Abstract
Objectives
To describe developmentally appropriate, specific body movements and other biobehavioral responses of preterm infants to a group of routine care giving tasks (Clustered Care), and to compare responses to acute pain with those of Clustered Care.
Methods
In a randomized design, 54 preterm infants were assessed at 32 weeks gestational age during 3 phases of blood collection (Baseline, Lance/squeeze, Recovery) and of diaper changing, measuring abdominal girth and axillary temperature, and mouth care (Baseline, Clustered Care, Recovery) in a neonatal intensive care unit. The Newborn Individualized Developmental Care and Assessment Program and 1 facial action from the Neonatal Facial Coding System, Brow Bulge, were coded from separate continuous bedside video recordings. Heart rate and oxygen saturation were also acquired continuously.
Results
Brow Bulge, heart rate, and a subset of 9 Newborn Individualized Developmental Care and Assessment Program movements increased and oxygen saturation decreased significantly to Lance/squeeze compared to Baseline. Similar facial and physiological changes occurred during Clustered Care, but with less intensity. However, infants showed greater frequencies and variety of Newborn Individualized Developmental Care and Assessment Program stress cues during Clustered Care than during Lance/squeeze. Stress cues persisted after Clustered Care, whereas the infants returned to Baseline following Lance/squeeze.
Discussion
Changes in facial activity and heart rate remain the most sensitive markers of pain in preterm infants. Tactile procedures, such as diaper changing, produce lower intensity facial and physiological responses than pain procedures, but greater body reactions. Also, the effects from tactile procedures appear to last longer. Adding observations of a small number of specific body movements to the assessment of pain and stress provides complementary information particularly for those infants who may show dampened facial reactivity as a result of repeated pain exposure.
Keywords: preterm infant, pain, NIDCAP®, NICU, stress
Preterm infants are exposed to multiple stressors such as unpredictable handling patterns and repeated painful procedures. Some hypothesize that chronic exposure to stressors such as these may alter brain development in very preterm infants.1,2 Indeed, in response to concerns with respect to difficulties in identifying and treating pain in preterm infants, considerable research in pain assessment has been carried out in recent years. Currently, researchers argue that multidimensional assessment of pain using both behavioral and physiological indicators is essential because different parameters provide different information.3 The most promising of these indicators in infants >28 weeks gestational age are changes in facial activity, shifts in infant sleep/waking state, and physiologic indices of heart rate and oxygen saturation. In addition to changes in facial activity and shifts in sleep/waking states, recent studies have shown that some specific body movements, described in the Newborn Individualized Developmental Care and Assessment Program (NIDCAP®), are associated with acute pain responses in preterm infants.4,5
One of the difficulties faced when assessing pain in preterm infants in the neonatal intensive care unit (NICU) is that the infants’ responses to pain and stress are nonspecific and can be misinterpreted. Although both stress and pain activate the autonomic nervous system and the hypothalamic-pituitary-adrenal axis, the continuum of tactile and invasive stimuli has cumulative effects. Some have suggested that distinguishing between pain and stress is not clinically relevant.6,7 However, appropriate management would differ depending on whether pain was present or not. Although not all tactile events produce deleterious neuroendocrinological responses,8 recent evidence suggests that nonpainful stimulation received during routine NICU care may be more stressful for preterm infants than painful interventions.9 Moreover, tactile events may become more stressful because preterm infants exhibit not only primary and secondary hyperalgesia, but also allodynia (pain arising from previously innocuous stimulation) as a result of central sensitization.10,11 Further, pharmacological interventions used for pain management may act differently if pain is or is not present.12 Conversely, sedatives often do not act specifically as analgesics. Using them when pain is present is inappropriate, because the detrimental physiological side effects of pain would not be controlled. Administering analgesics only when pain is present may be critical for preventing unwanted long-term side effects of opioid use.
Compared with the numbers of studies evaluating preterm infants’ responses to pain, few studies have provided detailed descriptions of preterm infants’ behavioral and physiological responses to nonpainful, but potentially stressful, care-giving tasks in the NICU.13–16 One study used diaper changing as a nonpainful event with which to evaluate construct validity of the Premature Infant Pain Profile (PIPP).17 These researchers reported lower pain scores during the nonpainful handling than with heel lance. However, when Blauer and Gerstmann used diapering as a “nonpainful” event with which to compare 3 infant pain scales, 2 of the scales rated diaper changing as more “painful” than endotracheal suctioning.18 These authors concluded that this finding represented a lack of specificity of the scales for measuring pain. More recently, Hellerud and Storm showed that diaper changing produced greater physiological changes than did heel lance in preterm infants.9 But the difficulty with using pain scales to measure infants’ more generalized stress response is that they may miss salient stress cues not associated with pain.
Studies using the NIDCAP® to evaluate the effects of routine care giving in the NICU are not only few in number, but have pooled all handling to make general comments regarding the infants’ responses,14 have not included the full range of NIDCAP® behaviors,19 have not specified the procedures observed in the study,20 or have not included diaper changing as one of the procedures being evaluated.13,15 Thus, the aims of this study are the following:
To describe, in detail, biobehavioral responses of preterm infants to a routine cluster of caregiving tasks (including diaper changing, measuring abdominal girth by placing a tape measure around the abdomen, mouth care, and taking an axillary temperature [Clustered Care])
To determine whether specific behaviors distinguish pain from tactile responses.
Because stress and pain reactions are on a continuum, for this study, stress will be defined as a reaction induced by tactile stimulation of noninvasive procedural handling; pain will be defined as a more severe form of stress that is associated with a tissue-breaking procedure.
MATERIALS AND METHODS
Participants
The study sample comprised 54 preterm neonates (24 female, 30 male) born at ≤32 completed weeks gestational age, in a major regional level III NICU at the Children’s & Women’s Health Center of British Columbia, Vancouver, Canada. Infants with a major congenital anomaly, significant intraventricular hemorrhage (IVH Grade III), and/or parenchymal brain injury (IVH Grade IV and/or periventricular leukomalacia [PVL]), as well as infants who had received analgesics or sedatives within 72 hours of the targeted study session, were excluded. All infants were 32 weeks postconceptional age (±7 days) at time of the study. Forty-four infants were appropriate for gestational age, 8 were small for gestational age, and 2 were large for gestational age. Sample size estimates were calculated as though we were using a between-groups design. This method provides a conservative estimate given that we used a repeated-measures design. GPOWER21 was used to calculate the estimate, and effect sizes entered into the program were based on differences in the Neonatal Facial Coding System (NFCS) scores in term infants between a painful and nonpainful event.22 Using this method, 16 infants were needed to detect differences between each phase for a power of 0.95 with the statistical significance set at 0.05 with a large effect size (1.23).
Procedures
The infants were recruited by an NICU research nurse, and written informed consent was obtained from the mother according to a protocol approved by the Clinical Research Ethics Board of the University of British Columbia. Videotaping and physiologic recording were carried out continuously, and methods are reported elsewhere.4 A single research nurse carried out clustered nursing procedures (Clustered Care) in a set order: changing the diaper, measuring girth, taking the axillary temperature, and cleaning the mouth with gauze and sterile water. Blood collection (Pain) following heel warming was carried out by a laboratory technician who cleansed the heel, applied a lancet, and squeezed the heel to collect blood. Each infant was tested on 2 separate occasions, these being no more than 13 days apart. Assignment to Pain versus Clustered Care as the first procedure was randomized when babies were entered into the study. For this study, 3 phases of Pain (Baseline, Lance, Recovery) and Clustered Care (Baseline, Clustered Care, Recovery) were analyzed.
Measures
Infant State
Infant sleep/wake state was coded every 2 minutes according to the NIDCAP® protocol.23 The predominant state over each 4-minute period was coded for each phase.
Facial Activity (Neonatal Facial Coding System: Brow Bulge)
The frequency of Brow Bulge (BB) was coded continuously for 12 minutes using the Noldus Observer system24 (throughout 4 minutes of Baseline, 4 minutes of the blood collection [Lance/squeeze] and Clustered Care, and 4 minutes after the last contact by the technician [Recovery]) to match the NIDCAP® coding. Traditionally, the full NFCS has been applied to brief periods (eg, 20 seconds per phase) to capture the acute pain response. Recently, continuous coding of individual NFCS behaviors has been used in preterm and full-term infants undergoing acutely painful procedures and surgery.4,5,25,26 Brow Bulge was selected as a proxy for upper facial actions because it has been shown to correlate highly with the other upper facial actions of the NFCS.27 Lower facial actions were not used because they are sometimes obscured by tape used to secure tubes to the face of preterm infants. Videotapes were edited for coding in random order of events, and coders were blind to all clinical information about the infants and to events. To establish reliability, both the primary NFCS coder (L.H.) and the reliability coder were trained on the entire tool with a reliability coefficient of 0.87.28 In addition, reliability coding was carried out on 20% of the sample with a reliability coefficient of 0.88. For data analysis, the frequency of BB was summed across all infants for each 4-minute phase.
Newborn Individualized Developmental Care and Assessment Program®
The NIDCAP® behaviors were coded continuously from video recordings of each infant for the 3 phases of blood collection (Baseline, Lance/squeeze, and Recovery), and Clustered Care (Baseline, Clustered Care, Recovery). Coding was carried out blind to all clinical information and the frequency of each infant’s movements was recorded systematically in 2-minute time blocks.23 A randomly selected sample of 5% of NIDCAP® video segments from the study (eg, Baseline segment, Lance/squeeze segment, Clustered Care segment or Recovery segment) was coded to evaluate reliability. NIDCAP® reliability was calculated by determining percent agreement of occurrence (both coders indicating the presence or absence of a behavior) within every 2-minute time segment during each 4-minute phase for each infant. Interrater agreement was 87%. Physiological measures were recorded by custom computer software and so were not scored using the NIDCAP® observation record.
Heart Rate
Mean heart rate (HR) was calculated for each 2-minute segment of each study period to correspond to the 2-minute NIDCAP® time blocks and averaged over 4 minutes of each of the 3 phases (Baseline, Lance/squeeze, Recovery or Baseline, Clustered Care, Recovery). Prior to statistical analysis, 42 (5%) of the 2-minute HR segments were dropped due to poor signal for that phase.
Oxygen Saturation
Continuous analog signals measures of oxygen saturation (O2 sat) were obtained using the same bedside computer apparatus as described elsewhere.4 Mean and standard deviations were calculated for each infant during each 2-minute segment of each study period, as detailed above for HR. Physiologic recordings were scrutinized for accuracy prior to analyses and 20 (3%) 2-minute segments were dropped due to poor signal.
Background Data
An NICU-trained research nurse completed the prospective clinical chart review and obtained information from birth to day of testing. Invasive procedures were defined as those involving skin breaking such as heel lance, venipuncture, insertion of arterial and venous lines, lumbar puncture, and chest-tube insertion. In addition, numbers of endotracheal intubations were collected. Infant characteristics are presented in Table 1. Study day characteristics of the infants are presented in Table 2 based upon data obtained up to the first observation.
TABLE 1.
Demographic Characteristics to Study Day 1 (n = 54)
| Mean (SD) | Range | N (%) | |
|---|---|---|---|
| Birth weight (grams) | 1257 (423) | 500–2345 | |
| Gestational age at birth (wks) | 29.3 (2.2) | 24–32 | |
| SNAP-II day 1* | 12 (9) | 0–38 | |
| SNAP-II day 3 | 3 (5) | 0–17 | |
| Ventilation (days) | 8.3 (12) | 0–46 | |
| Other respiratory support | 7.2 (7) | 0–28 | |
| Dexamethazone (days) | 0.32 (1.4) | 0–8 | |
| Pain exposure if Pain first procedure† | 67.17 (47) | 7–202 | |
| Pain exposure if Clustered Care first procedure† | 74.61 (56) | 9–246 | |
| Morphine exposure if Pain first procedure‡ | 0.87 (2.2) | 0–9.6 | |
| Morphine exposure if Clustered Care first procedure‡ | 0.66 (1.6) | 0–8.3 | |
| Ethnicity (Caucasian) | 39 (72) | ||
| Maternal age (yrs) | 31.4 (5.7) | 19–47 |
Score for Neonatal Acute Physiology.41
Number of invasive (skin-breaking) procedures from birth to the first study day.
Morphine exposure = (daily average/kg by mouth dose/3 + daily average intravenous mg/kg) × days.
TABLE 2.
Infant Characteristics on the First Study Day (n = 54)
| Mean (SD) | Range | N (%) | |
|---|---|---|---|
| Postconceptional age (wks) | 32 (0.7) | 31–33 | |
| Postnatal age (days) | 19 | 3–49 | |
| Mechanical ventilation | 9 (2) | ||
| Time since last feed (mins)* | 56.2 (32) | 0–152 | |
| No. painful procedures in 24 hrs prior to first study | 2 (2) | 0–11 |
Four infants were not on oral feeds.
Data Analysis
The frequencies of each NIDCAP® behavior were reviewed, and 31 movements that occurred in less than 25% of the infants were excluded from statistical analysis. Total frequencies of the remaining 26 NIDCAP® movements were summed for each 4-minute phase. Sleep-wake states were analyzed using nonparametric tests for related samples (Wilcoxon signed ranks and Friedman). Continuous measures (NIDCAP®, BB, HR, and O2 sat) were examined using repeated measures analysis of variance (ANOVA) to compare biobehavioral responses across the 3 phases of each procedure with gender as a between-patients factor. Because infants were positioned in 1 of 3 positions during the Pain procedure (18 in supine, 32 in prone, 4 in side lying), but all were in supine for the Clustered Care, position was entered as a covariate for the ANOVA for the Pain procedure only. In addition, because baseline behavioral state differed between the 2 procedures, this variable was also covaried. Bonferroni corrections for each test were used to correct for overall error with the P value set at 0.002 (when the Mauchley test indicated sphericity was violated, Greenhouse-Geisser correction was employed). Statistically significant ANOVA was followed by planned Student t tests for paired comparisons to identify differences between specific phases within each observation. Student t tests were also used to examine differences between frequencies of NIDCAP® movements occurring during Lance/squeeze with those occurring during Clustered Care. Pearson product-moment correlations were used to examine associations between perinatal variables and to describe relationships between the NIDCAP® and infant background characteristics during Lance/squeeze and Clustered Care.
RESULTS
Infant State
The infants differed in their behavioral state during the Baseline phases of the 2 procedures. Greater numbers of infants were in active sleep during the Baseline phase of the Pain procedure, whereas more infants were in quiet sleep during the Baseline phase of the Clustered Care procedure (z = −2.2, P < 0.03). State changed significantly across phases (Baseline, Lance or Clustered Care, Recovery) during both the Pain (χ2 = 62.2, P < 0.0001) and Clustered Care (χ2 = 69.2, P < 0.0001) conditions. Additionally, infants showed greater arousal during the Lance/squeeze compared to the Clustered Care Phase (z = −4.0, P < 0.0001). There were no statistically significant differences between Pain and Clustered Care Recovery states (Fig. 1). Finally, no Baseline behavioral state effects were found on any subsequent analyses.
FIGURE 1.
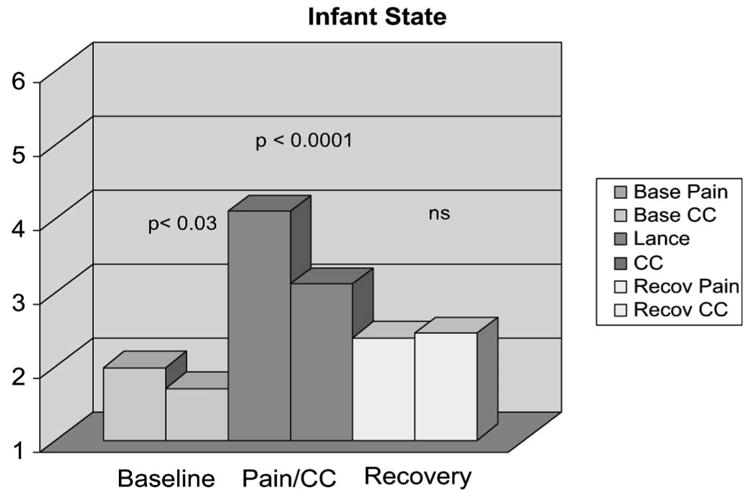
Infant sleep/wake state across 3 phases of Pain and Clustered Care (CC) procedures.
Facial Activity (Neonatal Facial Coding System: Brow Bulge)
There were no differences in the frequency of BB during the Baseline phases of the 2 procedures. The frequency of BB changed significantly across the 3 phases of both the Pain [F(1,52) = 52.0, P < 0.001] and Clustered Care [F(1,52) = 19.2, P < 0.0001] procedures, with infants showing greater frequencies of BB during the Lance/squeeze and Clustered Care phases. There were no gender differences. Finally, the infants showed greater numbers of BB during the Lance/squeeze phase compared to the Clustered Care phase (t = 3.8, P < 0.0001).
Newborn Individualized Developmental Care and Assessment Program®
Pain Procedure
Of the 26 NIDCAP® behaviors included in the statistical analysis (Table 3), a set of 5 NIDCAP® movements (flex legs, hand on face, finger splay, salute, and frown) showed an overall main effect with increased frequencies of movements during the Lance/squeeze Phase. There were no sex effects, nor position interactions with these 5 behaviors (Table 4). Moreover, there were no differences between the frequencies of these behaviors between Baseline and the Recovery phases. An additional behavior, flex arms [F(1,51) = 8.1; P < 0.001] also increased significantly during the Lance/squeeze phase with no gender effects, but those infants in the prone position had fewer instances of flex arms during the Lance/squeeze phase (P < 001). An eighth movement, yawning, also increased during the Lance/squeeze phase [F(1,50) = 3.4; P < 0.04], with those infants in the prone position yawning more than those in supine or side lying (P < 0.01). Further, a ninth movement, extend legs, increased during the Lance/squeeze Phase [F(1,51) = 8.6; P < 0.001], with girls showing greater frequencies of this movement than boys (P < 0.001) and those infants prone showing fewer instances of leg extensions (P < 0.009). Sitting-on-air (full extension of the legs into the air) increased significantly across the 3 phases with highest frequencies during the Recovery phase [F(1,51) = 3.6; P < 0.05); however, there were no statistically significant differences in frequencies between each individual phase. Finally, one movement, twitch extremities, decreased during Lance/squeeze [F(1,51) = 5.0; P < 0.01].
TABLE 3.
NIDCAP® Behaviors Included in Statistical Analyses
| NIDCAP® Behaviors | |
|---|---|
| Tremor | Tongue extension |
| Twitch face, twitch body, twitch extremities | Hand on face |
| Flex arms, flex legs | Mouthing |
| Extend arms, extend legs | Finger splay |
| Diffuse squirm | Airplane |
| Arch | Salute |
| Trunk tuck | Sit on air |
| Hand to mouth | Yawn |
| Grasping | Eye float |
| Fisting | Frown |
| Leg brace | Foot clasp |
TABLE 4.
Frequencies of NIDCAP® Behaviors That Increased Across Phases of Pain and Clustered Care
| Pain
|
Clustered Care
|
ANOVA
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain
|
Clustered Care
|
|||||||||
| NIDCAP® Behavior | Baseline Mean (SD) | Lance/squeeze Mean (SD) | Recovery Mean (SD) | Baseline Mean (SD) | Clustered Care Mean (SD) | Recovery Mean (SD) | F | P < | F | P < |
| Flex arms | 0.8 (1.7) | 1.4 (2.1) | 0.7 (1.8) | 0.3 (0.7) | 1.1 (1.3) | 0.7 (1.1) | 8.1 | 0.001 | 9.4 | 0.0001 |
| Flex legs | 1.8 (2.8) | 4.6 (5.5) | 1.8 (3.3) | 1.0 (2.0) | 2.6 (2.5) | 1.7 (3.5) | 5.5 | 0.01 | 5.6 | 0.01 |
| Extend legs | 1.6 (2.7) | 3.1 (5.0) | 1.1 (2.4) | 1.0 (2.3) | 3.5 (2.5) | 1.3 (3.0) | 8.6 | 0.001 | 16.9 | 0.0001 |
| Hand on face | 0.11 (0.4) | 0.6 (1.1) | 0.2 (0.4) | 0.1 (0.5) | 1.0 (1.2) | 0.2 (0.7) | 3.8 | 0.04 | 11.9 | 0.0001 |
| Finger splay | 0.5 (1.4) | 1.3 (1.9) | 0.8 (1.3) | 0.2 (0.7) | 2.9 (2.4) | 0.8 (1.4) | 5.1 | 0.009 | 40.3 | 0.0001 |
| Salute | 0 | 0.1 (0.3) | 0 | 0.1 (0.2) | 0.7 (1.4) | 0.1 (0.3) | 3.5 | 0.05 | 13.5 | 0.0001 |
| Frown | 0.2 (0.6) | 0.9 (1.2) | 0.2 (0.6) | 0.1 (0.3) | 0.6 (1.0) | 0.2 (0.5) | 11.4 | 0.0001 | 8.2 | 0.002 |
| Yawning | 0.2 (0.4) | 0.4 (1.0) | 0.3 (0.7) | 0.1 (0.3) | 1.5 (1.6) | 0.3 (0.6) | 3.4 | 0.04 | 36.9 | 0.0001 |
| Tongue extension | 0.4 (1.1) | 0.3 (0.8) | 0.4 (0.9) | 0.1 (0.5) | 1.2 (1.5) | 0.3 (1.3) | 0.9 | NS | 14.4 | 0.0001 |
| Extend arms | 0.6 (1.5) | 1.1 (1.6) | 0.5 (1.1) | 0.3 (0.7) | 2.0 (1.6) | 0.5 (1.1) | 1.1 | NS | 31.6 | 0.0001 |
| Airplane | 0.1 (0.5) | 0.1 (0.4) | 0.1 (0.4) | 0.1 (0.3) | 0.4 (1.0) | 0.1 (0.3) | 0.4 | NS | 6.1 | 0.01 |
| Sit on air | 0 | 0.1 (0.2) | 0.1 (0.5) | 0 | 0.3 (0.5) | 0.1 (0.6) | 3.2 | 0.05 | 6.0 | 0.005 |
| Hand to mouth | 0.4 (1.3) | 0.6 (1.1) | 0.4 (1.1) | 0.2 (0.4) | 0.1 (0.3) | 0.5 (1.3) | 1.2 | NS | 4.7 | 0.02 |
| Fisting | 0 | 0.2 (0.5) | 0.1 (0.4) | 0 | 0.3 (0.6) | 0.1 (0.5) | 1.6 | NS | 6.1 | 0.008 |
Clustered Care Procedure
There were no gender effects with any NIDCAP® movements during the Clustered Care procedure. Of the 26 NIDCAP® movements included in the statistical analyses, 14 movements increased during the Clustered Care procedure, 9 of which were those observed during the Lance/squeeze (Table 4). Grasping also increased between Baseline and Clustered Care and remained elevated during the Recovery phase [F(1,52) = 4.3; P < 0.02]. Eye floating showed increased frequencies during the Recovery phase [F(1,52) = 8.1; P < 0.003]. Furthermore, the infants continued to show increased finger splays (t = −2.7; P < 0.01), hand to mouth activity (t = −2.1, P < 0.04) and yawning (t = −2.5, P < 0.01) during Recovery when compared with Baseline frequencies.
Four NIDCAP® movements decreased significantly during the Clustered Care: twitch body [F(1,52) = 12.3; P < 0.0001], twitch face [F(1,52) = 19.6; P < 0.0001], twitch extremities [F(1,52) = 19.0; P < 0.0001], and mouthing [F(1,52) = 9.9; P < 0.001].
Newborn Individualized Developmental Care and Assessment Program® Pain Versus Clustered Care
Nineteen NIDCAP® movements were examined to compare the frequency of the movements occurring during Lance/squeeze versus Clustered Care phases. Infants showed greater numbers of twitch face and flex legs during the Lance/squeeze than during Clustered Care. However, infants extended their arms, extended their tongues, finger splayed, airplaned (infant extends arms laterally), saluted (extension of the arms into midair in front of the infant), sat on air, and yawned more frequently during the Clustered Care procedure (Table 5). Because the infants showed ongoing NIDCAP® stress cues following the Clustered Care, the data were further examined to compare the frequency of the movements during the Recovery phases of both procedures. The frequency of eye floating was statistically significantly higher during the Clustered Care Recovery phase than during the Pain Recovery phase (t = −2.98, P < 0.004).
TABLE 5.
Comparison of Frequencies of NIDCAP® Movements During Pain Versus Clustered Care
| NIDCAP® Behavior | Mean Differences (SD) | t | P < |
|---|---|---|---|
| Twitch face | 0.15 (0.5) | 2.2 | 0.03 |
| Flex legs | 1.94 (5.0) | 2.5 | 0.02 |
| Extend arms | −0.85 (2.1) | −2.9 | 0.005 |
| Tongue extension | −0.85 (1.7) | −3.6 | 0.001 |
| Hand on face | −0.3 (1.5) | −1.5 | 0.0001 |
| Finger splay | −1.6 (3.0) | −3.8 | 0.014 |
| Airplane | −0.4 (1.0) | −2.6 | 0.001 |
| Sitting on air | −0.2 (0.5) | −3.3 | 0.002 |
| Yawn | −1.1 (1.9) | −4.2 | 0.0001 |
Relationships Between Newborn Individualized Developmental Care and Assessment Program® Behaviors and Perinatal Variables
Examining the relationship between gestational age at birth and the frequencies of NIDCAP® behaviors during Lance/squeeze showed that increased body twitches during the Lance/squeeze phase were associated with infants who were born at earlier gestational ages (<30 weeks). Similarly, infants who were born at earlier gestational ages showed greater frequencies of flexing of legs, finger splays, and saluting during the Clustered Care procedure. Although less morphine exposure and less pain exposure was associated with increased frequencies of leg flexion during the Clustered Care phase, infants who had been exposed to fewer pain procedures showed increased leg extensions during the Clustered Care phase. Finally, fewer body twitches during Clustered Care were associated with infants who had been more stable on the first postnatal day (SNAP-I) (Table 6).
TABLE 6.
Correlations Between NIDCAP® Behaviors and Perinatal Variables
| Infant Characteristics | NIDCAP® Behavior | Procedure | r | P < |
|---|---|---|---|---|
| Gestational age at birth | Flex legs | CC | −0.40 | 0.003 |
| Finger splay | CC | −0.30 | 0.03 | |
| Saluting | CC | −0.30 | 0.03 | |
| Twitch body | P | −0.29 | 0.03 | |
| Illness severity (SNAP-II day 1) | Twitch body | CC | 0.33 | 0.02 |
| Pain exposure* | Flex legs | CC | −0.37 | 0.006 |
| Extend legs | CC | −0.27 | 0.05 | |
| Morphine exposure† | Flex legs | CC | −0.28 | 0.04 |
Number of invasive (skin breaking) procedures from birth to the first study day.
Morphine exposure = (daily average/kg by mouth dose/3 + daily average intravenous mg/kg) × days.
CC, Clustered Care; P, Pain.
Heart Rate
Mean HR changed significantly across the 3 phases of Blood Collection [F(1,49) = 69.2; P < 0.0001] and across the 3 phases of Clustered Care [F(1,53) = 41.7; P < 0.0001]. Heart rate (mean ± SD) increased from Baseline 157.2 ± 10 to Lance/squeeze 177.2 ± 15 (t = −11.1, P < 0.0001), and decreased during recovery 158.5 ± 13 (t = 9.2, P < 0.0001). Similarly, HR increased significantly from Baseline 155.7 ± 13 to Clustered Care 168.6 ± 16 (t = −8.9, P < 0.0001) and decreased significantly during Recovery 157.0 ± 14 (t = 6.6, P < 0.0001). Although there were no statistically significant differences between the Baseline phases of the 2 procedures or in the Recovery phases, the increase in HR was greater during Lance/squeeze compared to Clustered Care (t = 3.9, P < 0.0001). This finding was further confirmed by converting the changes from Baseline to Lance/squeeze and Baseline to Clustered Care Phases to change scores (Δ heart rate [Δ HR]; Baseline − Lance/squeeze or Clustered Care)/Baseline + Lance/squeeze or Clustered Care); this conversion takes into account the law of initial values.29 The Δ HR Lance/squeeze was significantly greater than the Δ HR Clustered Care (t = − 3.4, P < 0.001).
Oxygen Saturation
Mean O2 sat decreased across the three phases of Blood Collection [F(1,45) = 27.3; P < 0.0001] and Clustered Care [F(1,50) = 62.9; P < 0.0001]. During the pain procedure, O2 sats dropped from Baseline 96.1 ± 3 to 91.3 ± 6 during the Lance/squeeze phase (t = 6.2, P < 0.0001), and increased to 95.7 ± 6 during the Recovery Phase (t = −5.3, P < 0.0001). Likewise, during the Clustered Care procedure, O2 sats decreased from Baseline levels of 95.4 ± 3 to 88.0 ± 7 during Clustered Care (t = 8.2, P < 0.0001), and then increased during Recovery 96.1 ± 4 (t = −8.2, P < 0.0001). Baseline and Recovery changes in O2 sats did not differ between Pain and Clustered Care; however, O2 sats were lower during the Clustered Care procedure than during the Pain procedure (t = 2.6, P < 0.01).
DISCUSSION
Using multidimensional assessments, including the full NIDCAP® , this is the first study to examine preterm infants’ detailed physiological and behavioral responses to a common cluster of NICU procedures. We compare preterm infant responses to this tactile procedure with responses stimulated by an acutely painful procedure. The infants reacted to both procedures; however, the intensity of responses and the direction of the behavioral measures differed. The infants showed greater increases in behavioral state, facial reactivity (both NFCS BB and NIDCAP® frown), and HR during the painful procedure than during Clustered Care, a finding similar to others.17 Although there were similar differences in reduction of O2 sats between the Pain and Clustered Care procedures, Clustered Care produced greater drops. Although studies have also shown drops in O2 sats during acutely painful procedures,30–33 our findings differed: the drops in O2 sats were greater during the nonpainful handling. We speculate that this resulted from more vigorous handling during the Clustered Care. Alternatively, although the O2 sat data was carefully scrutinized for accuracy, movement artifact may have affected our results.
In contrast to the preceding findings, the infants exhibited not only a greater variety of body movements, but also greater frequencies of movements currently described as stress cues during Clustered Care. Increased body responses to a tactile procedure may have occurred either because the tactile procedures required greater physical manipulation of the infant’s body than did the heel lance or because the infants were sensitized to the tactile stimulation. Such sensitization has been reported in human and animal studies.9,34 Finally, during the Recovery phase, whereas the infants returned to baseline levels in all measures during the Pain procedure, they continued to show increased stress cues following the Clustered Care. This result may indicate the wind-up phenomenon, a progressive buildup of a response to repetitive low-frequency stimulation.35
When we examined the specific movements in more detail, we found that the infants responded to the Clustered Care with predominantly extensor movements such as extension of the arms and legs, finger splays, airplane, sitting on air, and salute. According to the NIDCAP® model, these movements are stress cues; this interpretation of these movements is supported by other studies.4,5,15,36 Another extensor action, tongue extension, also occurred more frequently to the Clustered Care. Tongue extension has been reported as a marker of pain response in preterm infants.22,37 But, given that the infants showed less intense facial and state responses to the Clustered Care, it is unlikely that the infants perceived the tactile procedure as “painful.” Rather, it is more likely that tongue extension is not a specific pain cue, but is a general indicator of stress.
Yawning, which increased significantly during Pain and Clustered Care, was also more prevalent during Clustered Care. Infants positioned in prone tended to yawn more during the Pain procedure than did infants in other positions. Yawning is thought to help increase the level of arousal in preterm infants and is associated with the drowsy state.38 In fact, in our study, more infants were classified as drowsy during the Clustered Care (96%) than during the Lance/squeeze (57%), and more who were prone during the Lance/squeeze phase were classified as drowsy (67%) than those in the supine/sidelying position (32%). However, according to the NIDCAP model® , yawning can be interpreted as a stress cue; therefore, this cue might be not only an indicator of a behavioral state, but also a sign of stress. This interpretation is supported physiologically in that adrenocorticotropic hormone (ACTH, a pituitary hormone that stimulates the release of cortisol, a stress hormone) facilitates yawning.39 Fisting also increased significantly to Clustered Care. Although it did not increase to statistically significant levels during the Pain procedure, fisting increased during the Lance/squeeze phase and remained at higher levels during Recovery. Even though fisting may be included as a pain indicator in some assessments, such as finger splays, it is more likely an indicator of stress. Finally, hand on face, a protective action whereby the infant places its hand on its face, interpreted as an attempt to create a barrier between the face and the stimulus, increased to both procedures, but with greater frequency to Clustered Care.
Flexor movements, observed during both Pain and Clustered Care procedures, generally are interpreted as attempts at self-regulation.15,23 Although flexor actions of the arms, such as hand to mouth, may provide increased stability, flexor actions of the legs may be reflex responses to pain and tactile stimulation in preterm infants.40 Finally, as in prior studies, twitches decreased during the Pain and Clustered Care phases, further supporting our contention that they are not stress indicators.4,36
In our study that assessed acute pain responses in preterm infants, earlier born infants showed greater numbers of stress cues than later born infants.4,5 Although these results were not replicated with the pain procedure in this study, infants who were born earlier did exhibit greater numbers of stress cues to Clustered Care. However, infants who had been exposed to less pain and morphine showed greater numbers of lower extremity flexion and extension during Clustered Care. It is possible that infants who experienced fewer painful procedures and were less ill (therefore needed less morphine) could mount more vigorous flexor withdrawal responses to tactile and painful stimuli. Although twitches do not appear to be stress cues, their presence during a tactile procedure may indicate relative instability: infants who were more ill on day 1 of life (SNAP-I) had body twitches during Clustered Care. Consonant with this finding, infants born earlier showed greater body twitches during Lance/squeeze.
Several limitations of our study should be mentioned. Although the NIDCAP® and NFCS reliability coders were blind to the purposes of the study, the primary coder was aware of the purposes of the study; consequently, observer bias may have affected the results. In addition, our study assumed that preterm infants would not perceive the tactile procedures as “painful.” Not only is controlling for prior pain exposure in this population difficult, but “true” pain perception in these infants can be assessed only indirectly through biobehavioral reactivity. The infants in this study did show graded facial and heart rate reactivity to each procedure, as others have shown32; nevertheless, their heightened motor responses may have occurred as a result of allodynia from their prior procedural pain exposure.
In conclusion, preterm infants exhibit clear, reproducible biobehavioral responses to both tactile and noxious stimulation. In fact, our findings indicate that, depending on the level of physical manipulation involved in the routine care giving tasks, even those as simple as changing a diaper, measuring abdominal girth and temperature, and performing mouth care, produce marked physiological and motor responses that indicate stress and that persist beyond the time of the handling. This outcome is critical because tactile procedures occur with much greater regularity than painful procedures. Our findings also highlight the need for further evaluation of the practice of clustering of care-giving tasks. Although clustering tasks may provide longer rest periods, a balance between shorter rest periods interspersed with 1 or 2 caregiving tasks might produce less intense biobehavioral responses in some infants. Finally, it appears that facial activity, changes in sleep/wake state, and HR are the most specific indicators of painful procedures; nevertheless, body movements reveal important information regarding the infants’ responses to painful events. Not only do further studies need to determine whether infants assessed at earlier gestational ages show similar responses to both painful and nonpainful nursery procedures, but clinicians working in NICUs must continue to study the cumulative effects of both painful and nonpainful handling as potential mechanisms for alterations in brain development in these vulnerable infants.
Acknowledgments
The authors would like to thank the staff and families of the Special Care Nursery at B. C.’s Children’s Hospital for their participation in this study, Colleen Fitzgerald, Study Coordinator, Gisella Gosse and Adi Amir for data collection, and Colleen Jantzen for carrying out reliability NFCS coding, all of whom are staff of the Biobehavioral Research Unit of Centre for Community Health and Health Evaluation Research, B. C. Research Institute for Children’s & Women’s Health.
Footnotes
Supported by the National Institutes of Health grant HD39783, Canadian Institutes of Health Research grant MOP42469, Canadian Institutes of Health Research/Canadian Occupational Therapy Foundation PostDoctoral Fellowship (L.H.), a Senior Scholar Award from the Michael Smith Foundation for Health Research (R.E.G.), and a Human Early Learning Partnership and the British Columbia Ministry of Children and Family Development grant 02-2410.
References
- 1.Grunau R. Early pain in preterm infants. A model of long-term effects. Clin Perinatol. 2002;29:373–394. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 2.Bhutta AT, Anand KJS. Vulnerability of the developing brain. Neuronal mechanisms. Clin Perinatol. 2002;29:357–372. doi: 10.1016/s0095-5108(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 3.Franck LS, Miaskowski C. Measurement of neonatal responses to painful stimuli: a research review. J Pain Symptom Manage. 1997;14:343–378. doi: 10.1016/s0885-3924(97)00222-4. [DOI] [PubMed] [Google Scholar]
- 4.Morison SJ, Holsti L, Grunau RE, et al. Are there developmentally distinct motor indicators of pain in preterm infants? Early Hum Dev. 2003;72:131–146. doi: 10.1016/s0378-3782(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 5.Holsti L, Grunau RVE, Oberlander TF, et al. Specific newborn individualized developmental care and assessment program movements are associated with acute pain in preterm infants in the NICU. Pediatrics. 2004;114(1):65–72. doi: 10.1542/peds.114.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntosh N. Pain in the newborn, a possible new starting point. Eur J Pediatr. 1997;156:173–177. doi: 10.1007/s004310050576. [DOI] [PubMed] [Google Scholar]
- 7.Andrews K, Fitzgerald M. Biological barriers to pediatric pain management. Clin J Pain. 1997;13:138–143. doi: 10.1097/00002508-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Acolet D, Modi N, Giannakoulopoulos X, et al. Changes in plasma cortisol and catecholamine concentrations in response to massage in preterm infants. Arch Dis Child. 1993;68:29–31. doi: 10.1136/adc.68.1_spec_no.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellerud BC, Storm H. Skin conductance and behavior during sensory stimulation of preterm and term infants. Early Hum Dev. 2002;20:35–46. doi: 10.1016/s0378-3782(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald M, Millard C, McIntosh N. Hyperalgesia in premature infants. Lancet. 1988:292. doi: 10.1016/s0140-6736(88)90365-0. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anesthesia. Pain. 1989;39:31–36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 12.Rahman W, Fitzgerald M, Aynsley-Green A, et al. The effects of neonatal exposure to inflammation and/or morphine on neuronal responses and morphine analgesia in adult rats. In: Jensen TS, Turner JA, Weisenfeld-Halling Z, editors. Progress in Pain Research and Management. 8. Seattle, WA: IASP Press; 1997. pp. 738–794. [Google Scholar]
- 13.Peters KL. Bathing premature infants: physiological and behavioral consequences. Am J Crit Care. 1998;7:90–100. [PubMed] [Google Scholar]
- 14.Sell EJ, Hill-Mangan S, Holberg CJ. Natural course of behavioral organization in premature infants. Infant Behav Dev. 1992;15:461–478. [Google Scholar]
- 15.Peters KL. Association between autonomic and motor systems in the preterm infant. Clin Nurs Res. 2001;10:82–91. doi: 10.1177/c10n1r8. [DOI] [PubMed] [Google Scholar]
- 16.Slevin M, Daly L, Murphy JEA. Preterm infants stress responses to an invasive NICU event: endotracheal suctioning. J Reprod Infant Psychol. 1998;16:285–292. [Google Scholar]
- 17.Ballantyne M, Stevens B, McAllister M, et al. Validation of the premature infant pain profile in the clinical setting. Clin J Pain. 1999;15:297–303. doi: 10.1097/00002508-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Blauer T, Gerstmann D. A simultaneous comparison of three neonatal pain scales during common NICU procedures. Clin J Pain. 1998;14:39–47. doi: 10.1097/00002508-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Stephens SE, Glazer G. Evaluating and monitoring the effects of the admission process on the premature infant. J Perinat Neonatal Nurs. 1992;5:46–57. doi: 10.1097/00005237-199203000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pressler JL, Helm JM, Hepworth JT, et al. Behaviors of very preterm neonates as documented using NIDCAP observations. Neonatal Netw. 2001;20:15–24. doi: 10.1891/0730-0832.20.8.15. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E. GPOWER: A Priori-, Post Hoc-, and Compromise Power Analyses for MS-DOS. Germany: Bonn University; 1998. [computer program] [Google Scholar]
- 22.Grunau RE, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 23.Als H. Manual for the Naturalistic Observation of Newborn Behavior (Preterm and Full Term) Boston, MA: The Children’s Hospital; 1984. [Google Scholar]
- 24.Base Package for Windows Reference Manual. Wageningen, The Netherlands: Noldus Information Technology; 1995. The Observer. [Google Scholar]
- 25.Porter FL, Wolf CM, Miller PJ. The effect of handling and immobilization on the response to acute pain in newborn infants. Pediatrics. 1998;102:1383–1389. doi: 10.1542/peds.102.6.1383. [DOI] [PubMed] [Google Scholar]
- 26.Peters JWB, Koot HM, Grunau RE, et al. Neonatal Facial Coding System for assessing postoperative pain in infants: item reduction is valid and feasible. Clin J Pain. 2003;19:353–363. doi: 10.1097/00002508-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Johnston CC, Stevens BJ, Yang F, et al. Differential response to pain by very premature neonates. Pain. 1995;61:471–479. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- 28.Grunau RE, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 29.Lacey JI. The evaluation of autonomic responses: towards a general solution. Ann N Y Acad Sci. 1956;67:125–163. doi: 10.1111/j.1749-6632.1956.tb46040.x. [DOI] [PubMed] [Google Scholar]
- 30.Stevens BJ, Johnston CC. Physiological responses of premature infants to a painful stimulus. Nurs Res. 1994;43:226–231. [PubMed] [Google Scholar]
- 31.Van Cleve L, Johnson L, Andrews S, et al. Pain responses of hospitalized neonates to venipuncture. Neonatal Netw. 1995;14:31–36. [PubMed] [Google Scholar]
- 32.Johnston CC, Stevens BJ, Yang F, et al. Differential response to pain by very premature neonates. Pain. 1995;61:471–479. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- 33.Craig KD, Whitfield MF, Grunau RVE, et al. Pain in the preterm neonate: behavioral and physiological indices. Pain. 1993;52:287–300. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 34.Jennings E, Fitzgerald M. C-fos can be induced in the neonatal rat spinal cord by both noxious and innocuous peripheral stimulation. Pain. 1996;68:301–306. doi: 10.1016/s0304-3959(96)03194-6. [DOI] [PubMed] [Google Scholar]
- 35.Woolf C. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- 36.Grunau RE, Holsti L, Whitfield MF, et al. Are twitches, startles and body movements pain indicators in extremely low birth weight infants? Clin J Pain. 2000;16:37–45. doi: 10.1097/00002508-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Grunau RE, Oberlander T, Holsti L, et al. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain. 1998;76:277–286. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 38.Giganti F, Hayes MJ, Akilesh MR, et al. Yawning and behavioral states in premature infants. Dev Psychobiol. 2002;41:289–296. doi: 10.1002/dev.10047. [DOI] [PubMed] [Google Scholar]
- 39.Argiolas A, Melis MR. The neuropharmacology of yawning. Eur J Pharm. 1998;343:1–16. doi: 10.1016/s0014-2999(97)01538-0. [DOI] [PubMed] [Google Scholar]
- 40.Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Dev Med Child Neuro. 1999;41:696–703. doi: 10.1017/s0012162299001425. [DOI] [PubMed] [Google Scholar]


