Abstract
Objective
The purpose of this study was to assess relations and concordance between behavioral and physiologic reactivity to pain in preterm neonates at 32 weeks postconceptional age as a function of gestational age at birth.
Setting
Level III neonatal intensive care unit.
Design/Patients
The study group comprised 136 preterm neonates (mean [range] birthweight, 1,020 g [445–1,500 g]; gestational age at birth, 28 weeks [23–32 weeks]) separated into three groups according to gestational age at birth as follows: 23 to 26 weeks (n = 48), 27 to 29 weeks (n = 52), and 30 to 32 weeks (n = 36).
Outcome Measures
Reactivity to routine blood collection at 32 weeks postconceptional age was assessed using bedside-recorded behavioral and autonomic measures. Coders who were blinded to the study design scored behavioral responses (facial activity using the Neonatal Facial Coding System, sleep/waking state, and finger splay). Autonomic reactivity was assessed by change in heart rate and spectral analysis of heart rate variability (change in low-frequency and high-frequency power, and the ratio of low-frequency to high-frequency power during blood collection).
Results
Facial activity and state correlated moderately with change in heart rate across gestational age groups (r = 0.41–0.62). Facial activity and state did not correlate significantly with change in low-frequency and high-frequency power, or the ratio of low-frequency to high-frequency power (r = 0.00–0.31). Finger splay did not correlate with any autonomic recording (r = 0.03–0.41). Concordance between established biobehavioral measures of pain revealed individual differences. Although some neonates showed high behavioral but low physiologic reactivity, other neonates displayed the opposite reaction; however, the majority displayed concordant reactions.
Conclusions
The study findings confirm the value of measuring domains independently, especially in neonates born at a very young gestational age.
Keywords: Behavioral measures, Biobehavioral relations, Pain, Physiologic measures, Premature neonates
Considerable research in pain assessment of neonates in recent years has resulted in the development of measures that assess acute procedural pain in full-term and preterm neonates. Changes in facial activity, shifts in the sleep/waking state, and physiologic indices of heart rate and oxygen saturation are viewed as the most promising pain indicators in neonates older than 28 weeks gestational age (GA).1,2 Because a cry is not vocalized under mechanical ventilation, it is precluded as a pain cue for the smaller and sicker patients in neonatal intensive care unit (NICU) care.
Multidimensional assessment of pain using both biological and behavioral measures is essential because different parameters can potentially yield different information.3–5 This assessment can be approached in two ways. Composite scales additively combine elements of physiologic and behavioral responses, providing a summary score. Alternatively, multiple sources of response are recorded, but are not combined into a single score. The purpose of this study was to examine the relations between behavioral and physiologic reactivity to further understand the development of pain-response systems. For the purpose of research, several univariate measures were studied.
Few studies have reported relations between behavioral and physiologic responses during painful procedures. Relations between responses may differ across GA and vary with background experience.5 In some studies with neonates of young postconceptional age (PCA) and with neonates born close to term, facial activity and measures of heart rate were moderately related. Among neonates with a mean PCA of 28–29 weeks, Johnston et al.6 observed a correlation of r = 0.55 between the facial action of brow bulge and maximum heart rate during heel stick, and of r = 0.36 between brow bulge and the standard deviation of heart rate. In more mature preterm neonates of 35 weeks GA at birth observed within the first 4 days of life, behavior and mean heart rate were highly correlated (r = 0.82) between the average number of facial movements and mean heart rate to heel stick.7 Porter et al.8 compared the amount of time neonates displayed an agitated behavioral state with the mean change in heart rate across nine procedures that varied in painfulness (as rated by clinicians), and found inconsistencies between the behavioral and physiologic responses.
Although blood collection was the pain procedure used in all of these studies except that of Porter et al.,8 heart rate parameters including mean heart rate, maximum heart rate, and heart rate variability differed. In addition, the PCA of the neonates studied was different among these studies. Johnston and Stevens9 tested neonates at 32 weeks PCA, and found that correlations between physiologic and facial variables were not significant. However, variation between these findings and the high correlations found close to term and at younger GA remains unexplained. The lack of concordance between behavioral and physiologic responses8–10 and the complexity of background and concurrent influences, which have been documented in several studies,9,11,12 underscore the challenges of measurement in this area.
To improve pain assessment in preterm neonates, studies that address biobehavioral relations within the complex context of previous and ongoing influences are needed.5,8 Gestational age at birth is a marker for a complex array of background variables, such as illness severity, previous exposure to painful procedures, medication exposure, and ventilation, all of which are highly interrelated. Gestational age at birth appears to affect subsequent pain response.9,12,13 Prolonged “wind up” may alter the underlying autonomic substrate and developmental course of the pain system and possibly other areas of brain development. Therefore, the relations between physiologic and behavioral responses may differ depending on GA at birth. Also, information regarding GA at birth is readily available in the clinical setting.
The most widely used univariate scale is the Neonatal Facial Coding System (NFCS),14,15 a fine-grained anatomically based measure of response to acute invasive procedures in neonates. Construct validity was established with term-born and preterm neonates because the NFCS facial actions discriminate tissue insult from tactile contact and nontissue-damaging procedures,16–21 and differentiate neonates receiving opioids20,22 or sucrose23 during invasive procedures. Convergent validity has been shown by comparison with comprehensive facial coding.24 The NFCS has recently been adapted for bedside use.21
Finger splay is proposed to be a motoric stress behavior in the Neonatal Individualized Developmental Care Assessment Program scale.25 This action occurs frequently during the invasive phases of blood collection21 and may be useful as a readily available, easily “read” stress cue.26
In term-born neonates, the sleep/waking state is a complex constellation of behavioral, physiologic, and electrophysiologic activity that is observed in predictable patterns.27 The construct of the sleep/waking state is unclear in neonates younger than 36 weeks GA because there is a lack of coupling between the behavioral and physiologic components at this age.28 Although controversial, cycles of behavioral activity are useful in the assessment of pain. This activity is observed in preterm neonates younger than 36 weeks PCA and appears to reflect shifts in arousal.21
Heart rate has frequently been used as a measure of physiologic reactivity to noxious events. Heart rate variability (measured by the standard deviation of mean heart rate) is increased in preterm neonates during blood collection.29 Techniques in spectral analysis of heart rate variability can be used to quantify changing levels of cardiac autonomic modulation. The high-frequency (HF) range (0.15–0.80 Hz) is mediated by changes in parasympathetic activity, whereas the low-frequency (LF) range (0.04–0.14 Hz) can reflect changes due to modulation of both cardiac vagal and sympathetic activity.30 The ratio of LF to HF power has been used in adults as a measure reflecting sympathovagal balance.31 Spectral analysis of heart rate variability has been used to assess cardiac autonomic response to pain in term and preterm infants.32–34 In preterm neonates, the autonomic response predominantly reflects changes in sympathetic cardiac modulation (LF changes),32 and with increasing GA there is a shift toward increasing parasympathetic modulation.35
The first aim of this study was to examine relations between physiologic (power spectral analysis of heart rate variability) and behavioral reactivity (NFCS facial activity, finger splay, and neonate sleep/waking state) to pain in preterm neonates at 32 weeks PCA as a function of GA at birth. Some researchers have postulated that although most people respond to stimuli to some extent behaviorally (externally) and physiologically (internally), some respond in a predominantly behavioral (“externalizers”) or physiologic (“internalizers”) way.36 A second aim of this study is to examine whether patterns of reactivity can be identified in neonates at 32 weeks PCA. These results may help to empirically determine relative weightings to derive an overall pain index that is applicable to this developmental age group.
METHODS
Subjects
Written informed consent was obtained from the mother or other legal guardian according to a protocol approved by the Clinical Research Ethics Committee of the University of British Columbia. A continuous series of 162 neonates with birth weight of 1,500 g or less and no major congenital anomaly or cardiac defect were recruited after admission to the tertiary level NICU in the British Columbia Children’s Hospital, and were available for testing at 32 weeks to 32 weeks 6 days PCA. The neonate sleep/waking state at baseline was examined and controlled by retaining only neonates in states 1 to 5 (deep sleep to active awake25). Conservative criteria for time since analgesia or sedation exposure were used based on the recent report that the clearance rate of morphine in premature neonates is considerably longer than previously thought.22 Anticholenergic eye drops were considered as well because this agent may affect heart rate. Neonates were excluded from the final data set because of questionable GA (one), crying during baseline (two), incomplete heart rate data due to technical difficulties (five), or not receiving a heel lance at 32 weeks PCA (one). Exclusions due to pharmacological exposure included neonates on morphine or receiving morphine within 75 hours of the test time (three), receiving midazolam within 24 hours of the test time (one), or receiving Tylenol within 18 hours of the test time (one). An additional 12 neonates were excluded because of significant neurologic changes during head ultrasonography (i.e., periventricular leukomalacia or grade IV intraventricular hemorrhage). The remaining 136 neonates with complete behavioral and physiological data comprised the study sample (mean [range] birthweight, 1,020 g [445–1,500 g]; GA at birth, 28 weeks [23–32 weeks]; number of previous pain procedures from birth to study at 32 weeks PCA, 86 [5–283]). Neonates were divided into three groups by GA at birth as follows: (1) 23 to 26 weeks (n = 48); (2) 27 to 29 weeks (n = 52); and (3) 30 to 32 weeks (n = 36). Subject characteristics are provided in Table 1.
TABLE 1.
Demographic characteristics of the three gestational age groups
| Gestation
|
|||
|---|---|---|---|
| 23–26 wks (n = 48) | 27–29 wks (n = 52) | 30–32 wks (n = 36) | |
| Birthweight (g) | 808 ± 118 | 1,053 ± 240 | 1,260 ± 199 |
| Sex | |||
| Male | 25 | 29 | 16 |
| Female | 23 | 23 | 20 |
| Apgar, 5 min | 7.5 ± 1.4 | 7.8 ± 1.6 | 8 ± 1.5 |
| Delivery | |||
| Vaginal | 21 (44%) | 22 (42%) | 8 (22%) |
| Vaginal breech | 7 (14%) | 5 (10%) | 2 (6%) |
| C-section | 20 (42%) | 25 (48%) | 26 (72%) |
| SNAP-II* day 3 | 11 ± 8 | 4 ± 0.5 | 0 ± 0 |
| Number of procedures† | 136 ± 51 | 79 ± 30 | 30 ± 17 |
Mean ± SD.
SNAP-II, Score of Neonatal Acute Physiology.
Number of invasive procedures from birth to 32-wk, postconceptional-age blood collection.
Measures
Neonate state was coded as defined by Als.25 The sleep/waking state was coded from 1 to 7 as follows: 1 = deep sleep, 2 = light sleep, 3 = drowsy, 4 = quiet awake, 5 = active awake, 6 = highly aroused, agitated, upset, or crying, and 7 = prolonged respiratory pause >8 seconds. Preterm neonates at times appear to go into a transitory state of “collapse” or “withdrawal” characterized by muscular flaccidity and prolonged pause in breathing, which is captured in state 7. Because the lance event is too brief to assess state, the squeeze phase was the period used and corresponded to the period used to measure the heart rate variability responses.
The 10 facial actions of the NFCS14,15 were coded as brow lowering, eyes squeezed shut, deepening of the nasolabial furrow, open lips, vertical mouth stretch, horizontal mouth stretch, taut tongue (cupping of the tongue), chin quiver (high-frequency vibration of the chin), lip purse (tightening the muscles around the lips to form “oo”), and tongue protrusion (tongue pushed forward). Each face action was coded as 1/0 (occurred/did not occur) during each event of blood collection.
The frequency distribution of individual NFCS face actions was examined. Frequency of a facial action of less than 5% during lance and squeeze was used as the cut-off point for including that facial action in the total score. Lip purse was excluded because fewer than 2% of neonates displayed this face action. Tongue protrusion appears to be a stress indicator in preterm but not term-born neonates.21 Tongue protrusion occurred frequently during the invasive phase (25% of neonates during squeeze) and was retained in the total score. Therefore, 9 of the possible 10 face actions were summed to provide a total facial activity score at lance and squeeze.
Finger splay is a motoric stress indicator in the Neonatal Individualized Developmental Care Assessment Program behavioral measurement system.25 Finger splay was defined as the backward extension of the fingers at squeeze and was coded as 1/0 (occurred/did not occur).
Continuous electrocardiographic activity was recorded from a single lead of a surface electrocardiogram (lead II) and digitally sampled at 360 Hz offline using a specially adapted computer-acquisition system and custom physiologic signal-processing software.37 R waves were detected from the sampled electrocardiograph and used to form a smoothed instantaneous 4-Hz heart rate time series as described elsewhere.38 Epochs of heart rate (2.2 minutes each) were selected from the resting baseline period within 5 minutes before the lance and a lance/squeeze period starting within 20 seconds after the lance. The epoch selection criteria were based on quantitative signal stationarity, the presence of a stable behavioral state, and the absence of gross movement artifact. Power spectral estimates of heart rate were quantified using the area (power) of the spectrum in an LF (0.04–0.15 Hz) and HF region (0.15–0.80 Hz), and by the ratio of LF and HF power (LFP/HFP), as previously described.39 To examine reactivity, change from baseline to squeeze (difference scores) was used for the heart rate variability variables (i.e., change in heart rate and LF or HF power, and LFP/HFP at squeeze.
Invasive procedures were defined as those involving tissue damage and included but were not limited to heel lance, venipuncture, insertion of arterial and venous lines, and injections. Intrusive procedures such as suctioning were not included. The procedures were summed to provide a single total, without considering the amount of potential pain from diverse types of experience. Severity of illness was measured using the Score for Neonatal Acute Physiology-II40,41 3 days after birth.
Procedures
A research nurse recruited neonates in the NICU. Real-time observations of the NFCS, neonate sleep/wake state, and hand movements were made by a coder at bedside during routine blood collection, which was performed by a laboratory technician. Data-collection methods using the NFCS at bedside have been described previously.21 The behavioral coders were blind to the purpose of the study and any background and NICU information about the neonates. Coders were trained using videotapes, followed by practice coding in the NICU at bedside, until interobserver agreement (≥85%) was achieved using the conservative formula described previously.14
The neonate’s NICU nurse applied the foot warmer, and the research nurse removed the electrodes from the bedside cardiac monitor cable and switched them to the study computer cable for heart rate data acquisition. Heart rate was recorded continuously during 200 seconds of baseline, throughout blood collection, and for a recovery period of 200 seconds after last contact. Cardiac activity was analyzed from the following three phases: 200 seconds of baseline, 200 seconds starting during squeeze (after the initial reaction to lance), and 200 seconds of recovery. Neonate state was also rated during the three phases of baseline, squeeze, and recovery. The following NFCS actions were rated during six events of blood collection: the last 60 seconds of baseline, first contact by the laboratory technician to remove the heel warmer, swab to cleanse the heel, lance, squeeze, and 60-second recovery period after last contact. The median time from first contact to last contact for the blood-collection procedure was 238 seconds. If a neonate required more than one heel lance, responses were used only from the first lance and squeeze phases.
Data-recording periods differed for physiologic and behavioral data because of the nature of measuring different aspects of neonate reactivity. For variability to be expressed, spectral recordings analysis of cardiac activity necessitates sufficient periods (2.2 minutes) of relative stability to eliminate artifactual effects; thus, broad-band periods are optimal. The neonate state is a construct for which sufficient time is needed to rate, usually a minimum of 60 seconds. In contrast, facial behavior changes rapidly from moment to moment, and brief recording times are needed to reflect changing events. Therefore, reactivity variables used for the correlational analyses included change in autonomic measures (mean heart rate, LFP, and HFP) from baseline to squeeze, LFP/HFP at squeeze, and behavioral measures (state, finger splay, and NFCS facial actions) at squeeze.
A medical chart review for each neonate was carried out prospectively by a neonatal nurse.
Data analysis
Associations between reactivity variables were examined using Pearson product moment correlations for interval data, point biserial correlations for the dichotomous variable (finger splay), and χ2 analyses for ordinal data for characteristics of concordant and discordant responders.
RESULTS
Relations between behavioral and autonomic responses
To compare these results with the few studies reporting correlations for entire samples of preterm neonates, correlations between and among the autonomic and behavioral reactivity variables across the whole sample of neonates to blood collection at 32 weeks PCA are presented in Table 2. When the sample is broken down into the three GA groups (Figs. 1–4), variability across GA groups was evident. Facial activity and sleep/waking state correlated moderately and significantly with change in heart rate for all the GA groups (range of Pearson correlations, 0.41–0.62; all p values <0.01) (Fig. 1), indicating that facial reactivity to blood collection and a more aroused state were related to greater increases in mean heart rate. Facial activity and sleep/waking state did not correlate significantly with changes in LF or HF power or LFP/HFP for any GA group (range of Pearson correlations, −0.31–0.00) (Fig. 1). Finger splay did not correlate significantly with any autonomic recordings in any GA group (range of point biserial correlations, −0.18–0.21) (Fig. 2).
TABLE 2.
Relation between behavioral and autonomic measures for the whole sample (N = 136)
| NFCS | Sleep/waking state | Finger splay | Change in HR | Change in LF power | Change in HF power | |
|---|---|---|---|---|---|---|
| Sleep/waking state | 0.557† | |||||
| Finger splay | 0.233* | 0.102 | ||||
| Change in HR | 0.550† | 0.508† | 0.094 | |||
| Change in LF power | −0.232* | −0.221* | −0.060 | −0.360† | ||
| Change in HF power | −0.155 | −0.152 | −0.096 | −0.330† | 0.680† | |
| LF/HF ratio | −0.111 | −0.153 | −0.012 | −0.120 | 0.326† | −0.053 |
p <0.01.
p <0.001.
NFCS, Neonatal Facial Coding System; HR, heart rate; LF, low-frequency; HF, high-frequency.
FIG. 1.
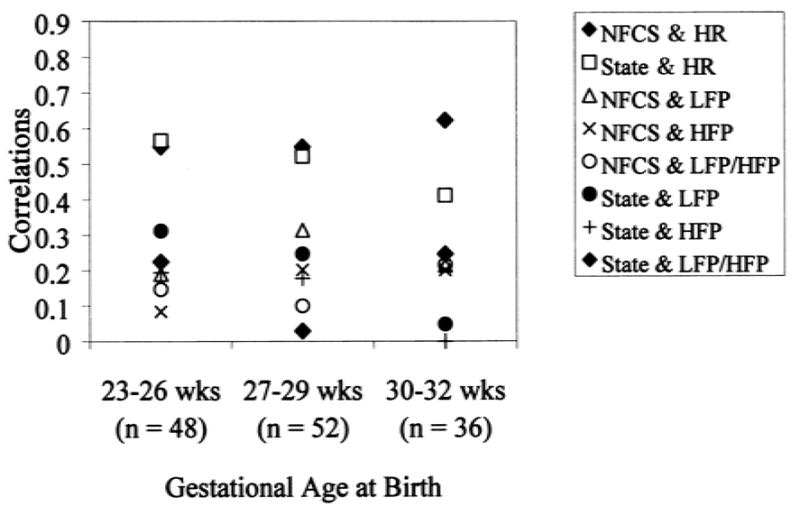
Associations between behavioral and autonomic reactivity at 32 weeks postconceptional age. Symbols greater than r = 0.4 are significant at p <0.01. NFCS, Neonatal Facial Coding System at squeeze; state, state at squeeze; HR, change in heart rate from baseline to squeeze; LFP, change in low-frequency power from baseline to squeeze; HFP, change in high-frequency power from baseline to squeeze; LFP/HFP, ratio of LFP to HFP at squeeze.
FIG. 4.
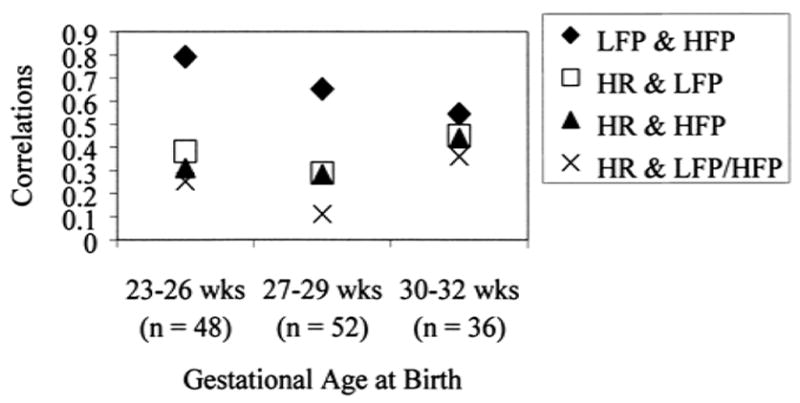
Associations among autonomic reactivity measures at 32 weeks postconceptional age. Symbols at r = 0.3 or greater are significant at p <0.05. HR, change in heart rate from baseline to squeeze; LFP, change in low-frequency power from baseline to squeeze; HFP, change in high-frequency power from baseline to squeeze; LFP/HFP, ratio of LFP to HFP at squeeze.
FIG. 2.
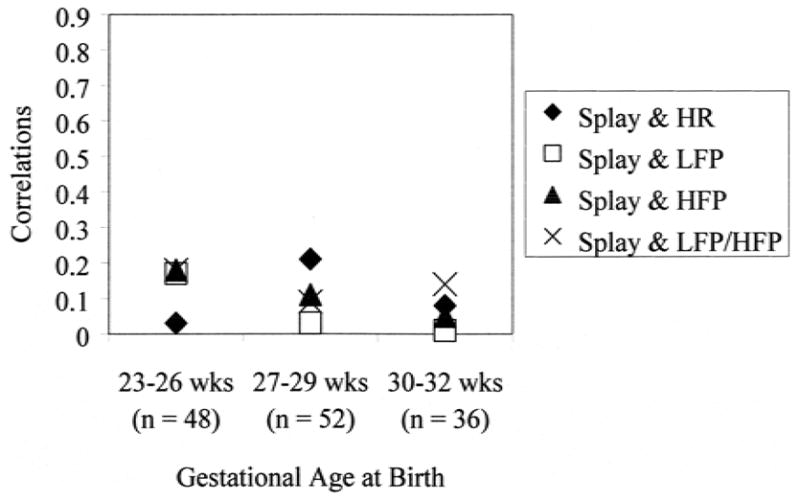
Associations between finger splay and autonomic reactivity at 32 weeks postconceptional age. Symbols greater than r = 0.25 are significant at p <0.01. Splay, finger splay at squeeze; HR, change in heart rate from baseline to squeeze; LFP, change in low-frequency power from baseline to squeeze; HFP, change in high-frequency power from baseline to squeeze; LFP/HFP, ratio of LFP to HFP at squeeze.
Relations among behavioral response measures
Facial activity correlated moderately and significantly with sleep/waking state at squeeze for all GA groups (range of Pearson correlations, 0.50–0.66; p <0.01), indicating that facial reactivity to blood collection was related to a more aroused state (Fig. 3). Facial activity correlated moderately and significantly with finger splay, but only in the 27 to 29 week GA group, in which facial reactivity was significantly related to the presence of finger splaying (point biserial r = 0.43, p <0.01). Finger splay was also correlated with sleep/waking state, but only marginally in the 27 to 29 week GA group, in which a more aroused state was related to finger splaying (point biserial r = 0.27, p = 0.05) (Fig. 3).
FIG. 3.
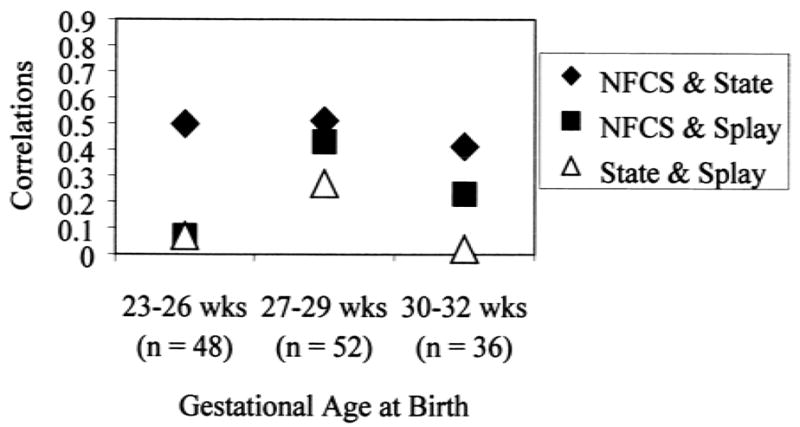
Associations among behavioral reactivity measures at 32 weeks postconceptional age. Symbols greater than r = 0.25 are significant at p ≤ 0.05. NFCS, Neonatal Facial Coding System; state, state at squeeze; splay, finger splay at squeeze.
Relations among autonomic response measures
Low-frequency and high-frequency power spectral estimates were moderately to highly correlated for all GA groups (range of Pearson correlations, 0.54–0.79; all p values <0.01) (Fig. 4). Change in heart rate was significantly related to change in LF and HF power spectral estimates across the GA groups (range of Pearson correlations, −0.44–−0.28), indicating that mean heart rate increases were moderately related to decreases in LF and HF power (Fig. 4). Mean heart rate change was significantly related to LFP/HFP, but only in the 30 to 32 week GA group (r = −0.36, p <0.05).
Characteristics of concordant and discordant responders
To examine the characteristics of neonates who react in a concordant or discordant fashion to blood collection, subjects were divided into four categories according to response above or below the median on facial reactivity to squeeze or change in mean heart rate. The tabulation of these median splits resulted in 52 neonates (38%) who scored above the median on facial and autonomic reactivity (high responders), 42 neonates (31%) who scored below the median on facial and autonomic reactivity (low responders), 26 neonates (19%) who responded with high facial reaction but low autonomic reaction (“externalizers”), and 16 neonates (12%) who responded with low facial reaction but high autonomic reaction (“internalizers”). We then examined whether there were any background or other variables that might differentiate the four groups. There were no differences between these groups regarding GA at birth, birthweight, postnatal age, number of invasive procedures from birth to blood collection at 32 weeks PCA, illness severity (Score for Neonatal Acute Physiology-II on day 3), time since last feeding, or days receiving morphine, fentanyl, pavulon, indomethacin, or midazolam. However, low responders were less likely to show facial activity to first contact by the technician (χ2 [df = 3] = 9.1, p <0.05) and to lance (χ2 [df = 3] = 26.7, p <0.001), and were less likely to show signs of arousal (state) at lance (χ 2 [df = 3] = 20.6, p <0.001).
DISCUSSION
This study was an initial attempt to unravel the relations of association and concordance among and between behavioral and autonomic reactivity to pain in premature neonates at 32 weeks PCA. The findings suggest that these relations are complex and mediated by multiple sources.
Consistent with previous work in older preterm neonates,7 facial reactivity to acute pain was only moderately related to mean heart rate change for the sample as a whole and within all GA groups. The common variance shared between facial and heart rate reactivity was only a modest 28%. Associations between facial reactivity and spectral measures of heart rate variability were much lower and significant only for the measure of change in LF power for the sample as a whole.
Concordance rates between the established biobehavioral response systems of facial reaction and mean heart rate change indicated that some neonates displayed a strong behavioral (external) response to pain with a low physiologic (internal) reaction, whereas others exhibited a high internal response with minimal external reaction. These behaviors may be an early demonstration of what Field36 referred to as “externalizing” and “internalizing.” However, the majority of neonates was concordant in their responses. Subjects exhibited moderate to high biobehavioral responses to painful stimuli in both the facial and cardiac domains, although there were many low responders. Examination of background characteristics of the neonates and their early experience revealed no predetermining factors to any of these reaction “types.” The only factors that differed between groups were the initial facial reactions and arousal to blood collection (lance); low responders were more likely to appear unaroused and have little facial reaction at the start of the procedure compared with neonates in the other three groups. Term-born14 and preterm neonates13 who were asleep during baseline were less likely to demonstrate behavioral and physiologic indicators to pain. Johnston et al.13 found that although the number of painful procedures since birth was not related to reaction, GA at birth and time since last painful procedure were indicators of nonresponse. The major difference between the results of our study and those of Johnston et al.13 could be because they differentiated neonates who did not respond to heel lance from those who did, even to a minimal degree. Johnston et al.13 also used the Premature Infant Pain Profile, a composite pain measure, to assess whether a neonate responded to heel lance, and their subjects were tested at different ages.
The finding of a relation between facial behavior and LF (not HF) power within the whole sample is in accordance with that of Lindh et al.,32 who demonstrated that autonomic response in preterm neonates predominantly reflects changes in sympathetic cardiac modulation (LF changes). Lack of an even modest relation between behavioral measures and specific indices of cardiac modulation might suggest that these autonomic and behavioral indices provide relatively unique information about premature neonates. These findings may also indicate that the cardiac modulation system is not as mature developmentally as the behavioral response system at 32 weeks PCA. A third explanation might be that these changes in HF and LF power estimates may not be adequately captured as single numbers. For example, Grunau et al.42 found that although numerical differences in spectral measures were not significant to a finger lance at 8 months corrected age between former extremely low GA neonates and full-term control neonates, differences between groups were evident in graphic displays of spectral responses.
In the current study, facial reactivity and sleep/waking state were moderately associated across GA groups. These behavioral measures do not appear to be redundant because only 34% of the variance is accounted for in the relation. Most preterm neonates at 32 weeks PCA are in “light sleep” or “active sleep” when undisturbed. Thus, at this stage of development, state appears to capture a global level of behavioral arousal and facial changes are likely to be used by the observer to rate state (e.g., highly aroused). State at this age might be best characterized as global “behavioral arousal” rather than “sleep/wake state,” with the latter meaning of state reserved for neonates older than 36 weeks GA.
Finger splay as a potential indicator of stress seemed to be independent of other behavioral (facial and sleep/waking states) and autonomic reactivity measures except in the 27 to 29 week gestational age group, in which facial reactivity and finger splay were moderately related. Little is known about finger splay and its relation with other behaviors during very early development, but it appears to be a stress indicator.21,26
The low to modest correlations between the behavioral and physiologic response systems to pain may be due to several reasons. In such a young population (extremely low GA neonates), different systems may not have matured and developed on a singular time clock. Response specificity and stimulus specificity may also play a role in discordance or low correlation.43,44 Further, some individuals respond more physiologically and less behaviorally, or vice versa, across all ages.36 Different measurement scales need to be used for the different systems; therefore, variability in means and variances of the responses are present and correlational analyses ignore the potential nonlinearity of relations.44,45 Finally, dissociations between behavioral and physiologic reactivity to pain or stress may occur because behavioral reactions could possibly be influenced by the robustness of the infant. Thus, a more overt behavioral reaction could be a sign of strength and robustness, whereas a less overt reaction could be a sign of weakness and illness (and not necessarily less pain or stress).46 All of these factors need to be considered when relations between different systems are examined.
In conclusion, the findings suggest that for neonates born at very low who are receiving neonatal intensive medical care, it is important to record the different systems separately to attempt to understand the nature of pain in this extremely fragile population. Moreover, it appears that concordance between behavioral and physiologic measures varies between neonates and is not systematically related to early experience. The finding that the majority of neonates (69%) displayed concordance supports the use of composite scales in the NICU. However, this still leaves a considerable proportion of neonates for whom a composite measure may not capture their pain entirely. For practical purposes, continued use of composite scales or simple bedside measures of pain or discomfort, such as changes in facial actions, sleep/waking state, and heart rate is recommended as an important aspect of pain management.
Acknowledgments
The authors thank the staff and families of the Special Care Nursery at British Columbia Children’s Hospital for their participation.
Footnotes
Supported by grant No. 96–0023 from the British Columbia Medical Services Foundation, a Mining for Miracles Postdoctoral Fellowship (to SJM), and a Scholar Award from the British Columbia Health Research Foundation and the British Columbia Research Institute for Children’s & Women’s Health (to REG).
References
- 1.Stevens BJ, Johnston CC, Grunau RVE. Issues of assessment of pain and discomfort in neonates. J Obstet Gynecol Neonatal Nurs. 1995;24:849–55. doi: 10.1111/j.1552-6909.1995.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 2.Stevens B, Johnston CC, Petryshen P, et al. The premature infant pain profile. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Franck LS, Miaskowski C. Measurement of neonatal responses to painful stimuli: a research review. J Pain Symptom Manage. 1997;14:343–78. doi: 10.1016/s0885-3924(97)00222-4. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Saad HH, Bours GJJW, Stevens B, et al. Assessment of pain in the neonate. Semin Perinatol. 1998;22:402–16. doi: 10.1016/s0146-0005(98)80056-6. [DOI] [PubMed] [Google Scholar]
- 5.Grunau RE. Current issues in infant pain assessment; Paper presented at the 9th World Congress on Pain; Vienna. August, 1999. [Google Scholar]
- 6.Johnston CC, Stevens B, Yang F, et al. Differential response to pain by very premature babies. Pain. 1995;61:471–9. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- 7.Porter FL, Wolf CM, Miller JP. The effect of handling and immobilization on the response to acute pain in newborn infants. Pediatrics. 1998;102:1383–9. doi: 10.1542/peds.102.6.1383. [DOI] [PubMed] [Google Scholar]
- 8.Porter FL, Grunau RE, Anand KJS. Long term effects of early pain. J Dev Behav Pediatr. 1999;20:253–61. doi: 10.1097/00004703-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98:925–30. [PubMed] [Google Scholar]
- 10.Rose SA, Schmidt K, Bridger WH. Cardiac and behavioral responsivity to tactile stimulation in premature and full-term infants. Dev Psychol. 1976;12:311–20. [Google Scholar]
- 11.Craig KD, Whitfield MF, Grunau RVE, et al. Pain in the preterm neonate: behavioral and physiological indices. Pain. 1993;52:287–99. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 12.Grunau RE, Oberlander T, Whitfield MF, et al. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks post-conceptional age. Pediatrics. 2001;107:105–12. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Johnston CC, Stevens BJ, Franck LS, et al. Factors explaining lack of response to heel stick in preterm newborns. J Obstet Gynecol Neonatal Nurs. 1999;28:587–94. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 14.Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 15.Grunau RVE, Craig KD. Facial activity as a measure of neonatal pain expression. In: Tyler DC, Krane EJ, editors. Advances in pain research and therapy. Vol. 15. New York: Raven Press; 1990. pp. 147–55. [Google Scholar]
- 16.Grunau RVE, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 17.Rushforth JA, Levene MI. Behavioral response to pain in healthy neonates. Arch Dis Child. 1994;70:F174–6. doi: 10.1136/fn.70.3.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens B, Johnston CC, Horton L. Factors that influence the behavioral pain responses of premature infants. Pain. 1994;59:101–9. doi: 10.1016/0304-3959(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 19.Johnston CC, Stevens B, Yang F, et al. Developmental changes of response to heelstick in preterm infants: a prospective cohort study. Dev Med Child Neurol. 1996;38:438–45. doi: 10.1111/j.1469-8749.1996.tb15101.x. [DOI] [PubMed] [Google Scholar]
- 20.Guinsburg R, Berenguel RC, de Cassia Xavier R, et al. Are behavioral scales suitable for preterm and term neonatal pain assessment? In: Jensen TS, Turner JA, Wiesenfeld-Hallin J, editors. Proceedings of the 8th World Congress on Pain, Progress in pain research and management. Vol. 8. Seattle: IASP Press; 1997. pp. 893–901. [Google Scholar]
- 21.Grunau RE, Oberlander T, Holsti L, et al. Bedside application of the Neonatal Facial Coding System. Pain. 1998;76:277–86. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 22.Scott CS, Riggs KW, Ling E, et al. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr. 1999;135:423–9. doi: 10.1016/s0022-3476(99)70163-0. [DOI] [PubMed] [Google Scholar]
- 23.Rushforth JA, Griffiths G, Thorpe H, et al. Can topical lidocaine reduce behavioral response to heelstick? Arch Dis Child. 1995;72:F49–51. doi: 10.1136/fn.72.1.f49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig KD, Hadjistavropoulos HD, Grunau RVE, et al. A comparison of two measures of facial activity during pain in the newborn child. J Pediatr Psychol. 1994;19:305–18. doi: 10.1093/jpepsy/19.3.305. [DOI] [PubMed] [Google Scholar]
- 25.Als H. Manual for the naturalistic observation of newborn behavior (preterm and fullterm infants) Boston: The Children’s Hospital; 1984. [Google Scholar]
- 26.Grunau RE, Holsti L, Whitfield MF, et al. Are twitches, startles and body movements pain indicators in extremely low birthweight infants? Clin J Pain. 2000;16:37–45. doi: 10.1097/00002508-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Prechtl HFR, O’Brien MJ. Behavioral states of the full-term newborn. The emergence of the concept. In: Stratton P, editor. Psychobiology of the human newborn. New York: Wiley and Sons; 1982. pp. 53–76. [Google Scholar]
- 28.Doussard-Roosevelt J, Porges SW, McClenny BD. Behavioral sleep states in very low birth weight preterm neonates: relation to neonatal health and vagal maturation. J Pediatr Psychol. 1996;21:785–802. doi: 10.1093/jpepsy/21.6.785. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh N, Van Veen L, Brameyer H. The pain of heel prick and its measurement in preterm infants. Pain. 1993;52:71–4. doi: 10.1016/0304-3959(93)90116-7. [DOI] [PubMed] [Google Scholar]
- 30.Hopf H-B, Skyschally A, Heusch G, et al. Low-frequency spectral power of heart rate variability is not a specific marker of cardiac sympathetic modulation. Anesthesiology. 1995;82:609–19. doi: 10.1097/00000542-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Malliani A, Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 32.Lindh V, Wiklund U, Sandman P-O, et al. Assessment of acute pain in preterm infants by evaluation of facial expression and frequency domain analysis of heart rate variability. Early Hum Dev. 1997;48:131–42. doi: 10.1016/s0378-3782(96)01851-8. [DOI] [PubMed] [Google Scholar]
- 33.Oberlander T, Grunau RE, Pitfield J, et al. The developmental character of cardiac autonomic responses to an acute noxious event in four and eight month old healthy infants. Pediatr Res. 1999;45:519–25. doi: 10.1203/00006450-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 34.Oberlander T, Grunau RVE, Whitfield MFW, et al. Biobehavioral pain responses in former extremely low birthweight infants at 4 months corrected age. Pediatrics. 2000;105:e6. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- 35.Aarimaa T, Oja R, Antila K, et al. Interaction of heart rate and respiration in newborn babies. Pediatr Res. 1988;24:745–50. doi: 10.1203/00006450-198812000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Field T. Individual differences in the expressivity of neonates and young infants. In: Feldman RS, editor. Development of nonverbal behavior in children. New York: Springer-Verlag; 1982. pp. 279–98. [Google Scholar]
- 37.Boston Medical Technologies. HRView software. Brighton, MA: Boston Medical Technologies; 1996. Version 2.0. [Google Scholar]
- 38.Berger RD, Saul JP, Cohen RJ. Transfer function analysis of autonomic regulation: I. Canine atrial rate response. Am J Physiol. 1989;256:H142–52. doi: 10.1152/ajpheart.1989.256.1.H142. [DOI] [PubMed] [Google Scholar]
- 39.Saul JP, Berger RD, Albrecht P, et al. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261(1):H1231–45. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- 40.Chien LY, Lee SK, Thiessen P, et al. Validation of the SNAP and SNAP-II on morbidity of infants less than 33 weeks gestational age. Pediatr Res. 1999;45:240. abstract. [Google Scholar]
- 41.Lee SK, Ohlsson A, Synnes AR, et al. Mortality variations and illness severity (SNAP-II) in Canadian NICUs. Pediatr Res. 1999;45:248. abstract. [Google Scholar]
- 42.Grunau RE, Oberlander TF, Whitfield MF, et al. Pain reactivity in former extremely low birthweight infants at corrected age 8 months compared with termborn controls. Infant Behavior Development. in press. [Google Scholar]
- 43.Eysenck HJ. Dimensions of personality: the biosocial approach to personality. In: Strelau J, Angleitner A, editors. Explorations in temperament: international perspectives on theory and measurement. New York: Plenum Press; 1991. pp. 87–103. [Google Scholar]
- 44.Kagan J. Behavior, biology, and the meanings of temperamental constructs. Pediatrics. 1992;90:510–3. [PubMed] [Google Scholar]
- 45.Green JA. Analyzing individual differences in development: correlations and cluster analysis. In: Colombo J, Fagen J, editors. Individual differences in infancy: reliability, stability, prediction. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 77–109. [Google Scholar]
- 46.Barr RG. Reflections on measuring pain in infants: dissociation in responsive systems and ‘honest signalling’ . Arch Dis Child. 1998;79:F152–6. doi: 10.1136/fn.79.2.f152. [DOI] [PMC free article] [PubMed] [Google Scholar]


