Abstract
Word-priming studies have suggested that the associative disturbance of schizophrenia may reflect aberrant spread of activation through the lexicon of the brain. To explore this, we examined lexical activation using a semantic word-priming paradigm coupled with functional magnetic resonance imaging (fMRI). We also wanted to determine whether brain activation to this paradigm correlated with relevant clinical symptom measures. In addition to completing clinical symptom measures, twelve chronic patients and twelve demographically-matched control subjects completed a lexical-decision semantic-priming paradigm developed as an event-related BOLD fMRI task. This paradigm consisted of words that differed in connectivity. Words with many connections between shared semantic associates are considered high in connectivity and produce the largest behavioral semantic priming effects in control subjects, while words with few connections between shared semantic associates are considered low in connectivity and produce a relatively smaller amount of semantic priming. In fMRI, a respective step-wise increase in activation from high connectivity to low connectivity to unrelated word pairs was expected for normal subjects. Controls showed the expected pattern of activation to word connectivity; however, patients showed a less robust pattern of activation to word connectivity. Furthermore, this aberrant response correlated with measures of Auditory Hallucinations, Distractive Speech, Illogicality, and Incoherence. The patients did not display left frontal and temporal activation as a function of the degree of word connectivity as seen in healthy controls. This may reflect a disease-related disturbance in functional connectivity of lexical activation, which in turn may be associated with clinical symptomatology.
INTRODUCTION
Bleuler (1911/1950) first suggested that a single cognitive deficit, association disturbances, underlie the majority of schizophrenia symptoms. A number of characteristics of schizophrenic speech, loose associations, indirect associations, and overly concrete or overly abstract thinking have suggested to investigators that semantic processing, and in particular, spreading activation between concepts, is abnormal in schizophrenia (e.g., Spitzer et al, 1993; Maher, 1983). Nestor et al. (1998) previously reported evidence of associational abnormalities using a cued word recall task with word pairs that varied according to an empirically derived concept called connectivity (Nelson et al., 1993). Assuming that word meanings are conceptually organized into semantically associated networks, connectivity refers to the number of connections between associate words of a particular target word (see Figure 1). Words that have many connections, (or are of high connectivity) are believed to be more salient and thus more easily recalled due to the benefit of spreading activation coalescing more focally and more strongly around the target word. Nestor et al. reported that schizophrenic subjects did not recall words according to connectivity as did control subjects, suggesting an abnormality in spreading activation within the schizophrenic semantic lexicon. We further investigated the implications of this study by developing a computer model to simulate the spreading activation patterns indicative of the schizophrenia illness (Han et al., 2003). Employing an empirically-derived database of word associations (Nelson et al., 1999), we were able to successfully simulate the word recall responses for both control and schizophrenic subjects. Furthermore, we were able to show evidence for an orderly degrading of network associational threads accounting for the behavioral response over and above random activation and disorganization (Han et al., 2003).
Figure 1.
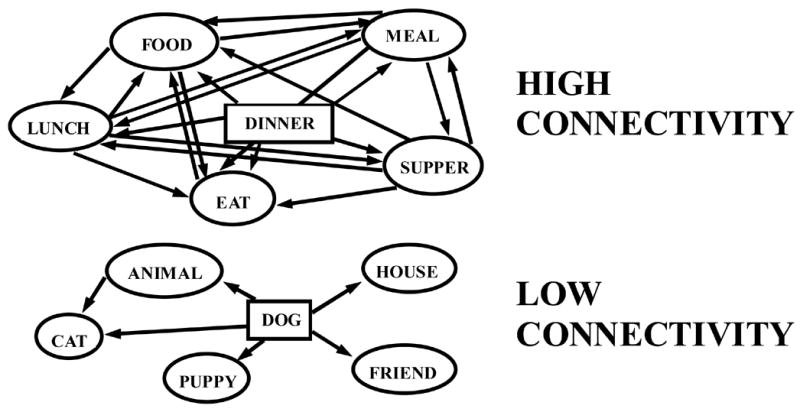
Examples of two lexical-semantic association networks that differ with respect to connectivity. The DINNER network is high in connectivity due to the greater number of associations that exist between associate words of the target word. The DOG network is low in connectivity due to the relatively fewer number of associations that exist between associate words of the target word.
A key behavioral task used to investigate this associational disturbance of schizophrenia is the semantic priming paradigm (Neely, 1991). Maher (1983), Spitzer et al. (1993), and Spitzer (1997) have suggested that the associative intrusions and derailments characteristic of schizophrenic speech might reflect, in part, an overactive semantic priming effect. Assuming for healthy individuals active associations quickly decay or are inhibited, thus preventing them from intruding into discourse, they believed that schizophrenic patients might have a disruption in the decay or inhibitory process and thus show an aberrant spread of activation through semantic networks. Because of this, schizophrenic subjects would show an even greater priming effect.
A number of researchers have provided evidence in support of this hypothesis (e.g., Moritz et al., 2001). However, a number of researchers have also provided evidence conflicting with Maher’s original hypothesis (e.g., Barch et al., 1996). A major methodological difficultly with using behavioral measures of semantic priming with schizophrenic subjects is that their reaction times are typically slower than control subjects. We reasoned that fMRI measures of brain activation during priming would provide a more direct measure of abnormalities in lexical activation.
While the controversy regarding semantic priming in schizophrenia has endured, there are a number of additional postulated methodological reasons for the contradictory findings. For example, Maher et al. (1996) found that priming effects were inversely correlated with length of illness such that patients with a longer history of disease generally showed reduced priming effects. Moritz et al. (1999) cited studies that show strong medication effects on semantic priming (Barch et al., 1996) and severe problems in processing quickly presented items among schizophrenic subjects (Spaulding et al., 1989). In a subsequent paper, Moritz et al. (2001) argued that the discrepancy in priming results may be reflective of differing levels of thought disorder, with thought disordered patients showing greater priming effects than non-thought disordered patients. Still another reason for the contradictory findings mentioned above may be due to the stimulus onset asynchrony (SOA) interval. Maher’s original hypothesis has been inferred specifically for automatic processing (Maher et al., 1987) implied with shorter SOA’s versus the controlled processes that play an increasing role with longer SOA’s. In a recently published paper, Maher et al., (2005) rated (coherent) narrative speech from schizophrenic and control subjects and found that schizophrenics had a greater number of normed associations in their speech and that this measure was correlated with the degree of facilitation in a long duration SOA semantic priming task.
In the healthy brain, functional magnetic resonance imaging (fMRI) studies have shown that semantic representations themselves may be distributed (at least in part) in right and left temporal regions (e.g., see Tranel, 2001). However, the regions linking semantic representations to their word form representations seem to be largely lateralized to the left temporal (especially middle temporal) and inferior parietal cortices (Boatman, 2004; Indefrey & Levelt, 2004). Semantic processing may also activate the inferior frontal cortex, although semantic representations are probably not stored there (Sylvester & Shimamura, 2002). Together, these findings argue for a distributed network of left frontal and temporal regions activated by word priming studies. In addition, the data have indicated decreased activation in response to priming for both visual and auditory modalities. The present study employed a lexical-decision, semantic priming paradigm with words that differed with respect to connectivity. The manipulation of connectivity was used as a probe of semantic/lexical processes. In accordance with previous priming imaging studies, we hypothesized a left-hemisphere stepwise reduction in activation from unrelated to low connectivity to high connectivity word-pairs in control subjects reflecting increased relative levels of semantic priming. In accordance with the “loose associational disturbance” of schizophrenia, we hypothesized (1) that schizophrenic subjects would show abnormal activity in left temporal/inferior parietal regions such that they would show an attenuation of the expected stepwise reduction in activation from unrelated to low connectivity to high connectivity word-pairs, and (2) that abnormal activity in the left temporal/inferior parietal regions would be correlated with clinical measures that could be related to abnormalities in semantic association such as thought disorder and symptoms of disorganization.
MATERIALS AND METHODS
Participants
Twenty-four right-handed subjects (12 chronic male schizophrenia patients and 12 controls) were recruited for the study. Patients were recruited from VA Boston HealthCare System, Brockton Campus, were medicated, and had a history of prior hospitalizations due to their symptoms. Control subjects were recruited from advertisements and group-matched to patients on age, gender, and parental socio-economic status. Inclusion criteria for both patients and controls were defined as ages 17–50; no previous history of ECT, neurological illness, alcohol or drug dependence; no present medication that would have deleterious effects on neurological and/or cognitive functioning; no hearing impairments; verbal I.Q. above 75; no alcohol use 24 hours prior to testing; and English as a first language.
All patients were diagnosed using the Structured Clinical Interview for DSM-IV (SCID-P; First et al., 1995) and chart information when applicable. Controls received a screening telephone interview, which was used to ascertain information regarding mental health, neurological illness, and developmental disabilities. All control subjects were interviewed with the SCID-NP (Spitzer et al., 1990) to rule out Axis I diagnoses, and the FH-RDC instrument (Andreasen et al., 1977) to rule out any history of mental illness in first-degree relatives. Handedness was assessed by a modified Oldfield Inventory (Oldfield, 1971), and parental SES by the Hollingshead Two-Factor Index of Social Position (Hollingshead, 1965). Clinical measures for patients included the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1981), the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) and the Positive and Negative Syndrome Scale (PANSS; Kay, Opler, and Fisbein, 1986). Clinical measures were administered at the time of entry into the study and at subsequent follow-up sessions. Chlorpromazine equivalent dosage for the two weeks previous to initial session was calculated for patients, as well as the dosage of anticholinergic medication, benzodiazepines, lithium, or novel anti-psychotic agents (Bezchilibnyk-Butler & Jefferies, 1996).
Priming Task
Sixty-four pairs of words were taken from the University of South Florida Word Association, Rhyme, and Word Fragment Norms (http://www.usf.edu/FreeAssociation) developed by Nelson, McEvoy, & Schreiber (1999). Distinctions between high and low connectivity word pairs were made in accordance with the convention outlined in Nelson et al (1993). Words were counterbalanced for the cue mean connectivity among associates, forward cue to target strength, mediated strength, overlapping associate strength, cue concreteness, target concreteness, number of syllables, number of phonemes, familiarity, set size, resonant strength, and frequency. Cue words and target words were counterbalanced according to Kucera-Francis frequency, familiarity, and concreteness. Pronounceable “non-words” were taken from Niznikiewicz et al (1997). The modality of presentation was auditory in congruence with (1) the previously described schizophrenic propensity for auditory processing abnormalities, (2) precautions against the possible confound of a grapheme-to-phoneme conversion process effect (Boatman, 2004), and (3) the successful control pilot study conducted by Wible et al. (2006) which served as a design for the current study. All words were recorded in the voice of a female using a sound editor (Sound Forge XP, Sonic Foundry, Inc. Madison, Wisconsin), and stored as wave files on a personal computer. Words were presented through sound-insulated earphones (Silent Scan, Avotec, Jensen Beach, FL) at a volume of approximately 85 db.
A lexical-decision task served as the behavioral task for the experiment. Subjects were presented with an auditory sequence of word pairs with a 650 millisecond SOA, an average of 100 milliseconds for the ISI, and an 8 second ITI, and were instructed to press a button as quickly as possible only if the second stimulus was a non-word (i.e., a word that they did not recognize as a real word). While previous investigators have identified an SOA convention to ensure automatic processing for visually presented stimuli (~ 50 ms), there is currently no precedent for an SOA range for aurally presented stimuli. The 650 millisecond SOA we employed was the shortest possible SOA before words started to conjoin or overlap. Schizophrenic subjects typically show slowed reaction times relative to controls limiting the ability to accurately assess priming-related changes in motor responses. This is a confounding factor in semantic priming studies, and while methods have been developed to reduce this confound for visual priming studies, it is more difficult to minimize with aurally presented words. Hence, we chose not to collect response data to the word-word pairs since we would not be able to interpret the results because of general response slowing. In addition, responses to word-word pairs were not recorded in order to minimize motor movement, motor slowing, and motor activation confounds. The word pairs were presented in two continuous, event-related fMRI runs. Each run contained a random mix of 24 related (12 high connectivity and 12 low connectivity) word pairs, 24 unrelated word pairs, 6 word/non-word pairs, and 12 null events (trials in which no word pair will be presented). The event-related design was used in order to be able to detect priming related decreases to individual word pairs. In addition, the blocked presentation of similar word pairs would result in possible strategic effects in addition to automatic spreading activation.1
fMRI
All images were acquired at the Brigham and Women’s Hospital (BWH) using a GE 3.0 Tesla Signa System with MR Image Acquisition Parameters, a HORIZON hardware/software package (GE Medical Systems, Milwaukee, WI).
Low-resolution anatomical images were acquired following initial sagittal localizer scans. They were spoiled-gradient-recalled (SPGR) images that were scanned in the same location and plane orientation as the functional images and using the same slice thickness. The low-resolution 3D SPGR images were acquired and reformatted into 6 mm contiguous (coronal) oblique slices. The protocol has the parameters TE = 3 msec, TR = 40 msec, 1 repetition, nutation angle = 45 degrees, FOV = 24 cm, and matrix = 256 X 192. The images were acquired perpendicular to the line of the left superior temporal sulcus. The images were prescribed to include most of the frontal lobe in addition to the areas of interest.
Functional images were acquired in a continuous manner using the EPIBOLD pulse sequence from GE. This gradient-echo, echo-planar sequence was used to acquire 28 contiguous 5 mm coronal slices of the whole brain with the parameters TE = 30 msec, TR = 2.5 sec, FOV = 24 cm, image resolution = 64 X 64, and in-plane voxel edge = 3.75 mm. Functional images were reconstructed on SUN workstations after scanning. Subsequent to reconstruction of the raw data into images, the first five images were taken and discarded (not used in the analyses) for all protocols due to initial recording burst.
High-resolution SPGR anatomical images with 124 1.5 mm thick coronal slices were acquired in a separate session and used for anatomical localization and 3D visualization of the activation. The parameters for the SPGR were TE = 3 ms, TR = 40 ms, FOV = 24 cm, acquisition matrix = 256 X 256, and voxel dimensions = 0.975 mm X 0.975 X 1.5 mm.
Functional data were analyzed using the SPM99 software package (Wellcome Department of Cognitive Neurology, London, UC). Each subject’s fMRI images were realigned to the first functional image in order to correct for head movement between scans. All images were spatially normalized into standard space using nonlinear three-dimensional transformations. Each image was then smoothed using an isotropic Gaussian kernel of 10 mm FWHM to accommodate differences in anatomy between subjects. The general linear model was used to estimate condition effects at each voxel in brain space with global intensity normalization. Linear contrasts were used to test hypotheses regarding conditions and groups, which produced a statistical parametric map of the t statistic generated for each voxel (SPM{t}). This map was then transformed into a map of corresponding Z values according to a threshold of 3.17 (p=0.001), and the resulting foci was characterized in terms of both spatial extend and peak height. For the group effect estimates, the resulting contrast images from all of the subjects were used for secondary statistical analyses that employed separate t tests for each condition across the subjects. This procedure again produced a statistical parametric map of the t statistic generated for each voxel (SPM{t}), and corresponding Z values with a threshold of 7.17 (p=0.001).
RESULTS
Behavioral
Both schizophrenic and control subjects were able to comprehend and complete the semantic priming paradigm without difficulty, and no subject was disqualified due to practice trial failure. Control subjects completed the priming paradigm with an accuracy rate of 94.5% (s.d. = 4.8), and schizophrenic subjects completed it with an accuracy rate of 93.1% (s.d. = 4.9). Accuracy data reflects both false alarms and correct rejections. Control subject accuracy performance did not significantly differ from schizophrenic subject performance (t=1.433, p=0.16). Reaction time to nonwords was 1.47 seconds (s.d. = 0.79) for schizophrenic subjects and 1.22 seconds (s.d. = 0.47) for control subjects, yielding a significant difference between groups according to a Mann-Whitney nonparametric analysis (z=−2.96, p=0.00).
fMRI
For the high connectivity condition, control subjects displayed within-group left hemisphere activity in and near the middle to superior temporal (x=−61, y=−14, z=−4, z=4.94) and the inferior frontal (x=−52, y=40, z=10, z=3.35) gyri, while schizophrenic subjects displayed activity near the middle to superior temporal gyri (x=−46, y=−28, z=−2, z=6.04). For the low connectivity condition, control subjects displayed greater left hemisphere activity again near the middle to superior temporal (x=−44, y=−34, z=6, z=5.41) and the inferior frontal (x=−46, y=36, z=4, z=3.75) gyri, while schizophrenic subjects displayed activity near the middle to superior temporal region (x=−60, y=−14, z=−4, z=5.87). For the unrelated condition, control subjects displayed even greater left hemisphere activity in the middle to superior temporal (x=−52, y=−20, z=2, z=5.07) and inferior frontal (x=−52, y=38, z=12, z=4.16) gyri, while schizophrenic subjects also displayed greater activity in the middle to superior temporal (z=−52, y=−22, z=−2, z=5.62) and inferior frontal (x=−44, y=28, z=14, z=4.09) gyri.
A linear regression analysis was applied to the current data set to more appropriately capture the expected step-wise reduction in activation according to word pair connectivity level. Covariates were entered as high connectivity = 1, low connectivity = 2, and unrelated word pairs = 3. Results revealed robust areas of activation in left middle to superior temporal (x=−62, y=−16, z=2, z=5.44) and inferior to middle frontal (x=−36, y=14, z=32, z=5.36) gyri for the control group, and less pronounced patterns of activation in these same areas (temporal: x=−48, y=−38, z=4, z=4.69; frontal: x=−44, y=28, z=12, z=4.43) for the schizophrenic group. Furthermore, between-group analyses of this regression model (here forward referred to as the high < low < unrelated regression or “HLU” abbreviated) revealed multiple areas of stronger left hemisphere activation in the middle to superior temporal (x=−46, y=−36, z=4, z=5.00) and middle to inferior frontal (x=−51, y=34, z=20, z=4.53) regions for the control group. No stronger areas of activation were indicated for the schizophrenic group.2
Correlation analyses were conducted using SPM between the HLU regression and various schizophrenic clinical global measures and single item responses. We hypothesized that measures of conceptual disorganization and thought disorder (as measured by the SAPS) would be correlated with abnormal activity in lateral temporal regions. We based these predictions on knowledge of functional anatomy and also on past findings correlating positive SAPS measures with fMRI activation and volumes (Kubicki et al., 2003; Wible et al., 1995). The correlation reflects a whole-brain regression analysis using SPM with the SAPS scores entered as a covariate. This analysis was done using the standard SPM tools and the same procedure has been used in other papers (e.g., Weinstein et al., 2006). Activation was observed in the left posterior middle temporal region for Distractive Speech (x=−46, y=−50, z=0, z=3.73), Illogicality (x=−42, y=−48, z=2, z=3.93), and Incoherence (x=−54, y=−50, z=−2, z=3.96; see figure 3). A small area of activation was also observed in the inferior frontal region (x=−40, y=36, z=2, z=3.73) for Distractive Speech. We also performed exploratory correlations with clinical measures of hallucinations and found significant areas of left-hemisphere activation in Broca’s area (x=−44, y=22, z=36, z=3.82), the inferior parietal region (x=−34, y=−36, z=34, z=4.13), and the middle temporal gyrus (x=−62, y=−66, z=8, z=3.27).
Figure 3.
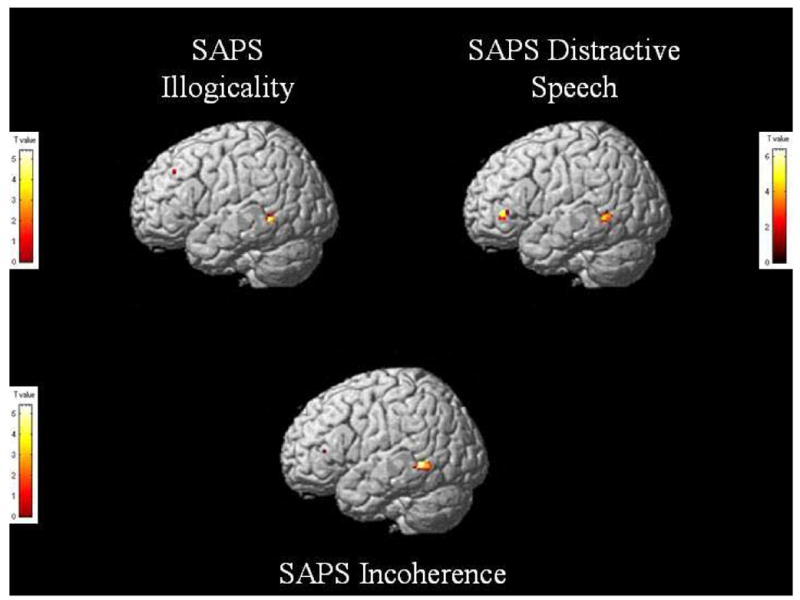
Significant areas of activation after a regression analysis of the high connectivity < low connectivity < unrelated contrast with SAPS Illogicality, Incoherence, and Distractive Speech in schizophrenic subjects. T value threshold at p=0.001, uncorrected.
Areas of activation were also observed in homologous portions of the right hemisphere (middle frontal gyrus: x=46, y=28, z=30, z=3.21; the inferior parietal region: x=48, y=−36, z=52, z=4.02; the middle temporal gyrus: x=58, y=−50, z=12, z=3.55). Other exploratory correlations with SANS and PANNS measures did not show significant activations. Incidentally, we have never found PANSS symptom scores to correlate with either fMRI or volume measures in our previous studies.
DISCUSSION
As expected, within-group BOLD activation reductions were observed in left temporal and frontal regions according to the connectivity level of the word pairs for the control subjects. That is, the most left hemisphere activation was observed for unrelated word pairs, slightly less activation was observed for low connectivity word pairs, and the least activation was observed for the high connectivity word pairs. As was noted in Weatley et al. (2005), the methods and findings of the published fMRI semantic priming papers are diverse and difficult to summarize. Many studies have found priming related decreases in the same regions as shown in our studies, however, some studies have shown semantic priming related increases in activity (e.g.. Rossell et al., 2003; Kotz et al., 2002). As was found in our study, the majority of semantic priming studies have found semantic priming related reductions in at least some portion of the middle temporal gyrus (Copeland et al., 2003; Giesbrecht et al., 2004; Rissman et al., 2003; Rossell et al., 2003; Kotz et al., 2002 and Gold et al., 2006) and of the inferior frontal region (Copeland et al., 2003; Giesbrecht et al., 2004; Kotz et al., 2002; Matsumo et al, 2005; Wheatley et al., 2005). In fact, the one region that showed activity related to both long and short SOA priming in Gold et al., (a portion of the posterior middle temporal gyrus) was neuranatomically very close to the region showing correlations with thought disorder in the current study. However, a number of other regions not found in the current study were also described to have shown priming effects in some studies. These include the superior temporal gyrus (Rissman et al., 2003; Kotz et al., 2002; Matsumo et al, 2005) and the posterior fusiform gyrus in studies using visual words (Wheatley et al., 2005; Gold et al., 2006).
Schizophrenic subjects displayed less clear within-group activation reductions in response to word pair connectivity in left frontal and temporal regions. These fMRI data suggested that the schizophrenic group did not process word connectivity relationships in the same manner as their demographically-matched control counterparts; showing a relatively less robust HLU regression activation pattern among language-specific left temporal and frontal regions. Given the strong empirical support for word connectivity as a parameter sensitive to the spreading of activation through lexical-semantic association networks broadly distributed across these areas (Nelson et al., 1993; Nestor et al., 1998; Han et al., 2003; Wible et al, in press), the current results suggest either a disease-related disturbance in lexical-semantic network organization, a disruption of activation through interrelated concepts, or a combination of these possibilities.
Warrington (1975) presented a hierarchical model of semantic knowledge and suggested that subordinate categorical information may be more sensitive to damage than superordinate information. Extending this understanding to the current neurobiological correlate analysis of word connectivity processing among schizophrenia patients, Bleuler’s original hypothesis of a disease-related associational breakdown is manifested either in an apparent degradation of highly associated lexical-semantic networks or “superconnectivity” of lowly associated networks such that differences between high and low connectivity networks become less salient and in effect produce a reduced discriminatory response. This coupled with a slight increase in network instability (Han et al., 2003) may serve as a foundational mechanism for specific thought disorder and speech pathology symptoms of the illness.
With regard to the semantic priming controversy in the schizophrenia literature, it is reasonable to theorize that a relatively more robust patient-specific HLU regressed BOLD signal might have been indicative of hyperpriming and a relatively less robust HLU regression signal might be indicative of hypopriming. The length of illness of the present population of patients is relatively long. According to Maher et al. (1996), a shorter length of illness (less than 15 years) should produce greater hyperpriming effects and a longer length of illness (greater than 15 years) should produce greater hypopriming effects. Although this initial assessment of the neurobiological correlates underlying a priming task may support the notion of hypopriming due to the apparent patient-specific decrease in signal affiliated with the HLU regression, further research will be needed to fully evaluate the behavioral findings with regard to semantic priming in schizophrenic subjects. For example, testing first episode patients in this priming paradigm would aid in evaluating the present set of findings in view of Maher et al.’s contribution. The SOA used in the present study was minimized (within the confines of the auditory fMRI priming paradigm) in order to maximize automatic processing of the word pairs (and protect against control processes confounding the results). Recent behavioral studies have shown that for semantic priming using auditory words, very short SOAs may not fully capture the priming phenomenon (Anderson et al., 1995). In addition, there is a trade-off between the ability to differentiate the response to the prime and target and the SOA: the shorter the SOA, the more likely it is that there is an overlap of hemodynamic response to the two words. Further applications of the current paradigm with variations in SOA would also provide important contributions to the semantic priming debate.
Of significant note are the set of clinical correlation findings. Though they must be considered exploratory due to multiple comparisons conducted, the correlations found in the current paper are consistent with those from a broad range of other methodologies, providing strong converging evidence for the association between brain abnormalities and symptoms. The regions showing abnormal activity also have functions in normal individuals that are very consistent with their role in clinical symptoms.
The HLU contrast should theoretically show activation from regions that were modulated by semantic priming. Regions subserving phonological word forms and lexical-semantic representations should be represented in this contrast. Measures of thought disorder such as distractive speech, incoherence, and illogicality correlated significantly with activation of the middle left temporal cortex within the HLU contrast. These findings are strikingly similar to findings reported using a completely different methodology from the current paper. In one report, Kirchner et al. (2001) used fMRI to measure activation while subjects spoke about visually presented Rorschach ink-blots. The left posterior middle temporal and posterior superior temporal gyrus activity showed an inverse correlation with the amount of thought disorder produced in schizophrenic subjects. The middle temporal region found to be abnormal in Kircher et al.’s study was almost identical to the region found to be correlated with measures of thought disorder in the current study. Distractive speech, in addition to correlating with abnormal activation in the middle temporal region, correlated with a small region of left inferior prefrontal cortex. This region (~BA 47) is thought to subserve working memory for semantic features and thematic structure, and may be used to re-analyze and repair utterance (Dronkers et al., 2004). Abnormal activity in this region might impair the ability of patients to use thematic structure and context to constrain their utterances.
Taken together, the lines of evidence converge to implicate abnormal activity in the left posterior middle/superior temporal region in producing thought disorder. Previous studies from our laboratory also found that the posterior superior temporal region showed decreased volume that correlated with thought disorder (Shenton et al., 1992) and abnormal fMRI activity in response to visual words that correlated with levels of positive symptoms in schizophrenic subjects (Kubicki et al, 2003). The middle temporal gyrus has been shown in lesion, stimulation, and neuroimaging studies to be the neural substrate for semantic comprehension at the single word level (Indefry & Levelt, 2000; Boatman, 2004; Bates et al., 2003; Dronkers et al, 2004; Hart & Gordon, 1990). For example, in a study of 101 patients with left hemisphere damage, comprehension was most affected by damage to the middle temporal gyrus (Bates et al., 2003; and see also Dronkers et al., 2004). This region contains either semantic representations or the link between semantic representations and lexical representations. These findings are thus consistent with Bleuler’s “loose association” hypothesis, where a breakdown in lexical-semantic associative relationships in the brain contributes to formal thought disorder.
Lifetime measures of auditory hallucinations were associated with abnormal activity in left hemisphere regions corresponding to Broca’s area, SMG, the posterior middle/superior temporal lobe, and homologous regions in the right hemisphere. Again, there is a remarkable correspondence with findings in the literature. Several papers have reported abnormalities in subsets of these regions using a variety of techniques (Gaser et al., 2004; Lennox et al., 1999; Shergill et al., 2003; Sibersweig et al., 1995; Stephane et al., 2001; Weiss & Heckers, 1999). This includes a report from our laboratory showing that volumes of the left middle temporal gyrus were correlated with levels of auditory hallucinations (Onitsuka et al., 2004). In another report, functional imaging was performed on a patient actively hallucinating (Lennox et al., 1999). This patient showed abnormal activity during hallucinations in the right posterior middle temporal and inferior parietal regions that were also found to be abnormal in the current study. Diffusion tensor imaging techniques have also found abnormalities in the lateral part of the temporoparietal section of the arcuate fasciculus which presumably connects temporal, inferior parietal and frontal speech production regions (Hubl et al., 2004).
The posterior part of the middle and superior temporal gyri (especially in the left), the left inferior parietal region, and Broca’s area are key components in a system for the production and monitoring of internally generated speech (Indefrey and Levelt, 2004). The present study provides converging evidence that abnormalities in the speech production system could underlie auditory hallucinations in schizophrenia. Sound based speech representations are thought to be processed in bilateral posterior superior temporal regions and then progress to an auditory-conceptual interface in the temporal-occipital-parietal junction of the left hemisphere that connects sound based speech representations with distributed semantic representations (Hickok and Poeppel, 2000). As discussed above, the middle temporal region is thought to be involved in the storage of representations that link semantic information with modality specific word forms (Hart & Gordon, 1990, Boatman, 2004; Binder et al., 1997; Damasio et al., 1996). The inferior parietal region, when damaged, results in deficits in auditory short- term memory (Dronkers et al., 2004), and Broca’s area is involved in the articulation of covert and overt speech (Indefrey and Levelt, 2004).
It is of note that our study as well as several others found that the right hemisphere analogs of these speech production regions also showed abnormal activity. A recent study of normal individuals found that the right posterior superior temporal sulcus displayed stronger responses to non-familiar than to familiar voices (Kriegstein & Giruad, 2004). Perhaps the right-sided activity contributes to the attribution of covert speech to an outside source in schizophrenic patients.
Although we have found evidence for abnormalities in those regions subserving lexical/semantic representation, this study is limited with regard to inferences about the exact nature of the abnormalities. Semantic priming related reductions in activity are presumably a result of both the prime and target words activating a similar set of associate concepts/lexical items. However, if there was overactivation of associates where the overactivated items differed to some extent for the prime and target, then one might actually predict increased fMRI activity with this type of overactivation (instead of the straightforward prediction of increased priming leading to decreased fMRI activity). Similar problems of interpretation hold for hypopriming such that predictions of increases or decreases in activity depend on the mechanisms underlying hypopriming. Consequently, behavioral measures of priming may not be reflected in fMRI activity in a straightforward way. However, relatively direct inferences can be made about what stages of lexical/semantic processing are abnormal by using information about that region’s normal role in processing, and these inferences do not have some of the limitations of behavioral measures for which inferences are made on the basis of response times.
In summary, the finding of a correlation between positive symptoms and activation in the posterior lateral temporal region is consistent with theories postulating abnormalities in lexical-semantic networks in schizophrenia. The current findings provide converging evidence for the role of abnormalities of the posterior superior and lateral temporal regions in producing positive symptoms in schizophrenia. At the psychological level, these findings point to abnormalities in lexical-semantic selection and activation. A recent paper presented evidence that schizophrenic subjects were deficient in identifying words with high lexical competition or words that were likely to be co-activated with a number of irrelevant words. This deficiency was associated with the levels of thought disorder and auditory hallucinations (Titone & Levy, 2004). That study, together with our present findings, implicates lexical activation as being abnormal in schizophrenia.
Supplementary Material
Figure 2.
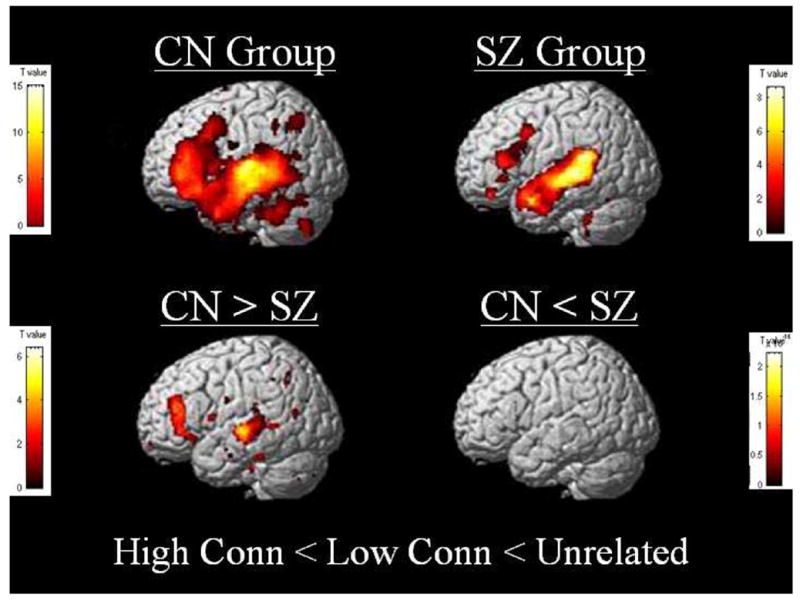
Within-group and between-group (second level) activation differences regressed according to word connectivity in left temporal and frontal areas for control and schizophrenic subjects. Control subjects showed multiple areas of greater activation regressed according to word connectivity compared to schizophrenia patients. T value threshold at p=0.001, uncorrected.
Figure 4.
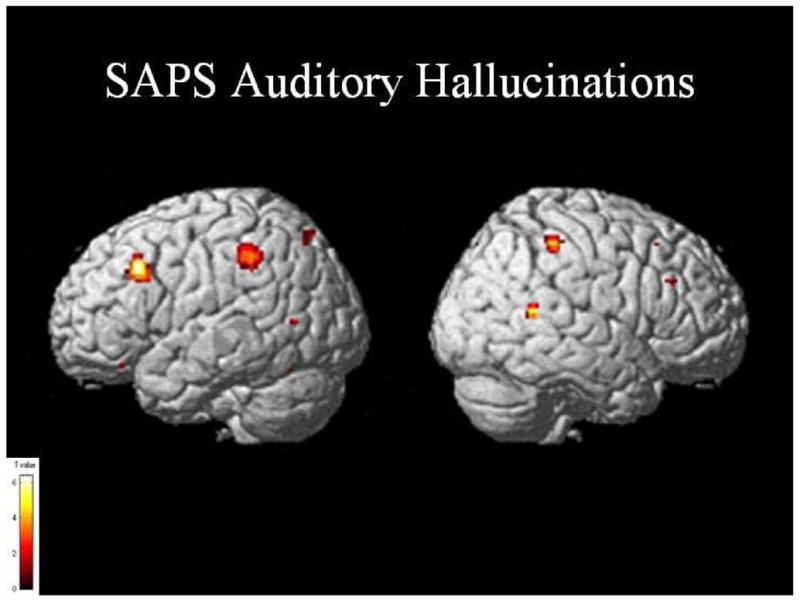
Significant areas of activation after a regression analysis of the high connectivity < low connectivity < unrelated contrast with SAPS Auditory Hallucinations in schizophrenic subjects. T value threshold at p=0.001, uncorrected.
Table 1.
Demographic Information
| Schizophrenic Subjects | Control Subjects | |
|---|---|---|
| Age | 39.75 +/− 11.89 | 45.5 +/− 6.47 |
| Education | 13.58 +/− 1.98 | 14.73 +/− 2.49 |
| Socioeconomic Status (SES) | 2.70 +/− 0.95 | 3.36 +/− 1.36 |
| Handedness | 0.72 +/− 0.29 | 0.77 +/− 0.27 |
| Mini-Mental Status Score | 28.75 +/− 1.48 | 29.64 +/− 0.67 |
| Length of Illness | 16.92 +/− 11.25 | |
| Age at Illness Onset | 22.83 +/− 5.49 | |
| Chlorpromazine equivalent of neuroleptic dose | 289.73 +/− 214.02 |
Demographic information for control and schizophrenic participant groups. Groups did not significantly differ according to years of education (t=1.23, p=0.23), parents’ socioeconomic status (t=1.28, p=0.22), age (t=1.50, p=0.15), or mini-mental status exam score (t=1.97, p=0.08). No other significant differences existed between groups for any of the other demographics. All patients were medicated with atypical antipsychotics with the exception of one patient who was medicated with a traditional antipsychotic.
Acknowledgments
This work was supported by the Veterans Administration REAP (RWM), with partial support to the primary author (SDH) by the Stanley Foundation through preceptor (CGW) and Department of Veterans Affairs Merit Awards (MN, PGN, RWM). This work was also supported by an NIMH grant: 1 R01 MH067080-01A2 (CGW). Portions of the results were previously presented at the Society for Biological Psychiatry 60th Annual Meeting in Atlanta, GA, on 5/21/05, and the International Neuropsychological Society 33rd Annual Meeting in St. Louis, MO, on 2/3/05.
Footnotes
In order to assess whether the priming paradigm utilized in this analysis elicited an appropriate behavioral priming response in control subjects, a separate set of 10 young control subjects were administered a reaction-time version of the priming paradigm. Results revealed an expected step-wise increase in mean response latencies for high connectivity, low connectivity, and unrelated word pairs sequentially, as indicated through a repeated measures linear contrast test (F=76.815; df=1; p<0.001); thus validating the use of the current paradigm.
Separate contrasts of high < low and low < unrelated were conducted to clarify and support the present results and are presented as Supplementary Material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JE, Holcomb PJ. Auditory and visual semantic priming using different stimulus onset asynchronies: An event-related brain potential study. Psychophysiology. 1995;32:177–190. doi: 10.1111/j.1469-8986.1995.tb03310.x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa College of Medicine; 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa College of Medicine; 1984. [Google Scholar]
- Barch DM, Servan-Schreiber D, Steingard S, Cohen JD, Steinhauer SS, van Kammen DP. Semantic priming in schizophrenia: An examination of spreading activation using word pronunciation and multiple SOAs. Journal of Abnormal Psychology. 1996;105(4):592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bezchilibnyk-Butler KZ, Jefferies JJ. Clinical handbook of psychotropic drugs. 6. Kirkland, WA: Hogrefe & Huber Publishers; 1996. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Preito T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox of the group of schizophrenias. New York: International University Press; 1911. 1950. [Google Scholar]
- Boatman D. Cortical bases of speech perception: evidence from functional lesion studies. Cognition. 2004;92:47–65. doi: 10.1016/j.cognition.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ. Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage. 2003;20:302–10. doi: 10.1016/s1053-8119(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Refern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P), Version 2.0. 722 W. 168th Street, New York, NY 10032: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Gaser C, Nedadic I, Volz HP, Buchel C, Sauer H. Neuranatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14:91–96. doi: 10.1093/cercor/bhg107. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Camblin CC, Swaab TY. Separable effects of semantic priming and imaginability on word processing in human cortex. Cerebral Cortex. 2004;14:521–529. doi: 10.1093/cercor/bhh014. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Anderson AH. Dissociation of Automatic and Strategic Lexical-Semantics: FMRI Evidence for Differing Roles of Multiple Frontotemporal Regions. The Journal of Neuroscience. 2006;26(24):6523– 6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Nestor PG, Shenton ME, Niznikiewicz M, Hannah G, McCarley R. Associative memory in chronic schizophrenia: A computational model. Schizophrenia Research. 2003;61(2–3):255–263. doi: 10.1016/s0920-9964(02)00289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a Functional Anatomy of Speech Perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Annals of Neurology. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Status. New Haven, CT: Yale Unversity Press; 1965. [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Indefry P, Levelt WJM. The neural correlates of language production. In: Ganzaniga MS, editor. The new cognitive neurosciences. 2. Cambridge, MA: MIT Press; 2000. pp. 845–865. [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(12):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. PANSS +/− Manual. North Tonawanda, NY: Multi-Health Systems, Inc.; 1986. [Google Scholar]
- Kirchner TTJ, Liddle PF, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Neural correlates of formal thought disorder in schizophrenia. Arch Gen Psychiatry. 2001;58:769–774. doi: 10.1001/archpsyc.58.8.769. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: an event-related functional MRI study. Neuroimage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Kriegstein KV, Giraud AL. Distinct functional substrates along the right superior temporal sulcus for the processing of voices. Neuroimage. 2004;22:948–955. doi: 10.1016/j.neuroimage.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG. An fMRI study of semantic processing in men with schizophrenia. Neuroimage. 2003;20:1923–1933. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Park SB, Jones PB, Morris PG. Spatial and temporal mapping of neural activity associated with auditory hallucinations. Lancet. 1999;353:644. doi: 10.1016/s0140-6736(98)05923-6. [DOI] [PubMed] [Google Scholar]
- Maher B, Manschrek TC, Hoover TM, Weisstein CC. Thought disorder and measured features of language production. In: Harvey PD, Walker, editors. Positive and negative symptoms in psychosis. Erlbaum; Hillsdale, NJ: 1987. pp. 195–215. [Google Scholar]
- Maher BA. A tentative theory of schizophrenic utterance. In: Maher BA, Maher WB, editors. Progress in experimental personality research: Psychopathology. Vol. 12. New York: Academic Press; 1983. pp. 1–52. [PubMed] [Google Scholar]
- Maher BA, Manschreck TC, Linnet J, Candela S. Quatitative assessment of the frequency of normal associations in the utterances of schizophrenia patients and healthy controls. Schizophrenia Research. 2005;78(2–3):219–224. doi: 10.1016/j.schres.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Moritz S, Andresen B, Domin F, Martin T, Probsthein E, Kretschmer G, Krausz M, Naber D, Spitzer M. Increased automatic spreading activation in healthy subjects with elevated scores in a scale assessing schizophrenic language disturbances. Psychological Medicine. 1999;29(1):161–170. doi: 10.1017/s0033291798007831. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, Kuppers D, Lausen A, Schickel M. Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophrenia Research. 2001;59:181–186. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: A selective review of current findings and theories. In: Besner D, Humphreys G, editors. Basic processes in reading: Visual word recognition. 1991. pp. 264–336. [Google Scholar]
- Nelson DL, Bennett DJ, Gee NR, Schreiber TA, McKinney VM. Implicit memory: Effects of network size and interconnectivity on cued recall. Journal of Experimental Psychology. 1993;19(4):747–764. doi: 10.1037//0278-7393.19.4.747. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. [Accessed October 1, 1999];The University of South Florida word association, rhyme, and word fragment norms. doi: 10.3758/bf03195588. Available at: http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW. Word recall in schizophrenia: A connectionist model. American Journal of Psychiatry. 1998;155(12):1685–1689. doi: 10.1176/ajp.155.12.1685. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz M, O’Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW. ERP assessment of visual and auditory language processing in schizophrenia. Journal of Abnormal Psychology. 1997;106:84–85. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. American Journal of Psychiatry. 2004;161(9):1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE. An event-related FMRI investigation of implicit semantic priming. Journal of Cognitive Neuroscience. 2003;15(8):1160–75. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: A quantitative magnetic resonance imaging study. New England Journal of Medicine. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry. 2003;182:525–531. doi: 10.1192/bjp.182.6.525. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill D, Holmes A, Crootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Spaulding W, Garbin CP, Dras SR. Cognitive abnormalities in schizophrenic patients and schizotypal college students. Journal of Nervous and Mental Disease. 1989;177:717–728. doi: 10.1097/00005053-198912000-00002. [DOI] [PubMed] [Google Scholar]
- Spitzer M. A cognitive neuroscience view of schizophrenic thought disorder. Schizophrenia Bulletin. 1997;23:29–50. doi: 10.1093/schbul/23.1.29. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: Direct evidence from indirect semantic priming. Biological Psychiatry. 1993;34:864–877. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R – Patient Version 1.0 (SCID-P) Washington, D.C.: American Psychiatric Press; 1990. [Google Scholar]
- Stephane M, Polis I, Barton SN. A subtype of auditory verbal hallucinations responds to fluvoxamine. J Neuropsychiatry Clin Neurosci. 2001;13:425–427. doi: 10.1176/jnp.13.3.425. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Shimamura AP. Evidence for intact semantic representations in patients with frontal lobe lesions. Neuropsychology. 2002;16:197–207. [PubMed] [Google Scholar]
- Titone D, Levy DL. Lexical competition and spoken word identification in schizophrenia. Schizophrenia Research. 2004;68:75–85. doi: 10.1016/S0920-9964(03)00212-3. [DOI] [PubMed] [Google Scholar]
- Tranel D. Combs, ducks, and the brain. Lancet. 2001;357:1818–9. doi: 10.1016/S0140-6736(00)05010-8. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wheatly T, Beauchamp M, Weisberg J, Martin, A Automatic Priming of Semantically Related Words Reduces Activity in the Fusiform Gyrus. Journal of Cognitive Neuroscience. 2005;17(12):1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- Weinstein S, Werker JF, Vouloumanos A, Woodward TS, Ngan ETC. Do you hear what I hear? Neural correlates of thought disorder during listening to speech in schizophrenia. Schizophrenia Research. 2006 doi: 10.1016/j.schres.2006.05.011. in press. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of hallucinations: a review of the literature. Psychiatry Res. 1999;92:61–74. doi: 10.1016/s0925-4927(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Kubicki M, Spenser MH, Niznikiewicz MH, Jolesz FA, McCarley RW, Nestor PG. Connectivity between semantic associates: An fMRI study of semantic priming. Brain and Language. 2006 doi: 10.1016/j.bandl.2005.11.006. in press. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW. Prefrontal cortex and schizophrenia: A quantitative MR study. Archives of General Psychiatry. 1995;52:279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


