Abstract
Background
Neurosurgical procedures are carried out routinely in health institutions across the world. A key issue to be considered during neurosurgical interventions is that there is always an element of inevitable brain injury that results from the procedure itself due to the unique nature of the nervous system. Brain tissue at the periphery of the operative site is at risk of injury by various means including incisions and direct trauma, electrocautery, hemorrhage, and retractor stretch.
Methods/Results
In the present review we will elaborate upon this surgically-induced brain injury and also present a novel animal model to study it. Additionally, we will summarize preliminary results obtained by pretreatment with PP1, a src tyrosine kinase inhibitor reported to have neuroprotective properties in in-vivo experimental studies. Any form of pretreatment to limit the damage to the susceptible functional brain tissue during neurosurgical procedures may have a significant impact on the patient recovery.
Conclusion
This brief review is intended to raise the question of ‘neuroprotection against surgically-induced brain injury’ in the neurosurgical scientific community and stimulate discussions.
Keywords: Neuroprotection, Brain Injury, Neurosurgery, Rat, Pretreatment
Rationale
There are over 800,000 cranial and spinal neurosurgery cases performed annually in US alone. The specialized field of neurosurgery has come a long way from its origins in trephination practiced by the ‘sirkaks’ (Inca neurosurgeons) hundreds of years ago [13,29] to recent advancements in endoscopic neurosurgeries [44,15]. Neurosurgical procedures, however, remain invasive procedures regardless of whether they are performed in elective or emergency settings. Some of the neurosurgical interventions such as surgeries for brain stem and spinal cord pathologies are intrinsically linked to postoperative neurological deficits and may lead to serious neurological injuries regardless of how carefully the operation is carried out [9].
A key issue during most neurosurgical procedures is that there is always an element of inevitable injury inflicted on the functional and normal brain while dissecting or eliminating the pathological tissue(s). This unavoidable injury may exist in many forms including predetermined cortical incisions to access any deeper pathological tissue [38], retraction of brain lobes or hemispheres [1,20], intra-operative bleeding [19] and thermal injury due to electrocoagulation. Endoscopic surgeries and stereotaxic guided procedures are designed to minimize the invasiveness of neurosurgical procedures. However, these procedures also lead to inevitable brain injuries and complications [8,12,19,37].
To reduce the post-operative effects of these brain injuries and decrease the subsequent neurological deficits, osmotic agents, diuretics and steroids are routinely used [30]. Steroids, though well established in ameliorating brain tumor associated edema, have not shown a definite therapeutic effect in clinical trials for ischemic stroke, in tracerebral hemorrhage, aneurysmal subarachnoid hemorrhage, and traumatic brain injury [18]. Some anesthetic agents themselves [23,36] and therapeutic modalities such as intraoperative hypothermia [4,43] are suggested to provide cerebral neuroprotection. Intraoperative hypothermia, however, is not widely practiced and not without it’s own drawbacks [4,40,43].
Even with adjunct treatments, the standard neurosurgical maneuvers, whether planned or performed in an emergency can cause damage to the surrounding brain tissue and may contribute to or result in critical early postoperative complications such as brain edema and ischemia or delayed healing [11,28,41]. Even if there are no life threatening and serious complications in most cases, neurosurgical patients have to be monitored closely, especially in the intensive care unit. This translates into longer hospital stay with significant implications for the patient, the healthcare system and the society. The neurological and neurobehavioral functional deficits can additionally be a source of long term emotional, social, physical and financial duress. Moreover, the concerns of causing injury to the surrounding normal and functional brain, especially in vulnerable areas such as brain stem, [9] may hamper the approach of the neurosurgeons.
In the past and at present, the inevitable brain injury resulting from neurosurgical interventions is not treated separately or covered under any separate neuroprotective regimen but rather is left to be healed on its own. Presently, there are established therapeutic agents such as erythropoietin and statins used clinically in patients for different non-neurological disorders that have also shown promise as neuroprotectants in experimental studies [6,10,39]. These agents, with proven safety in patients, are poised for clinical trials in cerebrovascular disorders [10]. We want to take advantage of such therapeutic modalities for use as pretreatment against surgically-induced brain injury due to their effectiveness when used prior to brain injury (such as stroke or traumatic brain injury) and their reduced or lack of efficacy if used after brain injury. Neuroprotection in the form of some pretreatment may not only guard the susceptible normal brain tissue from surgically-induced brain injury, but is also clinically relevant in our model.
Animal Model for surgically-induced brain injury
An extensive literature search did not yield any animal model used to study surgically-induced brain injury. We have created a novel in vivo model to study brain injury caused exclusively by neurosurgical procedures. The frontal lobe surgical injury rat model allows us to simulate the surgically-induced brain injury by causing both cortical and parenchymal damage, and to study the postoperative complications that follow neurosurgical procedures. Additionally, it allows the study of molecular mechanisms and signaling pathways involved in surgically-induced brain injury and the testing of different pretreatment modalities for neuroprotection.
Adult male Sprague Dawley rats, each weighing 300-350 grams were used for the procedure. Following anesthesia with isoflurane (4.5% for induction and 2.5% maintenance by intubation) or ketamine (100 mg/kg) plus xylazine (10mg/kg) i.p, the rats were placed in prone position in a stereotaxic frame (Benchmark™) under a surgical operating microscope. After dissecting the skin and connective tissue, the periosteum was reflected with a periosteal elevator to expose the right frontal skull. An operating square area (5mm edge) was identified on the frontal skull bone such that the left lower corner of the square was at the bregma (Figure 1). The margins of the square were thinned out to translucency with a micro-drill without penetrating the skull. Using a bone lifter and forceps, this piece of bone was gently lifted to expose a square window displaying the underlying right frontal lobe of the brain covered by dura. The dura was carefully incised with no. 20 needle to minimize bleeding and gently flipped over to expose the right frontal lobe. Using a flat blade (6 mm × 1.5 mm), two incisions were made leading away from the bregma along sagittal and coronal planes to sever an area of brain 2mm lateral of sagittal and 1mm proximal of coronal planes. The sectioned brain was removed and weighed immediately (approximately 35 mg). In preliminary studies we observed that the variability in the weights of the sectioned brain tissue was not significantly different (unpublished data). Intraoperative packing and saline irrigation was used to control bleeding. Hemostasis was confirmed by close observation after removal of packing. Subsequently the dura was placed loosely back in original position, as was the skull cap and overlying connective tissue. Skin was sutured using 3-0 silk (Ethicon) on a reverse cutting needle. Vital signs were monitored throughout the procedure which lasts about 20-25 minutes. Sham surgery included only craniotomy and replacement of the bone flap without any dural incisions. Preliminary studies have shown zero mortality in this model.
Figure 1.
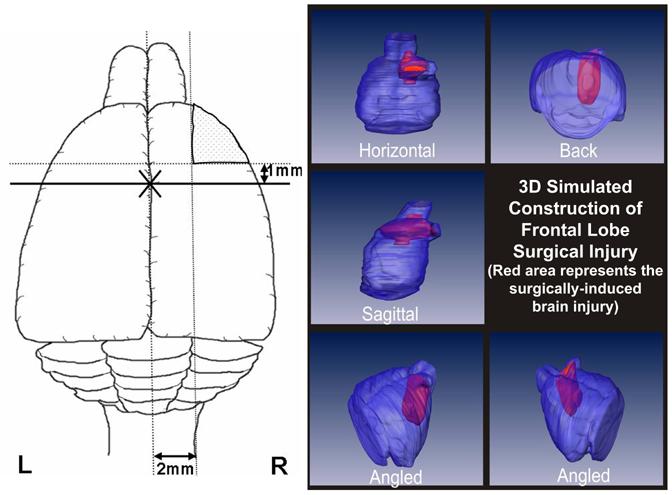
Left panel depicts a rat brain cut along horizontal plane and shows the frontal lobe surgical injury in relation to the bregma (marked by X). The two incisions are made leading away from the bregma along the sagittal and coronal planes 2 mm lateral and 1 mm proximal to the sagittal and coronal sutures respectively. The right panel is a 3D image of the proposed model showing the frontal lobe surgical injury (in red) from different angles and planes.
Caveats in the animal model
Ideally, we intend to develop this model to simulate most, if not all, surgically-induced brain injuries in a reproducible manner. The present model incorporates cortical and parenchymal damage, including axotomy usually caused by surgical tools either inadvertently or in a premeditated approach. Secondly, there is intraoperative bleeding which is controlled by packing and saline irrigation. Electrocoagulation is also used to control bleeding and to make incisions to gain access to deeper structures. There are variations in its usage in terms of voltage, frequency and duration of application. Brain injuries caused by lobe retraction were not considered due to variability in usage of retractors. Moreover, miniature instruments producing consistent pressure and stretch injuries seen with retractor usage are required for this particular brain injury component. Further improvements of this model, however, should address these issues.
Even though the scale of frontal lobe injury may be comparable to brain tumor resection/debulking [34] or epilepsy surgery (mostly in the temporal lobe) to some degree, this model is not intended to mimic any specific neurosurgery operations. Instead the goal is to produce a standard and reproducible model with certain amount of brain tissue loss and injury that will produce neuronal death, and blood brain barrier dysfunction leading to brain edema which occurs during routine neurosurgical operations in clinical practice.
Brain injuries produced by this animal model
Preliminary studies on the animal model presented above revealed that there is presence of localized edema around the operative site, i.e. surgically-induced brain injury. This edema indicated by brain water content [(wet weight-dry weight)/wet weight] × 100 and calculated using standard methods [46] was present only in the ipsilateral frontal area, i.e the area contiguous to the surgically-induced brain injury (Figure 2). Time course performed experiments further revealed that this localized edema peaked at 24 hours, started declining after 3 days and almost entirely resolved by 1-2 weeks after the procedure (unpublished data), thus mimicking a clinical situation appropriately. Further studies also revealed that there was a disruption of the blood brain barrier (BBB) as indicated by standard methods such as IgG staining (Figure 3) and Evan’s blue dye extravasation (unpublished data) [35,42]. Additionally, using Nissl’s staining we identified neuronal death in the area bordering the surgically-induced brain injury (Figure 4). Moreover, a clear transition zone i.e. gradual change from dead cells to viable cells could be identified thus enabling us to measure and quantify the extent of injury. Preliminary experiments involving pretreatment with neuroprotectants have showed a decrease in the extent of injury (Figure 2). Thus, this model allows to study BBB dysfunction leading to brain edema, and neuronal death in the brain tissue susceptible to surgically-induced brain injury.
Figure 2.
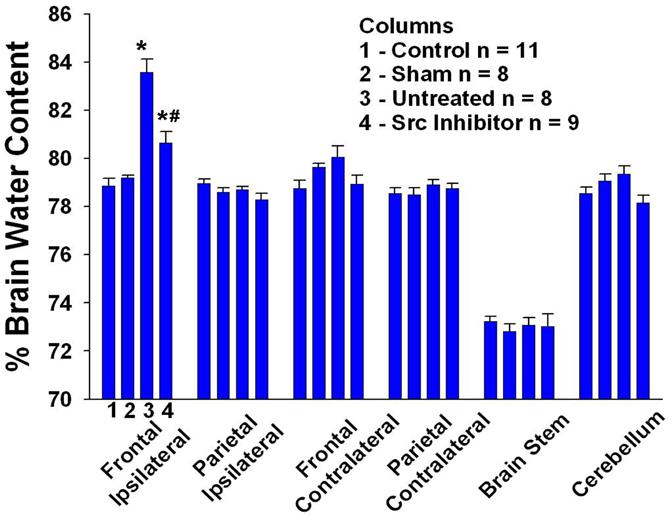
The figure shows marked edema in the frontal ipsilateral (bordering the surgical-injury) brain in untreated and PP1-treated groups as compared to the control group 24 hrs after the surgical-injury. The brain edema, however, is significantly lowered in the group that is pretreated (45 mins before surgery) with src tyrosine kinase inhibitor, PP1 (1.5mg/kg, i.p.) as compared to untreated group. The other brain regions did not differ statistically from each other after the injury. p< 0.05 for both * and # which represent vs control and vs untreated respectively. Data are expressed as mean ± S.E.M. Statistical significance was verified by one-way analysis of variance (ANOVA) for multiple comparisons.
Figure 3.
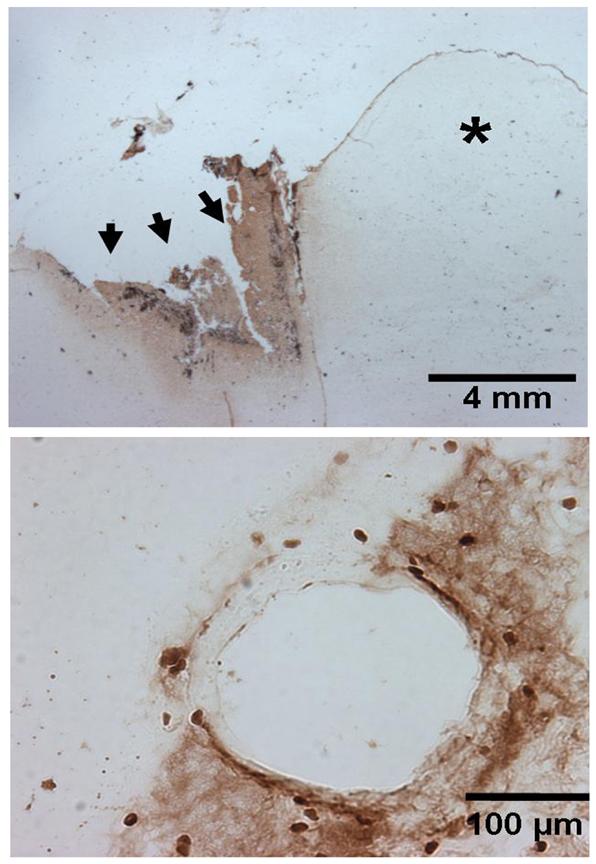
The representative figure (n=4) in the upper panel shows IgG staining in a horizontal section of the brain 24 hours after the frontal lobe surgical injury. There is increased IgG staining surrounding the surgically-induced brain injury (marked by arrows) on the ipsilateral side as compared to the unaffected contralateral side indicated by the asterisk (*). The lower panel shows an individual affected blood vessel at high magnification depicting disruption of blood brain barrier as indicated by IgG staining. Scale represents 4 mm and 100 μm in upper and lower panels respectively.
Figure 4.
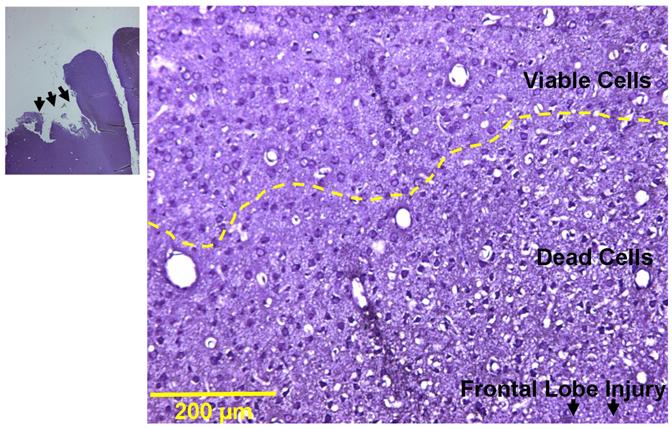
The representative figures (n=4) show Nissl’s staining in a horizontal section of the brain 24 hours after the frontal lobe surgical injury. There is a marked area comprising of dead cells adjoining the surgically-induced brain injury (marked by arrows at low magnification) on the ipsilateral side. A transition zone marks the merging of this affected area with the unaffected viable cells as marked by the yellow broken line. Scale represents 200 μm.
More importantly, this model also allows studying the cellular signaling mechanisms by performing molecular techniques on the affected brain tissue. Delineating the signaling pathways will elucidate the key molecular targets for neuroprotection against surgically-induced brain injury. Preliminary experiments using inhibitors of key molecular targets such as src tyrosine kinase have yielded very promising results (Figure 2). PP1 is a selective inhibitor for src tyrosine kinase [33], which is an upstream regulator of mitogen activated protein kinases (MAPKs). Src tyrosine kinase and the ubiquitous MAPKs are well implicated in brain injury resulting from different causes such as cerebral ischemia, trauma and hemorrhage [2,21,22,24,26,27,31,32,45] and have an important role in cerebral edema [26,31]. Inhibition of src tyrosine kinase with PP1 reduces the expression of vascular endothelial growth factor (VEGF), protects blood-brain barrier, and reduces brain edema immediately after subarachnoid hemorrhage [26] and also offers cerebral protection against stroke by influencing the VEGF-mediated vascular permeability and cerebral edema [33]. In this animal model, we have observed a marked effect of PP1 on brain edema as shown in Figure 2. Neurological scoring is routinely employed in animal models studying neurological disorders, such as middle cerebral artery occlusion model for ischemic stroke [14], endovascular perforation model for subarachnoid hemorrhage [25], and spinal cord injury models [3]. However, these scoring systems were inappropriate for the present model as the affected brain area is localized and the type of injury is different. Sensorimotor neurological deficits were observed in the form of contralateral fore-limb weakness and decreased response to contralateral vibrissae stimulation and side stroking. The animals had lesser appetite reflected in loss in body weight after surgical injury (92 ± 0.4% of preoperative weight) as compared to the sham-operated animals (98.2 ± 0.7% of preoperative weight) and were sometimes aggressive. These neurobehavioral deficits can be attributed to the ipsilateral frontal lobe injury, as the brain frontal lobe lesions have been linked to aggressive behavior and loss of appetite and weight loss [5,7,16,17]. Interestingly, the sensorimotor deficits seemed to resolve almost completely by end of 1 week parallel to the recovery from brain edema.
Conclusion
The issue of neuroprotection against surgically-induced brain injury has not been addressed before due to lack of any studies in humans and to the unavailability of an appropriate animal model to study it. The new model simulates injuries caused during neurosurgical procedures and produces commonly seen post-operative complications. Additionally, it allows us to study the cellular signaling pathways and identify key molecular targets to focus on for neuroprotective pretreatment before neurosurgical intervention. A successful therapeutic intervention for ‘surgically-induced brain injury’ may result in significant benefits for patients and healthcare organizations.
Acknowledgement
This study was partially supported by grants from NIH NS45694, NS53407, and NS43338 to JHZ.
Abbreviations
- BBB
Blood Brain Barrier
- MAPK
Mitogen Activated Protein Kinase
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews RJ, Muto RP. Retraction brain ischaemia: cerebral blood flow, evoked potentials, hypotension and hyperventilation in a new animal model. Neurol. Res. 1992;14:12–18. doi: 10.1080/01616412.1992.11740004. [DOI] [PubMed] [Google Scholar]
- 2.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J. Cereb. Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 4.Baughman VL. Brain protection during neurosurgery. Anesthesiol. Clin. North America. 2002;20 doi: 10.1016/s0889-8537(01)00004-9. [DOI] [PubMed] [Google Scholar]
- 5.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 6.Dawson TM. Preconditioning-mediated neuroprotection through erythropoietin? Lancet. 2002;359:96–97. doi: 10.1016/S0140-6736(02)07335-X. [DOI] [PubMed] [Google Scholar]
- 7.de Bruin JP, van Oyen HG, Van de PN. Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behav. Brain Res. 1983;10:209–232. doi: 10.1016/0166-4328(83)90032-3. [DOI] [PubMed] [Google Scholar]
- 8.Decq P, Le Guerinel C, Brugieres P, Djindjian M, Silva D, Keravel Y, Melon E, Nguyen JP. Endoscopic management of colloid cysts. Neurosurgery. 1998;42:1288–1294. doi: 10.1097/00006123-199806000-00051. [DOI] [PubMed] [Google Scholar]
- 9.Deletis V, Sala F. The role of intraoperative neurophysiology in the protection or documentation of surgically induced injury to the spinal cord. Ann. N. Y. Acad. Sci. 2001;939:137–144. doi: 10.1111/j.1749-6632.2001.tb03620.x. [DOI] [PubMed] [Google Scholar]
- 10.Fagan SC, Hess DC, Machado LS, Hohnadel EJ, Pollock DM, Ergul A. Tactics for vascular protection after acute ischemic stroke. Pharmacotherapy. 2005;25:387–395. doi: 10.1592/phco.25.3.387.61592. [DOI] [PubMed] [Google Scholar]
- 11.Fasano VA, Penna G. [Postoperative complications in neurosurgery] Minerva Anestesiol. 1992;58:15–21. [PubMed] [Google Scholar]
- 12.Freudenstein D, Wagner A, Ernemann U, Duffner F. Subdural hygroma as a complication of endoscopic neurosurgery--two case reports. Neurol. Med. Chir (Tokyo) 2002;42:554–559. doi: 10.2176/nmc.42.554. [DOI] [PubMed] [Google Scholar]
- 13.Froeschner EH. Two examples of ancient skull surgery. J. Neurosurg. 1992;76:550–552. doi: 10.3171/jns.1992.76.3.0550. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 15.Gerzeny M, Cohen AR. Advances in endoscopic neurosurgery. AORN J. 1998;67:957–5. doi: 10.1016/s0001-2092(06)62621-5. [DOI] [PubMed] [Google Scholar]
- 16.Glick SD. Modulation of food and water intake by frontal cortex in the rat. Commun. Behav. Biol. 1971;5:365–370. [PubMed] [Google Scholar]
- 17.Glick SD, Greenstein S. Recovery of weight regulation following ablation of frontal cortex in rats. Physiol Behav. 1973;10:491–496. doi: 10.1016/0031-9384(73)90211-4. [DOI] [PubMed] [Google Scholar]
- 18.Gomes JA, Stevens RD, Lewin JJ, III, Mirski MA, Bhardwaj A. Glucocorticoid therapy in neurologic critical care. Crit Care Med. 2005;33:1214–1224. doi: 10.1097/01.ccm.0000166389.85273.38. [DOI] [PubMed] [Google Scholar]
- 19.Hellwig D, Bertalanffy H, Bauer BL, Tirakotai W. Pontine hemorrhage. J. Neurosurg. 2003;99:796–797. doi: 10.3171/jns.2003.99.4.0796. [DOI] [PubMed] [Google Scholar]
- 20.Hernesniemi J, Leivo S. Management outcome in third ventricular colloid cysts in a defined population: a series of 40 patients treated mainly by transcallosal microsurgery. Surg. Neurol. 1996;45:2–14. doi: 10.1016/0090-3019(95)00379-7. [DOI] [PubMed] [Google Scholar]
- 21.Hu BR, Liu CL, Park DJ. Alteration of MAP kinase pathways after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2000;20:1089–1095. doi: 10.1097/00004647-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res. Mol. Brain Res. 2000;77:65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi M, Furuya H, Patel PM. Neuroprotective effects of anesthetic agents. J. Anesth. 2005;19:150–156. doi: 10.1007/s00540-005-0305-5. [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa H, Warita H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K. Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci. Lett. 1999;274:45–48. doi: 10.1016/s0304-3940(99)00676-x. [DOI] [PubMed] [Google Scholar]
- 25.Kusaka G, Calvert JW, Smelley C, Nanda A, Zhang JH. New lumbar method for monitoring cerebrospinal fluid pressure in rats. J. Neurosci. Methods. 2004a;135:121–127. doi: 10.1016/j.jneumeth.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2004b;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 27.Kusaka G, Kimura H, Kusaka I, Perkins E, Nanda A, Zhang JH. Contribution of Src tyrosine kinase to cerebral vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2003;99:383–390. doi: 10.3171/jns.2003.99.2.0383. [DOI] [PubMed] [Google Scholar]
- 28.Manninen PH, Raman SK, Boyle K, El Beheiry H. Early postoperative complications following neurosurgical procedures. Can. J. Anaesth. 1999;46:7–14. doi: 10.1007/BF03012507. [DOI] [PubMed] [Google Scholar]
- 29.Marino R, Jr., Gonzales-Portillo M. Preconquest Peruvian neurosurgeons: a study of Inca and pre-Columbian trephination and the art of medicine in ancient Peru. Neurosurgery. 2000;47:940–950. doi: 10.1097/00006123-200010000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Menon D. Critical care medicine: Management of raised intracranial pressure. In: Warrell DA, editor. Oxford Textbook of Medicine. 4th edition Vol. 2. New York; Cox TM Firth JD; Oxford University Press: p. 1256. [Google Scholar]
- 31.Mori T, Wang X, Aoki T, Lo EH. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J. Neurotrauma. 2002;19:1411–1419. doi: 10.1089/089771502320914642. [DOI] [PubMed] [Google Scholar]
- 32.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol. Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 33.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat. Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 34.Peker S, Abacioglu U, Sun I, Yuksel M, Pamir MN. Irradiation after surgically induced brain injury in the rat: timing in relation to severity of radiation damage. J. Neurooncol. 2004;70:17–21. doi: 10.1023/b:neon.0000040820.78643.0a. [DOI] [PubMed] [Google Scholar]
- 35.Rossner W, Tempel K. [Quantitative determination of the permeability of the so-called blood-brain barrier of Evans blue (T 1824)] Med. Pharmacol. Exp. Int. J. Exp. Med. 1966;14:169–182. [PubMed] [Google Scholar]
- 36.Sanders RD, Ma D, Maze M. Anaesthesia induced neuroprotection. Best. Pract. Res. Clin. Anaesthesiol. 2005;19:461–474. doi: 10.1016/j.bpa.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder HW, Niendorf WR, Gaab MR. Complications of endoscopic third ventriculostomy. J. Neurosurg. 2002;96:1032–1040. doi: 10.3171/jns.2002.96.6.1032. [DOI] [PubMed] [Google Scholar]
- 38.Solaroglu I, Beskonakli E, Kaptanoglu E, Okutan O, Ak F, Taskin Y. Transcortical-transventricular approach in colloid cysts of the third ventricle: surgical experience with 26 cases. Neurosurg. Rev. 2004;27:89–92. doi: 10.1007/s10143-003-0309-2. [DOI] [PubMed] [Google Scholar]
- 39.Stone TW. Adenosine, neurodegeneration and neuroprotection. Neurol. Res. 2005;27:161–168. doi: 10.1179/016164105X21896. [DOI] [PubMed] [Google Scholar]
- 40.Todd MM, Hindman BJ, Clarke WR, Torner JC. Intraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators, 2005. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 13;352(2):135–45. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- 41.Tommasino C. [Postoperative cerebral edema. Physiopathology of the edema and medical therapy] Minerva Anestesiol. 1992;58:35–42. [PubMed] [Google Scholar]
- 42.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J. Cereb. Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 43.Wagner KR, Zuccarello M. Local brain hypothermia for neuroprotection in stroke treatment and aneurysm repair. Neurol. Res. 2005;27:238–245. doi: 10.1179/016164105X25261. [DOI] [PubMed] [Google Scholar]
- 44.Wang E, Yong NP, Ng I. Endoscopic assisted microneurosurgery for cerebral aneurysms. J. Clin. Neurosci. 2003;10:174–176. doi: 10.1016/s0967-5868(02)00320-x. [DOI] [PubMed] [Google Scholar]
- 45.Wu DC, Ye W, Che XM, Yang GY. Activation of mitogen-activated protein kinases after permanent cerebral artery occlusion in mouse brain. J. Cereb. Blood Flow Metab. 2000;20:1320–1330. doi: 10.1097/00004647-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Brain edema after intracerebral hemorrhage: the effects of systemic complement depletion. Acta Neurochir. 2002;(Suppl 81):253–256. doi: 10.1007/978-3-7091-6738-0_66. [DOI] [PubMed] [Google Scholar]


