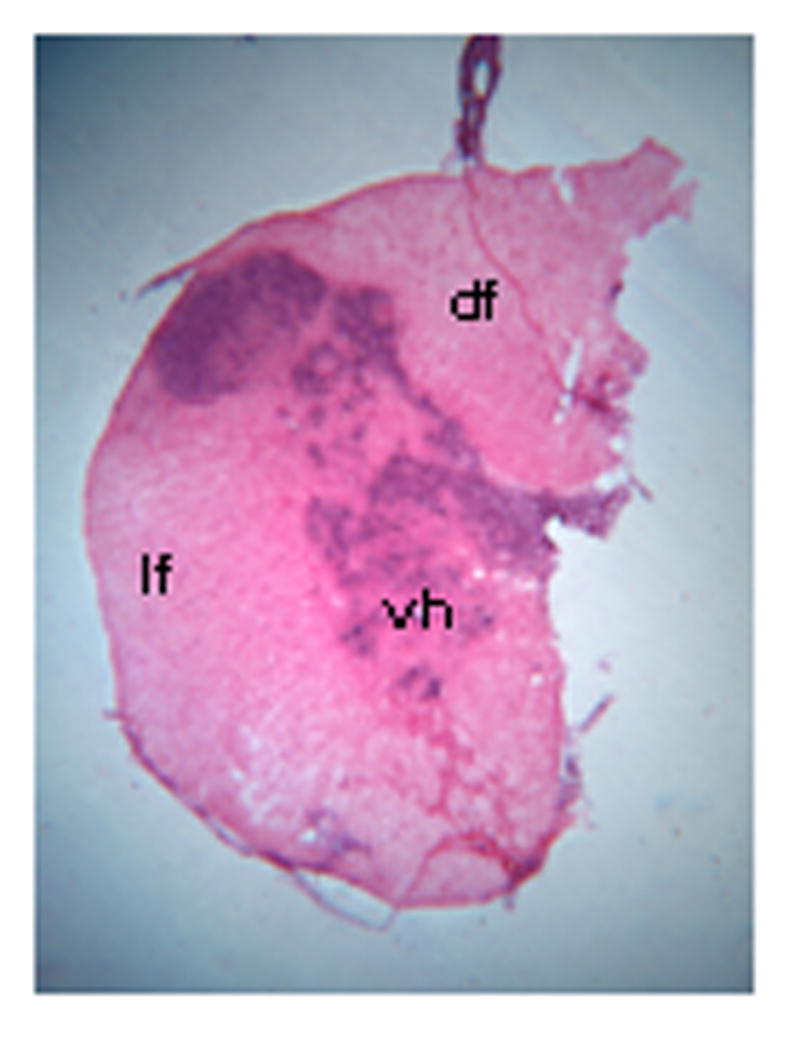
Photomicrograph showing an example of the extent of a hemisection lesion in a hemisected rat, one week post injury; note that one half of the spinal cord is missing with the exception of some slight sparing of the dorsal funiculus. df = dorsal funiculus; lf = lateral funiculus; vh = ventral horn. Magnification: 25X.
GABA-A receptor antagonists
The GABA-A antagonist, bicuculline, applied to the cervical spinal cord did not significantly affect respiratory frequency and therefore, most likely did not affect medullary respiratory centers (Fig. 2). Bicuculline also did not affect the burst duration in controls and the contralateral output of hemisected rats. Bicuculline did, however, significantly increase the peak amplitude of the respiratory motor output bilaterally in both sham controls and spinal cord injured rats (Fig. 2). Also, the burst area of the control rats and the contralateral nerve of hemisected rats were significantly elevated. In hemisected rats, application of bicuculline to the cervical spinal cord induced crossed phrenic activity (Fig. 2); phrenic motor output was evident in the previously quiescent nerve in all rats examined.
Figure 2.
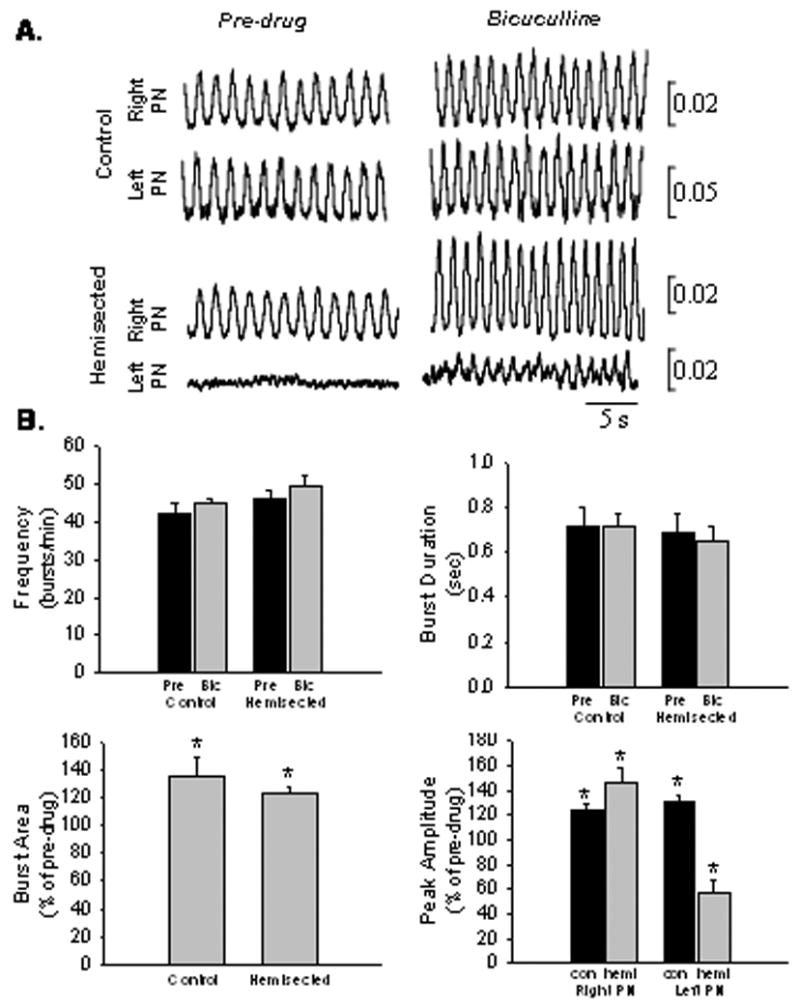
A. Representative tracings of integrated, right and left phrenic neurograms (PN) taken before and after bicuculline was applied to the cervical spinal cord. Note that bicuculline increased the peak height of the phrenic burst in both control and spinal cord injured rats. In addition, bicuculline resulted in the induction of crossed phrenic activity in all spinal cord injured rats. B. Graphs showing that bicuculline caused no significant change in respiratory frequency (p=0.243) or the burst duration of controls (p=0.804) and hemisected rats (p=0.884). Bicuculline significantly increased burst area in both control and contralateral output in hemisected rats (p=0.006), as well as the burst peak amplitude bilaterally in control and hemisected rats (p=0.007). Since there was no neural activity in the left phrenic nerve of hemisected rats before drug was applied, the crossed phrenic activity that was induced by bicuculline application was expressed as a percentage of the right phrenic nerve after bicuculline.
Application of the selective GABA-A receptor antagonist, Gabazine, (SR-95531, Sigma) to the cervical spinal cord resulted in a similar response as bicuculline application; burst peak amplitude and burst area of the phrenic motor output increased, while there was no change in respiratory frequency or burst duration (Fig. 3A, C). This response was observed bilaterally and thus, the drug activated crossed phrenic pathways in hemisected rats (Fig. 3C).
Figure 3.
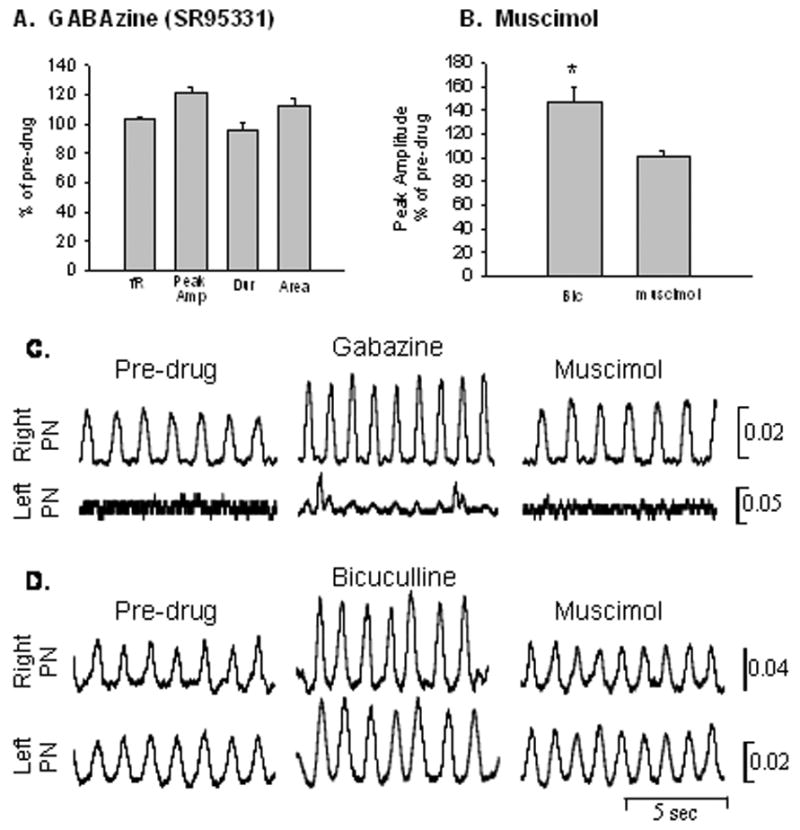
A. Gabazine application to the cervical spinal cord resulted in an increase in burst peak amplitude (peak Amp) and burst area (area), while there was no effect on frequency (fR) or burst duration (dur); similar to the effects observed with bicuculline application. B. Muscimol blocked the effects of bicuculline and peak amplitude was significantly reduced back to control values. C. Rectified and integrated traces of the respiratory motor output from one hemisected rat showing before drug, after Gabazine administration to the spinal cord followed by muscimol application. Note that Gabazine application induced crossed phrenic activity in the phrenic nerve ipsilateral to the hemisection and muscimol eliminated this effect. D. Rectified and integrated traces of the respiratory motor output from one control rat before drug, after bicuculline, and followed by muscimol application. Note that the peak amplitude of the phrenic bursts is increased after the GABA-A antagonist and this effect is blocked by muscimol.
To verify that bicuculline was acting via the GABA-A receptor, muscimol, a selective GABA-A agonist was applied approximately 5 min after bicuculline to block the effect of bicuculline. Muscimol application resulted in the subsequent reduction in peak amplitude back to control values (Fig. 3B, C).
Bicuculline administration sometimes caused convulsive-like bursting bilaterally in both control (20%) and hemisected rats (43%). There were no significant differences between the convulsive bursting observed between the controls and hemisected rats. The convulsive bursting pattern was often a rapid onset, long duration type of burst (Fig. 4A). Sometimes the bursting was larger than the “normal” phrenic bursts, and sometimes it was the same size or even smaller. The convulsive bursts sometimes coincided with a normal phrenic burst (Fig. 4A-2) and some of these bursts appeared between the normal phrenic bursts (Fig. 4A-1). Transection of the spinal cord after the bicuculline-induced convulsive bursting reduced the amount and frequency of the large, convulsive bursting patterns observed in the phrenic motor output (Fig. 4B), but it did not affect the peak height of the ‘normal’ phrenic motor bursts indicating that the effect of bicuculline on respiratory-related phrenic motor output is limited to the cervical spinal cord (Fig. 4C).
Figure 4.
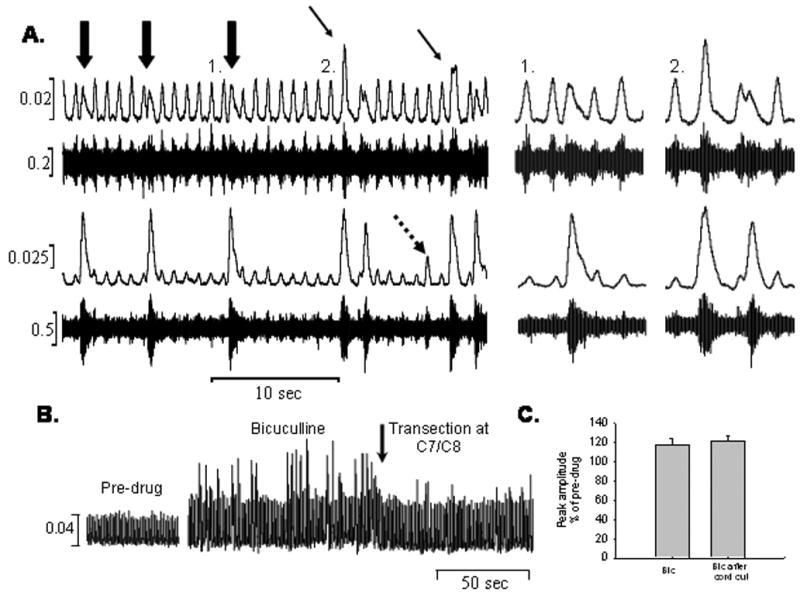
A. Raw and integrated neural recordings from the right (top) and left (bottom) phrenic nerves showing the convulsive-like bursting patterns that were observed after the application of bicuculline. Note that these bursts occurred either between “normal” phrenic bursts (large arrows, 1) or coincided with the phrenic bursts (small arrows, 2). Sometimes small bursts were only visible on the left side (dotted arrow). B. Integrated neural recordings before bicuculline, 7 ½ minutes after bicuculline was applied showing increased “convulsive” bursts, and after transection of the spinal cord at C7/C8. Transection attenuated the convulsive bursting, but did not affect the peak amplitude of the phrenic bursts (C).
Glycine receptor antagonists
Application of strychnine directly to the cervical spinal cord did not affect respiratory motor output at any dose examined. There was no change in respiratory frequency, burst peak amplitude, burst area or burst duration at any dose, (Fig. 5). In order to demonstrate that the strychnine used in the present study was active, we injected the strychnine systemically so that the strychnine could affect medullary respiratory centers as well. Strychnine, administered systemically, significantly altered respiratory motor output, as expected. There was a transient seizure-like discharge followed by an increase in respiratory frequency and burst peak amplitude as well as a decrease in burst duration (Fig. 6). There was no effect on burst area.
Figure 5.
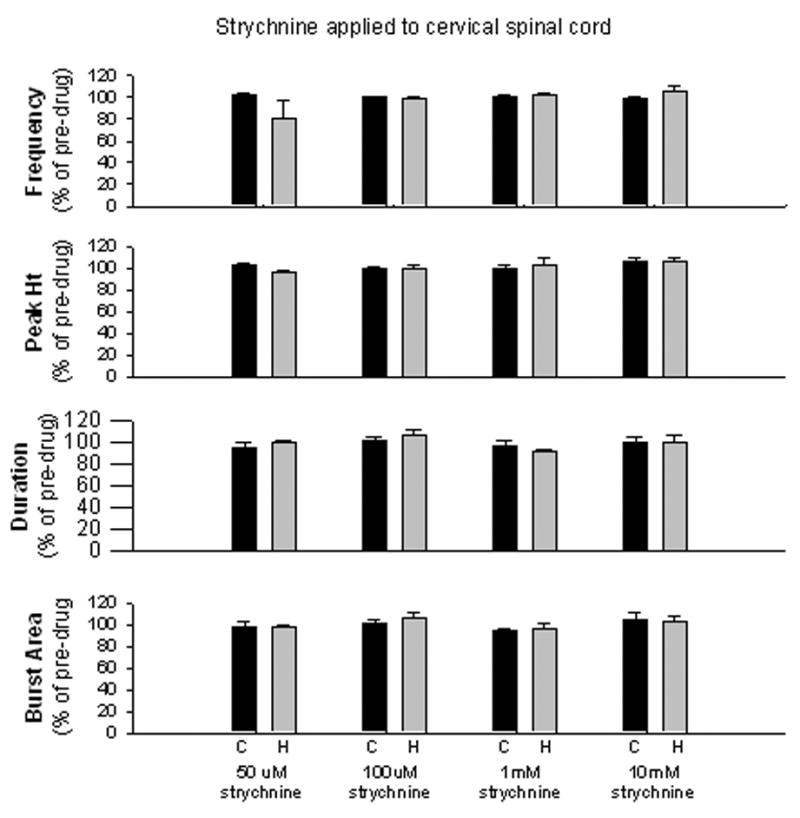
Application of strychnine to the cervical spinal cord of control (C) and hemisected (H) rats did not affect respiratory frequency (fR), burst peak amplitude (peak Amp), burst duration (dur) or burst area (area) at any dose applied.
Figure 6.
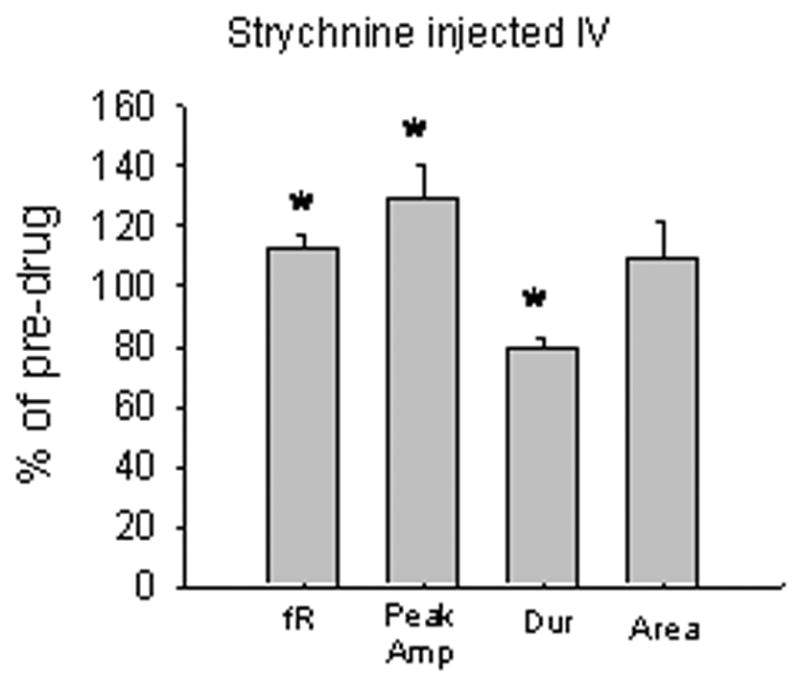
Injection of strychnine (IV) caused a transient increase in respiratory frequency (fR) (p=0.02) and burst peak amplitude (peak Amp) (p=0.05), and a decrease in burst duration (dur) (p=0.005), while burst area (area) was not affected.
Discussion
GABA-A receptors
Fast synaptic inhibition is mediated by γ–aminobutyric acid (GABA) and glycine, and both are involved in the inhibitory control of ventilation (Hayashi and Lispski 1992). Electrophysiological studies have shown that GABA-A receptors are involved in the inhibition of phrenic motor neurons during the expiratory phase of respiration (Fedorko et al., 1987; Merrill and Fedorko, 1984), but more recently, GABAergic inhibition has also been demonstrated during the inspiratory phase (Parkis et al. 1999; Funk et al., 2000). Immunohistochemistry has established a dense network of GABAergic terminals surrounding phrenic motor neuron somata and proximal dendrites as well as throughout the phrenic dendrite bundles (Zahn et al. 1989). Furthermore, electron microscopy has confirmed a direct GABAergic synaptic contact with phrenic motor neurons (Murphy et al. 1998; Tai and Goshgarian 1996). Sources of phrenic GABAergic inputs arise from multiple brainstem respiratory centers, such as the Bötzinger complex, Kölliker Fuse nucleus and the ventral respiratory group (VRG) (Ellenberger 1999; Song et al. 2000). Sources of GABAergic inputs onto phrenic motor neurons that arise from spinal cord origins have not been definitively established, but it is known that neurons containing GABA, as well as nerve terminals containing GAD are found in all laminae and segments of the spinal cord (Mugnaini and Oertel 1985; Mackie et al. 2003). Moreover, electrophysiological studies have shown that spinal inhibitory mechanisms affect phrenic motor neurons, such as the phrenic-to-phrenic inhibitory reflex (Cleland and Getting, 1993; Iscoe and Duffin, 1996; Speck and Revelette 1987), as well as studies that indirectly suggest that interneurons may be involved in the GABAergic inhibition of phrenic motor neurons (Chitravanshi and Sapru 1999).
We recently demonstrated that serotonin 1A receptor activation in the dorsal horn of the spinal cord resulted in the expression of crossed phrenic activity probably through the disinhibition of phrenic motor neurons (Zimmer and Goshgarian, 2006). The data suggested that dorsal horn sensory neurons may be involved in the increased inhibition of phrenic motor neurons after high cervical spinal cord hemisection. In addition, other studies (Goshgarian 1981; Fuller et al. 2002) have shown that cervical dorsal root rhizotomy can activate crossed phrenic pathways supporting the idea that primary afferent fibers in the dorsal roots excite the dorsal horn sensory neurons which, in turn, inhibit phrenic motor neurons. Therefore, we hypothesized that GABA may originate from dorsal horn neurons either directly or indirectly to inhibit phrenic motor neurons. The results from this study do show that the GABA-A receptors located within the spinal cord are involved in the inhibition of phrenic motor output. However, the data do not show that the GABA-mediated inhibition originates from within the spinal cord. The source of the GABA-mediated inhibition may come from local sources originating in the dorsal horn, but they may also originate from bulbospinal GABA inputs.
In the present study, both bicuculline and Gabazine were used to block GABA-A receptors. Since bicuculline can block apamin-sensitive Ca2+-activated K+ currents (Johnson and Seutin 1997) at high doses, Gabazine (SR-95531) was used to verify the data obtained using bicuculline. The results from this study show that, indeed, blocking GABA-A receptors in the cervical spinal cord, using either bicuculline or Gabazine, resulted in the disinhibition of the crossed phrenic pathway. Furthermore, muscimol, a specific GABA-A agonist, was applied after bicuculline treatment. Muscimol was sufficient in blocking the responses observed with bicuculline, again verifying that the effects observed after bicuculline treatment in this study were due to specifically blocking the GABA-A receptor.
The phrenic response to bicuculline application was rapid, and peak amplitude started to increase within minutes after drug application. Intrathecal administration of drugs can penetrate tissue to depths of 1000 μM within minutes after application (Hains et al., 2002). Therefore, bicuculline most likely acted directly on phrenic motor neurons to enhance phrenic motor output. Studies have shown that microinjection of bicuculline directly into the phrenic motor nucleus acts to enhance motor output (Chitravanshi and Sapru 1999) and similarly using the neonatal in vitro preparation, application of bicuculline to the cervical spinal cord caused a significant increase in phrenic motor output (Parkis et al., 1999). However, we cannot rule out the possibility that bicuculline acted on other sites within the cervical spinal cord to aid in the disinhibition of phrenic motor neurons.
It is unlikely that bicuculline acted on receptors located on supraspinal respiratory structures for two reasons. First, in the present study, bicuculline was applied to the cervical spinal cord. The design of the experimental setup, with the head supported by ear bars in a stereotaxic frame and the body and neck lower than the head, prevented the flow of drug rostrally, against gravity, to medullary structures; in fact cerebral spinal fluid pooled in the caudal region of the cervical cord, toward C8-T1. Secondly, there was no significant increase in respiratory frequency. If bicuculline is applied systemically, an increase in breathing frequency is observed (Hayashi and Lipski 1992) and muscimol (a GABA-A agonist) applied to brainstem respiratory regions consistently decreases respiratory frequency (Brockhaus and Ballanyi 1998; Hayashi and Lipski 1992).
Bicuculline has also been shown to affect phrenic nerve activity via spinal mechanisms originating below C8. In non-injured rats, bicuculline was applied intrathecally to the lower thoracic spinal cord, T8-T11 (Goodchild et al. 2000) to examine the effects of GABA-A receptor antagonism on sympathetic nerve activity (SNA). The authors noted that a significant increase in phrenic nerve amplitude and irregular ventilatory patterns coincided with bicuculline’s effect on SNA. When the spinal cord was transected at C8, the effect of bicuculline on phrenic nerve activity was abolished (Goodchild et al. 2000). In the present study, we found that transection of the spinal cord at C7/8 decreased the irregular ventilatory patterns observed after the application of bicuculline. It did not, however, result in a significant reduction in the phrenic motor output. Thus, it appears that phrenic motor neurons are modulated by a bicuculline-sensitive GABA-A receptor mechanism localized to the cervical spinal cord.
Finally, it is known that crossed phrenic activity becomes spontaneously active over time (Nantwi et al., 1999). It is possible that the GABA-mediated inhibition is slowly down-regulated over time after injury and this may be one mechanism underlying the spontaneous appearance of crossed phrenic activity. Currently, studies are ongoing to determine if GABA influences are reduced over time after spinal cord hemisection injury.
Glycine receptors
During the respiratory cycle, inhibitory inputs are involved in counteracting synaptic excitation and in shaping the firing patterns. While studies show that glycine does not affect phrenic motor neurons directly to modulate resting motor output (Parkis et al., 1999; Su and Chai, 1998), under certain conditions, glycine does play a role in the inhibition of phrenic motor neurons. Stimulation of leg afferents induces a spinal reflex which inhibits phrenic motor neurons (Eldridge et al., 1981) through the release of glycine (Eldridge et al., 1987), and Renshaw cells inhibit phrenic motor neurons (Lipski et al., 1985) also using glycine. It is known that glycine and GABA are often co-localized (Todd and Sullivan 1990) and are simultaneously released at synapses between interneurons and motor neurons (Jonas et al. 1998). In fact, both GABA and glycine are coreleased by synaptic terminals onto hypoglossal motor neurons (O’Brien and Berger 1999), but it is not known whether this is the case for phrenic motor neurons. Under normal resting conditions, glycine may not play an active role in the inhibition of phrenic motor neurons, but it is not clear whether spinal cord injury alters this. Indeed, recurrent inhibition via Renshaw cells is increased after spinal cord injury (Shefner et al., 1992).
We found that application of strychnine to the cervical spinal cord caused no significant change in phrenic motor output in spinal-injured rats. Furthermore, we saw no change in respiratory frequency indicating that the drug was not reaching medullary respiratory centers. Since no significant responses were observed with any of the doses of strychnine used in the present study, we injected strychnine systemically to show that the drug itself does affect respiration. Strychnine injected systemically caused convulsive-like discharges followed by a transient increase in respiration similar to the results of Ling et al., 1993.
Summary
The primary cause of all deaths after spinal cord injury is due to respiratory insufficiency (National Spinal Cord Injury Statistical Center 2005). Therefore, it is extremely important that we understand how spinal cord injury affects all aspects of ventilatory control. A previous study showed that activation of serotonin 1A receptors in the dorsal horn induced crossed phrenic activity, probably via disinhibition of phrenic motor neurons. The present study demonstrated that GABA may be involved in this inhibition, since blocking the GABA-A receptor enhanced phrenic motor output and induced crossed phrenic activity after injury. Glycine receptors do not appear to be involved in the inhibition of crossed phrenic pathways since strychnine applied to the cervical spinal cord had no effect on phrenic motor output.
Acknowledgments
This research was supported by NIH grant HD 31550 (H.G. Goshgarian).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharm. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. GABA receptors in the phrenic nucleus of the rat. Am J Physiol. 1999;276:R420–R428. doi: 10.1152/ajpregu.1999.276.2.R420. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Getting PA. Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4–C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res. 1993;93:307–311. doi: 10.1007/BF00228399. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motorneurons in rat. J Comp Neurol. 1994;347(1):64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE, Waldrop TG. Spinal inhibition of phrenic motoneurons by stimulation of afferents from peripheral muscles. J Physiol. 1981;311:67–79. doi: 10.1113/jphysiol.1981.sp013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop T. Spinal inhibition of phrenic motoneurons by stimulation of afferents from leg muscle in the cat: blockade by strychnine. J Physiol. 1987;389:137–146. doi: 10.1113/jphysiol.1987.sp016650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH. Distribution of bulbospinal γ-aminobutyric acid-synthesizing neurons of the ventral respiratory group of the rat. J Comp Neurol. 1999;411:130–144. doi: 10.1002/(sici)1096-9861(19990816)411:1<130::aid-cne10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Connelly CA, Remmers JE. Neurotransmitters mediating synaptic inhibition of phrenic motoneurons. In: Sieck GC, Gandevia SC, Cameron WE, Alan R, editors. Respiratory muscles and their neuromotor control. Liss, Inc.; New York: 1987. pp. 167–173. [Google Scholar]
- Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett. 2002;323:25–28. doi: 10.1016/s0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23(7):29933000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Parkis MA, Selvaratnam SR, Robinson DM, Miles GB, Peebles KC. Synaptic control of motoneurons excitability in rodents: from months to milliseconds. Clin Exp Pharm Physiol. 2000;27:120–125. doi: 10.1046/j.1440-1681.2000.03202.x. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25(11):2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Forero D, Alvarez FJ. Differential postnatal maturation of GABAA, Glycine receptor, and mixed synaptic currents in Renshaw cells and ventral spinal interneurons. J Neurosci. 2005;25(8):2010–2023. doi: 10.1523/JNEUROSCI.2383-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild AK, Van Deurzen BTM, Sun QJ, Chalmers J, Pilowsky PM. Spinal GABA receptors do not mediate the sympathetic baroreceptor reflex in the rat. Am J Physiol Regul Intergr Comp Physiol. 2000;279:320–331. doi: 10.1152/ajpregu.2000.279.1.R320. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp Neurol. 2002;175:347–362. doi: 10.1006/exnr.2002.7892. [DOI] [PubMed] [Google Scholar]
- Hains BC, Willis WD, Hulsebosch CE. Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res. 2003;970:238–241. doi: 10.1016/s0006-8993(03)02347-3. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Lipski J. Tthe role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol. 1992;89:47–63. doi: 10.1016/0034-5687(92)90070-d. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Duffin J. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic motoneurones in cats. J Physiol. 1996;497.3:803–812. doi: 10.1113/jphysiol.1996.sp021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+ -activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Ling L, Back KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Ling L, Karius DR, Speck DF. Pontine-evoked inspiratory inhibitions after antagonism of NMDA, GABAA, or glycine receptor. J Appl Physiol. 1993;74(3):1265–1273. doi: 10.1152/jappl.1993.74.3.1265. [DOI] [PubMed] [Google Scholar]
- Lipski J, Fyffe REW, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci. 1985;5(6):1545–1555. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie M, Huges DI, Maxwell DJ, Tillakaratne NJK, Todd AJ. Distribution and colocalisation of glutamate decarbosylase isoforms in the rat spinal cord. Neurosci. 2003;119:461–472. doi: 10.1016/s0306-4522(03)00174-x. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Dekin MS, Mitchell GS. Glutamate, GABA, and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Lung Biology in Health and Disease, Regulation of Breathing. Vol. 79. Marcel Dekker; New York: 1995. pp. 151–218. [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CFL, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Fedorko L. Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. J Neurosci. 1984;4:2350–2353. doi: 10.1523/JNEUROSCI.04-09-02350.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABA-ergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. part 1. Vol. 4. Elsevier; Amsterdam: 1985. pp. 436–608. [Google Scholar]
- Murphy SM, Pilowsky PM, Llewellyn-Smith IJ. Pre-embedding staining for GAD67 versus postembedding staining for GABA as markers for central GABAergic terminals. J Histochem Cytochem. 1998;46(11):1261–1268. doi: 10.1177/002215549804601106. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center Birmingham AL. Spinal cord injury facts and figures. J Spinal Cord Med. 2005;28(3):296–297. [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a pparalyzed hemidiaphragm following cervical spinal cord hemisection. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- Nantwi K, Goshgarian HG. Alkylxanthine-induced recovery of respiratory function following cervical spinal cord injury in adult rats. Exp Neurol. 2001;168:123–134. doi: 10.1006/exnr.2000.7581. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Dong X-W, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: Phase-specific control of excitability. J Neurosci. 1999;19(6):2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Botzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol. 1998;509.1:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter WT. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol. 17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP, Fyffe REW. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol. 1992;68(2):397–406. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Berman SA, Sarkarati M, Young RR. Recurrent inhibition is increased in patients with spinal cord injury. Neurology. 1992;42(11):2162–2168. doi: 10.1212/wnl.42.11.2162. [DOI] [PubMed] [Google Scholar]
- Song G, Qin L, Feng-Zhi S. GABAergic neurons in Kölliker-Fuse nucleus and Bötzinger complex with axons projecting to phrenic nucleus. Acta Physiol Sinica. 2000;52:167–169. [PubMed] [Google Scholar]
- Speck DF, Revelette WR. Attenuation of phrenic motor discharge by phrenic nerve afferents. J Appl Physiol. 1987;62(3):941–945. doi: 10.1152/jappl.1987.62.3.941. [DOI] [PubMed] [Google Scholar]
- Su CK, Chai CY. GABAergic inhibition of neonatal rat phrenic motoneurons. Neurosci Lett. 1998;248:191–194. doi: 10.1016/s0304-3940(98)00361-9. [DOI] [PubMed] [Google Scholar]
- Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J Comp Neurol. 1996;372:343–355. doi: 10.1002/(SICI)1096-9861(19960826)372:3<343::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296(3):496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- Zahn WZ, Ellenberger HH, Feldman JL. Monoaminergic and gabaergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neurosci. 1989;31(1):105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29(2):147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]


