Abstract
Studies have shown that smoked and intravenous cocaine’s effects differ in cocaine-dependent women compared to men and across the menstrual cycle. However, this has not been systematically investigated with intranasal cocaine. Thus, a range of intranasal cocaine doses was examined in cocaine-dependent women across the menstrual cycle. Female cocaine users were admitted to the hospital once during the luteal phase and once during the follicular phase of their menstrual cycle; menstrual cycle phase during admissions was counterbalanced. During each admission, an intranasal cocaine dose–response curve (0.06, 0.34, 0.69 and 1.37 mg/kg) was determined during four laboratory sessions. Cocaine produced similar dose-related increases in ratings of ‘‘positive’’ subjective effects, cardiovascular effects and cocaine plasma levels in women in both menstrual cycle phases. To assess sex differences in the effects of intranasal cocaine, the current data were compared to published data collected in men using an identical procedure. Cocaine produced similar dose-related increases in ratings of positive subjective effects, cardiovascular effects and cocaine plasma levels in men and women. Thus, in contrast to studies examining smoked or intravenous cocaine administration, there were no sex differences or menstrual cycle effects on the subjective or cardiovascular response to intranasal cocaine, suggesting that the influence of sex and menstrual cycle on cocaine’s effects vary as a function of route of administration.
Keywords: Cocaine, Humans, Sex Differences, Menstrual Cycle, Plasma levels, Subjective effects, Cardiovascular effects
1. Introduction
Recently, cocaine has been responsible for more emergency room visits nationwide than any other illegal drug (DAWN, 2004; CEWG, 2006), demonstrating that cocaine use is still a widespread public health issue. Although there are a number of studies on the abuse liability of smoked cocaine (e.g. Sofuoglu et al., 2000; Evans et al., 2002; Foltin et al., 2003), many cocaine-dependent individuals initiated cocaine use via the intranasal route (Gorelick, 1992). However, there are few published studies systematically examining intranasal cocaine.
A 2003 nationwide household survey found that 30% of individuals who used cocaine in the past year were women (SAMHSA, 2004) and that more women initiated cocaine use at a younger age than men (NSDUH, 2002). Further, length of time from initiation of cocaine use to onset of abuse and dependence has been shown to be shorter in women compared to men (Ridenour et al., 2005). Thus, women are more vulnerable to cocaine dependence after initiation of cocaine use compared to men. It is not clear whether women have a different subjective response to intranasal cocaine than men, as the effects of intranasal cocaine in females compared to males has yet to be systematically examined.
Intravenous and smoked cocaine’s effects differ in cocaine-dependent women compared to men and across the menstrual cycle, but the results have varied as a function of route of administration and experimental paradigm (for review, see Turner & de Wit, 2006). In response to a single administration of intravenous cocaine, no difference was found in the subjective and cardiovascular effects of 0.2 mg/kg or 0.4 mg/kg cocaine between men and women in the follicular or luteal phase of their menstrual cycle (Mendelson et al., 1999). In contrast, repeated administration of a range of intravenous cocaine doses produced a greater response in ratings of “Stimulated” in cocaine-dependent men compared to cocaine-dependent women, and greater cardiovascular effect in cocaine-dependent women compared to cocaine-dependent men (Haney et al., 1998).
Repeated administration of a single dose of smoked cocaine (0.4 mg/kg; Sofuoglu et al., 1999) or a range of smoked cocaine doses (Evans et al., 2002; Evans & Foltin, 2006) produced a similar increase in the positive subjective effects in men and women in their follicular phase. However, the response to cocaine was reduced in women in their luteal phase compared to when they were in their follicular phase or compared to men (Sofuoglu et al., 1999; Evans et al., 2002; Evans & Foltin, 2006). Together, these studies suggest that sex or menstrual cycle phase effects may emerge in response to repeated administration of a range of intravenous or smoked cocaine doses. However, the influence of sex and menstrual cycle on the subjective and cardiovascular effects of a range of intranasal cocaine doses has not been determined.
In response to a single administration of one dose (0.9 mg/kg) of intranasal cocaine, men had higher plasma cocaine levels than women, but there was no difference in subjective and cardiovascular effects between men and women in the follicular or luteal phase of their menstrual cycle (Lukas et al., 1996). However, repeated administration of one dose of intranasal cocaine (2 mg/kg) produced a greater increase in ratings of “Nervous” (Kosten et al., 1996) and four administrations of a single dose of cocaine (1 mg/kg) produced a greater increase in ratings “Feel Good” (McCance-Katz et al., 2005) in women compared to men. These data suggest that women are more sensitive to cocaine’s subjective effects, yet no conclusions can be drawn without a full dose-response curve determination.
The primary purpose of the current study was to examine the subjective and cardiovascular effects of two administrations of a range of intranasal cocaine doses in cocaine-dependent women in the luteal phase compared to the follicular phase of the menstrual cycle. A secondary purpose was to compare the same cocaine-dependent women to a group of cocaine-dependent men to determine if sex differences are present. Although the effects of intranasal cocaine in the cocaine-dependent men have been presented elsewhere (Foltin & Haney, 2004), the data will be presented here as well, for comparison purposes, since the same experimental conditions were used as in the current study. We hypothesized that cocaine-dependent women would have a greater response to intranasal cocaine during the follicular phase than the luteal phase of their menstrual cycle. Additionally, we hypothesized that cocaine-dependent women in the follicular phase would have a similar response to intranasal cocaine compared to cocaine-dependent men, but an attenuated response during the luteal phase.
2. Methods
2.1. Participants
Eight female research volunteers (five African-American, two Caucasian, one Hispanic), 26 to 44 years of age (mean = 33.4 years) and with an average of 14.3 ± 2.3 (mean ± S.D.) years of education, participated in this study. Seven of the participants smoked an average of 13.3 ± 8.3 tobacco cigarettes per day. The participants reported using cocaine by the intranasal route for the past 9.9 ± 8.9 years, using cocaine 3.1 ± 1.9 days per week, and spending US $150 ± 113 per week on cocaine (the cost of cocaine was about US $30/g in the New York City area when these data were collected). Four additional participants were enrolled in the protocol: three participants left after the first inpatient phase for personal reasons and one participant was excluded from the analyses because hormone levels indicated that ovulation had not occurred.
The data for the group of men who participated in this study are presented in detail in a previously published paper (Foltin & Haney, 2004), and will be briefly described here. Ten male research volunteers (six African-American, three Hispanic, one Caucasian), 25 to 44 years of age (mean = 35.8 years) and with an average of 12.1 ± 0.04 (mean ± S.D.) years of education, participated in this study. Seven of the participants smoked an average of 16.0 ± 3.0 tobacco cigarettes per day. The participants reported using cocaine for the past 17.9 ± 2.5 years, using cocaine 4.2 ± 0.4 days per week, and spending US$260 ± 53 per week on cocaine. One additional participant was enrolled in the protocol but left after the first inpatient phase for personal reasons.
All participants met DSM-IV criteria for cocaine dependence but did not meet criteria for dependence on any other illicit drug or on alcohol. All the participants passed the medical evaluation prior to the study, and no one was receiving psychiatric treatment. None were seeking treatment for their drug use, and none of the participants were using hormonal contraceptives, or any other prescription medication. Also, women were not pregnant (based on blood pregnancy tests) or lactating, and had not had an abortion or been pregnant within the previous 6 months. Lastly, none of the female participants suffered from premenstrual dysphoric disorder. Each signed a consent form, approved by the Institutional Review Boards of The College of Physicians and Surgeons of Columbia University and The New York State Psychiatric Institute, which described the study, outlined the possible risks, and indicated that cocaine would be administered.
2.2. Procedure
After signing the study consent form, all participants began filling out daily rating forms each evening; they were paid to report to the laboratory twice a week to return completed forms and pick up new forms. For women, this was done to ensure continued outpatient contact in order to monitor the menstrual cycle and schedule the various inpatient phases. The forms asked questions related to daily mood and physical symptoms that vary across the menstrual cycle and women indicated whether they were menstruating. Female participants were prospectively tracked for several weeks before the first inpatient admission, and throughout the study, to determine menstrual cycle length and time of ovulation. They were instructed to notify the research nurse when menstruation started. During the midfollicular phase, female participants provided daily urine samples to determine the time of ovulation using OvuQuick® (QUIDEL Corp., San Diego, CA; Martini et al., 1994). This test is simple to use and is 96–99% accurate at detecting luteinizing hormone (LH) in urine. The day of ovulation was used to schedule the midluteal admission. To provide consistency, men filled out a comparable daily rating form (excluding questions regarding menses), and where appropriate questions were modified (e.g. breast tenderness for women became skin tenderness for men).
The participants were admitted to the Irving Center for Clinical Research in the Presbyterian Hospital. Their private hospital rooms were equipped with a television, radio, and VCR; videotaped movies were provided to them. The rooms contained an air purification system, and the participants were free to smoke tobacco cigarettes in their rooms. Visitors were prohibited. Female cocaine users completed a set of sessions during two different inpatient admissions, during two different menstrual cycle phases. One inpatient admission was scheduled during the follicular phase so that cocaine administration sessions occurred between 6–10 days after the onset of menstruation. The second inpatient admission was scheduled during a normal midluteal phase (approximately 7–12 days after the urinary ovulation test kit indicated that ovulation had occurred) characterized by elevated estradiol and progesterone levels.
Participants were admitted in counter-balanced order with regard to menstrual cycle phase. Of the female cocaine users who completed the study, three were in the luteal phase and five were in the follicular phase for their first hospital admission. Male cocaine users also completed a set of sessions during two different inpatient admissions. For both women and men, the day following each inpatient admission, participants engaged in laboratory sessions twice each day for 2 days and were discharged on the fourth inpatient day. A different dose of cocaine was tested during each laboratory session. In the natural ecology, cocaine is used repeatedly within one episode of use; thus to better mimic this pattern, participants snorted 4 'lines' of cocaine twice per session.
2.3. Experimental sessions
Volunteers participated in laboratory sessions twice/day for 2 days: The first session each day began at 0900 h, and the second session each day began at 1500 h. Each session lasted approximately 2 hr. There were 5 hr 20 min between the last dose of cocaine administered during the first session and the first dose of cocaine administered during the second session.
During the experimental sessions, the participants were seated in a reclining chair in front of a Macintosh computer and video monitor with a mouse manipulandum. An 18-gauge catheter (Quik-Cath, Travenol Laboratories, Deerfield, IL) was inserted in a subcutaneous vein on one arm: The intravenous line was available for drawing blood to determine hormone levels and if needed in an emergency during the cocaine sessions. Electrocardiograms (ECGs) were monitored continuously with chest electrodes (MAC PC, Marquette Electronics, Milwaukee, WI), and heart rate (HR) and blood pressure (systolic, SP; diastolic, DP) were recorded every 2 min (Sentry II-Model 6100 automated vital signs monitor, NBS Medical, Costa Mesa, CA) beginning 20 min prior to cocaine administration. An Apple IIci computer located in an adjacent control room was used for automated data collection. The participants were monitored via a one-way mirror by a physician and research nurses located in the adjacent room, with whom they could communicate via an intercom system.
Each of the four laboratory sessions during each admission consisted of a 20-min resting baseline, followed by the insufflation of one cocaine dose (0.06, 0.34, 0.69, 1.37 mg/kg) at Time 0, and a second insufflation of the same dose at Time 40 min. Sessions continued for 110 min after the first dose was self-administered. Subjective-effects questionnaires were completed at baseline and at 4, 14, 34, 44, 54, 74, and 104 min. The corresponding time points were calculated for the cardiovascular measures by obtaining the mean of the data collected in 10-min bins beginning 2 min before each mood scale was started, e.g. −14 to −4 min, 2 to 12 min. The dosing order and time of testing (a.m. vs. p.m.) were balanced across participants within each hospital stay and varied between the two times that each participant was in the hospital.
Cocaine hydrochloride was administered as 120 mg of white powder (cocaine and lactose), handed to the participants on a 50 x 50 cm mirror. The participants prepared four similar size lines of powder with a single-edged razor blade and insufflated the powder, two lines per nostril, within 60 s using a 7.5-cm straw (e.g. Foltin et al., 1988). Cocaine was not given if cardiovascular activity was above the criteria for safe drug administration (i.e. HR>130, DP>100, SP>165). Participants could always refuse a dose at each trial, but no participant refused a dose.
The subjective-effects questionnaire consisted of a series of 100-mm visual analog scales (VAS) anchored by not at all (0 mm) at one end and extremely (100 mm) at the other end. To reduce the number of dependent variables, our laboratory has been publishing data using cluster analysis for our VAS which measures the subjective effects of cocaine since 2002 (e.g. Evans et al., 2002; Foltin & Haney, 2004). A cluster analysis of previous subjective effects of cocaine yielded five clusters of adjectives that are correlated with the changes in one item being predictive of the changes in the other items in the same cluster, but not predictive of changes in items in the other clusters (Evans et al., 2002). Each cluster was derived by taking the arithmetic average of the items in the cluster. In all, 20 of the VAS items resulted in the following five clusters: ‘Bad drug effect’ consisted of seven items related to negative drug effects (e.g. ‘bad drug effect’, ‘anxious’), ‘self-esteem’ consisted of five items (e.g. ‘self-confident’, ‘social), ‘calm’ consisted of two items (‘calm’ and ‘able to concentrate’), ‘good drug effect’ consisted of three items (‘high’, ‘good drug effect’, and ‘stimulated’), and ‘drug quality’ consisted of three items related to the cocaine dose the participant had just received (‘drug quality’, ‘drug potency’, and ‘drug liking’). Four VAS were used to operationalize drug craving, and were labeled ‘I want…’ ‘…cocaine’, ‘…heroin’, ‘…ethanol’, and ‘…nicotine’. A final question asked the participants ‘How much would you pay for the dose you just received?’ with a range of $0–25.
Venous blood was collected for the determination of cocaine and metabolite plasma levels at −20 min (before the first dose), 14 and 34 min after the first dose, and 14, 34, 64, 94, and 140 min after the second dose. Blood drawn 94 and 140 min after the second dose (session time 136, 180 min) was obtained in the participants’ rooms. At each blood withdrawal, 6 cc of whole blood was placed in a glass centrifuge with a small amount of sodium fluoride. The bloods were then centrifuged, yielding 3 cc of plasma for the determination of cocaine levels (Isenschmid et al., 1988; Cone, 1995).
2.4. Hormone Assays
Each experimental day, before cocaine administration, venous blood samples (approximately 6 ml) for estradiol and progesterone were drawn from an indwelling catheter into tubes containing SST® gel and clot activator. Samples were centrifuged within 30 min of collection, yielding approximately 3 ml of plasma, and stored frozen until the time of analysis. Estradiol and progesterone levels were determined by Dr. Michel Ferin at the College of Physicians and Surgeons of Columbia University, Department of Obstetrics and Gynecology (New York, NY). Estradiol and progesterone were measured by a commercial solid-phase, chemiluminescent immunoassay (Immulite, Diagnostic Products Co., DPC, Los Angeles, CA). For estradiol, the assay sensitivity was 4 pg/ml and the intra- and interassay coefficients of variation were 4.3 and 10.5%. For progesterone, the assay sensitivity was 0.2 ng/ml and the intra- and interassay coefficients of variation were 4.8 and 9.1%.
2.5. Cocaine
Cocaine hydrochloride (provided by The National Institute on Drug Abuse) was weighed out by the Presbyterian Hospital Manufacturing Pharmacy to yield prepackaged doses of 0.06, 0.34, 0.69, and 1.37 mg/kg (rounded to the nearest 10 kg), with a maximal cocaine dose of 120 mg (participants had to weigh < 200 lbs). The cocaine was combined with lactose to yield 120 mg of total powder. The 0.06-mg/kg dose, which, when insufflated, produces minimal cardiovascular and subjective effects but does produce a slight numbing of the nasal mucosa (Foltin et al., 1988; Javaid et al., 1978), served as an active placebo dose.
2.6. Data analysis
2.6.1 Menstrual Cycle Phase Effects
Due to technical difficulties, the cocaine plasma samples were not obtained during one session for one woman. To not exclude the data from this participant, the plasma levels from the 0.06 mg/kg session during the luteal phase was also used for the 0.06 mg/kg session for the follicular phase. Twelve additional plasma samples for cocaine were not collected due to technical difficulties with blood drawing, such that, including the missing sessions, 20 blood samples out of 384 were not collected, i.e. 5% plasma samples were missing. Because the pattern of the missing values was not related to cocaine dose or maintenance condition, i.e. data were missing at random (Little & Rubin, 1987), plausible estimates of the missing values were calculated using multiple imputation (Rubin & Schenker, 1991).
The subjective effects clusters and cardiovascular effects data were collapsed over time within session for the analyses. Mean subjective clusters, mean cardiovascular effects and peak cocaine plasma levels were compared using an analyses of variance (ANOVA) with two within-participants factors: menstrual phase (follicular and luteal hospital admission) and cocaine dose (four levels). Estradiol and progesterone levels were compared using an ANOVA with menstrual phase as a within-subject factor. The results were considered statistically significant at P ≤ 0.05, using Huynh-Feldt adjustments as a conservative measure to control for potentially uncorrelated within-subject data. Helmhert orthogonal contrasts were accomplished following a significant dose effect: The 0.06 mg/kg dose was compared with the 0.34, 0.69, and 1.37 mg/kg doses; the 0.34 mg/kg dose was compared with the 0.69 and 1.37 mg/kg doses; and the 0.69 mg/kg dose was compared with the 1.37 mg/kg dose. Planned contrasts were used to compare the luteal and follicular phase at each cocaine dose. The planned contrasts were single degree of freedom comparisons that used the error term for the Dose x Phase interaction.
2.6.2. Sex Differences
In the male group, one of the second cocaine doses during one session was withheld due to elevated cardiovascular activity. Because this was the only dose out of the 160 total possible doses that was not given, the data from this session were still included in the analyses rather than excluding all of the data from this participant.
Due to technical difficulties, the plasma samples were not obtained during one session for two participants. To not exclude the data from these two participants, the values from the same dosing condition during the other testing phase were used in the analysis; that is, results from the second 0.69 mg/kg session were also used for the first 0.69 mg/kg session for one participant, and results from the first 0.06 mg/kg session were also used for the second 0.06 mg/kg session for one participant. Thirteen additional plasma samples were not collected due to technical difficulties with blood drawing, such that, including the two missing sessions, 29 blood samples out of 640 were not collected, i.e. < 5% plasma samples were missing.
The subjective effects clusters and cardiovascular effects data were collapsed over time within session for the analyses. Because there were no significant effects of test session for men (first vs. second hospital admission) and no significant menstrual cycle phase effects in women, the data were collapsed across admission. Mean subjective clusters, mean cardiovascular effects and peak cocaine plasma levels were compared using analyses of variance (ANOVA) with sex as the between-participants factor and cocaine dose as the within-participants factors. The results were considered statistically significant at P ≤ 0.05. Helmhert orthogonal contrasts were accomplished following a significant dose effect, as described above. Planned contrasts were used to compare men and women at each cocaine dose. The planned contrasts were single degree of freedom comparisons that used the error term for the Dose x Sex interaction.
3. Results
3.1. Menstrual Cycle Phase Effects
Women had normal ovulatory menstrual cycles ranging from 23 to 30 days. In women, estradiol levels were not significantly different in the luteal phase compared to the follicular phase (81.29 ± 17.74 ng/ml vs. 73.64 ± 27.70 ng/ml). Inspection of individual participant estrogen levels revealed that this was due to one participant who had elevated estradiol levels (210 pg/ml) in the follicular phase despite the fact that she was tested on days 8 and 9 of the follicular phase. Progesterone levels in the luteal phase were significantly higher than in the follicular phase (6.14 ± 1.37 ng/ml vs. 0.66 ± 0.15 ng/ml; p = 0.003; only p-values will be presented for planned comparisons).
Women in their luteal and follicular phases had a similar significant cocaine dose-related increase in HR, SP and DP [F(3,21) > 5.06, p ≤ 0.009]. During the follicular phase, there was an average peak increase of 8 bpm in HR, 6 mm/Hg in SP and 6 mm/Hg in DP between the lowest (0.06 mg/kg) and highest (1.37 mg/kg) dose of cocaine. During the luteal phase, there was an average peak increase of 14 bpm in HR, 6 mm/Hg in SP and 7 mm/Hg in DP between the lowest and highest dose of cocaine. There was no significant menstrual cycle phase effect on any of the cardiovascular measures.
Figure 1 shows selected mean subjective effects clusters as a function of cocaine dose and menstrual cycle phase. Women in their luteal and follicular phases had a similar significant cocaine dose-related increase in ratings of the Good Drug cluster and the Cocaine Dose cluster [F(3,21) = 5.21, p ≤ 0.05]. Women in their luteal phase rated the Good Drug cluster and the Cocaine Dose cluster greater after 1.37 mg/kg cocaine compared to 0.06 mg/kg cocaine (p ≤ 0.02). There was a single menstrual phase effect [F(3,21) = 8.00, p = 0.03] with women in their luteal phase rating the Bad Drug cluster greater than during their follicular phase after 0.06 mg/kg cocaine (p ≤ 0.01). There was no significant menstrual cycle phase or cocaine dose effect in ratings of the Calm and Self-Esteem clusters.
Figure 1.
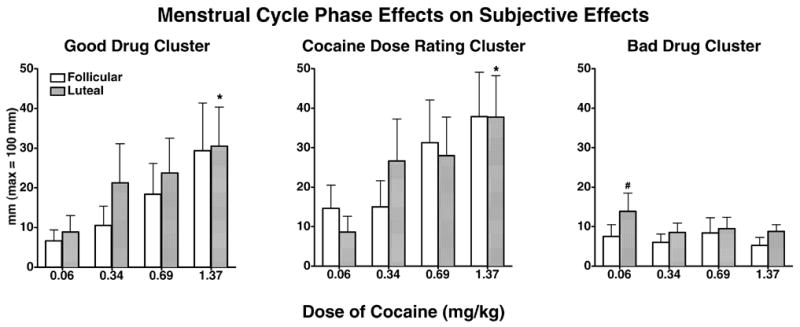
Mean scores on the good drug effect cluster, cocaine dose rating cluster and bad drug effect cluster as a function of cocaine dose and menstrual cycle phase. Bars represent the mean + 1 S.E.M. # represents p ≤ 0.05 when comparing 0.06 mg/kg cocaine in the luteal phase to 0.06 mg/kg cocaine in the follicular phase; * represents p ≤ 0.05 when comparing 1.37 mg/kg cocaine in the luteal phase to 0.06 mg/kg cocaine in the luteal phase.
Table 1 shows that women in their luteal and follicular phases had a similar significant cocaine dose-related increase in peak cocaine plasma levels [F(3,15) = 14.64, p = 0.004]. There were also significant cocaine dose-related increases in peak BZ and EME levels [F(3,15) > 6.83, p < 0.02]. There were no significant phase effects in peak cocaine plasma or metabolite levels.
Table 1.
Comparison of Peak Cocaine Plasma Levels as a Function of Intranasal Cocaine Dose and Menstrual Cycle Phase in Females
| Follicular | Luteal | |
|---|---|---|
| Cocaine levels (ng/ml)a | ||
| 0.06 mg/kg | 28.2 (± 29.8) | 44.5 (± 33.5) |
| 0.34 mg/kg | 80.7 (± 15.6) | 72.7 (± 19.3) |
| 0.69 mg/kg | 114.7 (± 16.0) | 129.7 (± 17.4) |
| 1.37 mg/kg | 284.7 (± 73.7) | 354.3 (± 94.4) |
Values represent the peak and ± 1 SEM.
3.2. Sex Differences
There was a significant cocaine dose-related increase in HR, SP and DP [F(3,48) > 17.46, p = 0.0001] in men and women. In women, there was an average peak increase of 10 bpm in HR, 7 mm/Hg in SP and 6 mm/Hg in DP between the lowest and highest dose of cocaine. In men, there was an average peak increase of 14 bpm in HR, 14 mm/Hg in SP and 10 mm/Hg in DP between the lowest and highest dose of cocaine. SP was significantly higher in women after 0.06 mg/kg cocaine only, compared to men (121 mm/Hg vs. 113 mm/Hg; p = 0.020). There was no significant sex difference in HR and DP between men and women after any cocaine dose.
Figure 2 shows selected mean subjective effects clusters as a function of cocaine dose and sex. Men and women had a similar significant cocaine dose-related increase in ratings of the Good Drug cluster and the Cocaine Dose cluster [F(3,48) > 14.88, p = 0.0001]. Post-hoc tests showed that the Good Drug cluster and the Cocaine Dose cluster were rated significantly greater after the highest dose of cocaine compared to 0.06 mg/kg cocaine in both men and women (p = 0.03). There was a significant effect of sex on ratings of the Self-Esteem [F(1,16) = 6.62, p = 0.020] and Calm [F(1,16) = 8.91, p = 0.009] clusters, with men rating both clusters significantly greater than women, regardless of the cocaine dose (p < 0.05). There was no significant sex or cocaine dose effect in ratings of the Bad Drug cluster.
Figure 2.
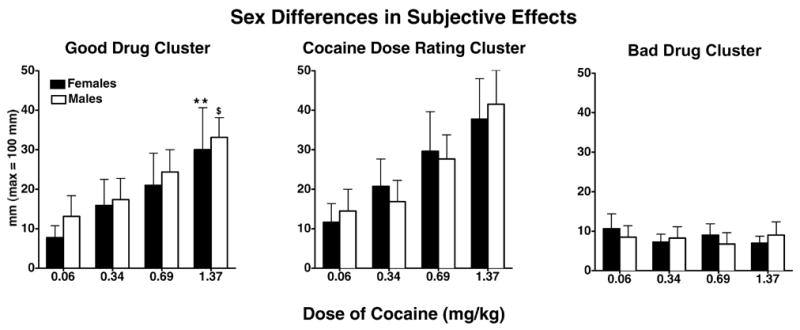
Mean scores on the good drug effect cluster, cocaine dose rating cluster and bad drug effect cluster as a function of cocaine dose and sex. Bars represent the mean + 1 S.E.M. ** represents p ≤ 0.05 when comparing 1.37 mg/kg cocaine in women to 0.06 mg/kg cocaine in women; $ represents p ≤ 0.05 when comparing 1.37 mg/kg cocaine in men to 0.06 mg/kg cocaine in men.
Table 2 shows that there was a similar significant cocaine dose-related increase in peak cocaine plasma levels in men and women [F(3,90) = 69.31, p = 0.0001], but no significant effect of sex. Additionally, for peak BZ levels, there was a cocaine dose effect [F(3,42) = 28.21, p = 0.0001], but no sex effect. However, for peak EME levels, there was a cocaine dose effect [F(3,42) = 24.70, p = 0.0001] and a sex effect [F(1,42) = 10.30, p = 0.006]; men had significantly higher EME levels than women only after the highest cocaine dose (p = 0.02).
Table 2.
Comparison of Peak Cocaine Plasma Levels as a Function of Cocaine Dose and Sex
| Females | Males | |
|---|---|---|
| Cocaine levels (ng/ml)a | ||
| 0.06 mg/kg | 31.1 (± 18.8) | 36.0 (± 6.4) |
| 0.34 mg/kg | 71.2 (± 9.1) | 84.7 (± 8.6) |
| 0.69 mg/kg | 115.7 (± 8.3) | 155.4 (± 19.6) |
| 1.37 mg/kg | 319.5 (± 55.5) | 342.4 (± 32.0) |
Values represent the peak and ± 1 SEM collapsed across phase (2 phases).
4. Discussion
In the current study, there were few differences in subjective or cardiovascular effects or cocaine plasma levels after repeated administration of a range of intranasal cocaine doses in women in their follicular phase compared to when they were in their luteal phase. In contrast, the positive subjective effects of repeated administration of a range of smoked cocaine doses were greater during the follicular phase than the luteal phase in women (Sofuoglu et al., 1999; Evans et al., 2002; Evans & Foltin, 2006). Further, there were menstrual cycle phase effects in diastolic pressure after smoked cocaine (Evans et al., 2002; Evans & Foltin, 2006; but see Sofuoglu et al., 1999). Together, these studies show that the effects of intranasal cocaine do not differ as a function of menstrual cycle phase, whereas the effects of smoked cocaine do differ between the luteal and follicular phases. Thus, it appears that route of administration plays a role in interaction between menstrual cycle phase and the subjective and cardiovascular effects of cocaine in women.
In terms of sex differences, there was no difference in positive subjective effects, cardiovascular effects or cocaine plasma levels after intranasal cocaine in men compared to women in either menstrual phase in the current study. In contrast, greater increases in positive ratings of cocaine and systolic pressure were observed in men compared to women after a range of self-administered intravenous cocaine doses (Haney et al., 1998). In response to smoked cocaine, subjective and cardiovascular effects of repeated doses of a range of cocaine doses were reduced in women during their luteal phase compared to men (Sofuoglu et al., 1999; Evans et al., 2002; Evans & Foltin, 2006). These studies show that smoked and intravenous cocaine produced differential effects, whereas intranasal cocaine produced similar effects, in men and women. Thus, similar to the effects of cocaine across the menstrual cycle, these studies suggest that sex differences in cocaine’s subjective and cardiovascular effects vary as a function of route of administration. Interestingly, there were no menstrual cycle phase or sex differences in cocaine plasma levels after intranasal (current study) or smoked (Sofuoglu et al., 1999; Evans et al., 2002; Evans & Foltin, 2006) cocaine, suggesting a dissociation between cocaine plasma levels and subjective and cardiovascular effects.
The current study is the first to comprehensively examine the effects of range of repeated intranasal cocaine doses in women across their menstrual cycle and compared to men. Numerous studies have shown sex differences in cocaine-related behavior in laboratory rodents (e.g. Lynch and Carroll, 2000; Roth and Carroll, 2004). For example, responding to i.v. cocaine self-administration was more variable in females than males, particularly during the estrus phase of their estrous cycle (Lynch et al., 2000). These differences may be a result of gonadal hormone influence. Estradiol administration enhanced acquisition of cocaine self-administration in ovariectomized female rats, and progesterone inhibited this enhancement (Jackson et al., 2006). These studies suggest that hormone levels modulate the reinforcing effects of cocaine. However, the results of the current study and other studies examining the effects of repeated doses of a range of cocaine doses in women across menstrual cycle and compared to men vary as a function of route of cocaine administration.
With respect to intranasal cocaine, the current results do not support the hypothesis that women have a greater response to cocaine than men or that there are differences in cocaine’s effects across menstrual cycle. However, other studies have shown that women have a greater response to the positive effects of smoked cocaine than men (e.g. Evans & Foltin, 2006). Thus, these findings suggest smoked cocaine has a differential effect than intranasal route. This may be due to the differential pharmacokinetics of the intranasal and smoked routes of cocaine administration. For example, intranasal cocaine has a slower onset of action after than smoked cocaine (Cone, 1995, 1998). Further, drug concentrations in the brain may be higher after smoked compared to the intravenous or intranasal cocaine (Cone, 1995, 1998), which in turn may produce a more pronounced difference in subjective effects in women in different stages of their menstrual cycle or compared to men.
The strengths of the present study are that 1) a full dose–response function for intranasal cocaine (0.06, 0.34, 0.69, and 1.37 mg/kg) was determined; 2) data collection was within subject, which reduced error variance and increased statistical power; and 3) menstrual cycle was prospectively determined, verifying ovulation. The results show that neither sex nor menstrual cycle status significantly influenced the subjective or cardiovascular effects of intranasal cocaine.
Acknowledgments
This research was supported by Grant No. DA-08105 from the National Institute on Drug Abuse. The participants resided on the Irving Center for Clinical Research at the Columbia-Presbyterian Medical Center, supported by Grant No. MOI-RR-00645 from the National Institutes of Health. The authors gratefully acknowledge the expert assistance of Brenda Faye, R.N., Alyce Stephens, R.N. and Laura Burr R.N. and Drs. Carl Hart, Suzanne Vosburg, Eric Rubin and Eric Collins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- CEWG. Community Epidemiology Work Group. Epidemiologic Trends in Drug Abuse Advance Report. 2006. [Google Scholar]
- Cone EJ. Pharmacokinetics and pharmacodynamics of cocaine. J Anal Toxicol. 1995;19:459–78. doi: 10.1093/jat/19.6.459. [DOI] [PubMed] [Google Scholar]
- Cone EJ. Recent discoveries in pharmacokinetics of drugs of abuse. Toxicol Lett. 1998;102–103:97–101. doi: 10.1016/s0378-4274(98)00292-6. [DOI] [PubMed] [Google Scholar]
- DAWN. Drug Abuse Warning Network. Drug-related visits to hospital emergency departments and drug-related deaths investigated by medical examiners and coroners. 2004. [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous Progesterone Attenuates the Subjective Effects of Smoked Cocaine in Women, but not in Men. Neuropsychopharmacology. 2006;31:659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Intranasal cocaine in humans: acute tolerance, cardiovascular and subjective effects. Pharmacol Biochem Behav. 2004;78:93–101. doi: 10.1016/j.pbb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Repeated intranasal cocaine administration: lack of tolerance to pressor effects. Drug Alcohol Depend. 1988;22:169–77. doi: 10.1016/0376-8716(88)90015-4. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Ward AS, Haney M, Hart CL, Collins ED. The effects of escalating doses of smoked cocaine in humans. Drug Alcohol Depend. 2003;70:149–57. doi: 10.1016/s0376-8716(02)00343-5. [DOI] [PubMed] [Google Scholar]
- Gorelick DA. Progression of dependence in male cocaine addicts. Am J Drug Alcohol Abuse. 1992;18:13–19. doi: 10.3109/00952999209001607. [DOI] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Isenschmid DS, Levine BS, Caplan YH. A method for the simultaneous determination of cocaine, benzoylecgonine, and ecgonine methyl ester in blood and urine using GC/EIMS with derivatization to produce high mass molecular ions. J Anal Toxicol. 1988;12:242–5. doi: 10.1093/jat/12.5.242. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Javaid JI, Fischman MW, Schuster CR, Dekirmenjian H, Davis JM. Cocaine plasma concentration: relation to physiological and subjective effects in humans. Science. 1978;202:227–8. doi: 10.1126/science.694530. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–8. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: John Wiley Press; 1987. [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–54. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of the menstrual cycle on energy and nutrient intake. Am J Clin Nutr. 1994;60:895–9. doi: 10.1093/ajcn/60.6.895. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40:511–28. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- NSDUH. Results from the 2001 National Survey on Drug Use and Health. Office of Applied Studies; Rockville, MD: 2002. [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB. Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug Alcohol Depend. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Rubin DB, Schenker N. Multiple Imputation in Health-Care Data Bases: An Overview and Some Applications. Stat Med. 1991;10:585–98. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2003 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. Office of Applied Studies; Rockville, MD: 2004. [Google Scholar]
- Sofuoglu M, Brown S, Dudish-Poulsen S, Hatsukami DK. Individual differences in the subjective response to smoked cocaine in humans. Am J Drug Alcohol Abuse. 2000;26:591–602. doi: 10.1081/ada-100101897. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Turner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]


