Abstract
A phase I study of Dimethylamino Benzoylphenylurea (BPU), a tubulin inhibitor, was performed using a weekly continuous schedule. Patients with refractory solid tumours received oral BPU once weekly without interruption at doses ranging from 5 to 320mg using an accelerated titration design. Nineteen subjects received 54 cycles of BPU. Early pharmacokinetic findings of decreased clearance with increasing dose and plasma accumulation led to the expansion of the 320mg dose level. Two subjects then developed late haematologic dose-limiting toxicities (DLTs) that were associated with the highest plasma exposure to BPU and metabolites. Study enrollment resumed at dose 150mg with real-time pharmacokinetic monitoring. Seven additional subjects (6 evaluable) were treated for a median of 2 cycles (range, 1.5–4) without further myelotoxicity. A long half-life and accumulation of BPU and active metabolites were observed, recommending against a continuous administration. Weekly oral BPU therapy should be further tested using an interrupted schedule.
Keywords: tubulin inhibitor, pharmacokinetics, accelerated titration
Introduction
Dimethylamino Benzoylphenylurea (BPU, NSC #639829) is a novel, small-molecule, oral tubulin-interactive agent with potent anti-tumour activity [1-3]. Benzolyphenylureas were originally developed as insecticides, and after their anti-tumour properties were discovered a derivative, BPU (N-[4-(5-bromo-2-pyrimidinyloxy)-3-methylphenyl]-N′-(2-dimethylaminobenzoyl)urea) with improved physicochemical properties was synthesised [4, 5]. The mechanism of action of BPU is not completely elucidated. BPU inhibits tubulin polymerization and microtubule depolymerization by weakly binding to the colchicine site of tubulin [2, 4, 6]. It also inhibits DNA polymerase-α, and HL-60 leukemia cells treated with BPU accumulate in the G1-S phase of the cell cycle [7].
BPU demonstrated in vivo activity against a wide range of human xenografts [8]. It was more effective than paclitaxel in the PC-3 prostate cancer model, and produced a sustained anti-tumour effect following discontinuation in several animal models [9]. Animal toxicity studies showed GI toxicity and dose-limiting myelosuppression. Human hematopoietic progenitor cells were found in vitro to be less sensitive than canine or murine progenitors. Animal pharmacokinetic (PK) studies showed the drug to be poorly bioavailable [10]. BPU was found to be extensively metabolised to two metabolites with antitumour activity, desmethyl-BPU (mmBPU) and di-desmethyl-BPU (aminoBPU), which showed variable but higher exposure than parent compound [11]. AminoBPU is the most potent metabolite, with BPU and mmBPU demonstrating intermediary potency, and four metabolites (G280, G308, G322, and G373) demonstrated no cytoxicity (for chemical structures see Ref 11) [11, 12].
The present trial was designed to evaluate BPU administered on a continuous weekly schedule in patients with refractory solid tumours using an accelerated titration design [13]. The study objectives were to define the maximum tolerated dose (MTD) of BPU and to characterise the toxicities of BPU on this schedule as well as its PK profile and any preliminary evidence of antitumour activity. A separate study is evaluating a weekly, interrupted schedule [14].
Patients and Methods
Eligibility
Patients with histologically-confirmed advanced solid malignancies without conventional treatment options were eligible. Inclusion criteria included age≥18; ECOG performance status (PS) of 0-2; at least 4 weeks elapsed since prior chemotherapy or radiation therapy (6 weeks if the regimen included nitrosoureas or mitomycin C); and adequate haematologic, hepatic, and renal function. Patients were excluded if they had known brain metastases, active infections, chronic diarrhoea, malabsorption, peripheral neuropathy > grade 1 [National Cancer Institute Common Toxicity Criteria version 2 (NCI CTCv2)], pregnancy, HIV infection, or serious concurrent medical conditions.
The clinical protocol was approved by the Johns Hopkins Institutional Review Board and NCI Cancer Therapeutics Evaluation Program (CTEP). All subjects provided written informed consent prior to study drug administration.
Drug Dosage and Administration
Oral BPU was administered continuously as a fixed, non BSA-adjusted, dose once weekly. One cycle was defined as 28 days. An accelerated titration design was used starting at 1/10th the LD10 in dogs. Subsequent dose levels were double the previous dose. One patient was enrolled at each dose level, with plans of cohort expansion to three patients if grade 2 or greater toxicity (any cycle) or DLT (first cycle) occurred. Intra-patient dose-escalation was permitted for patients enrolled to the initial dose levels only. The highest dose level evaluated, 320mg, was expanded to six patients.
BPU was supplied by the CTEP in conjunction with the Division of Cancer Treatment and Diagnosis (DCTD) and Ishihara Sangyo Kaisha, Ltd. Initially 5mg capsules were used, but due to the large number of capsules required, a 25mg capsule was subsequently made available for the 150mg dose level. The 5 mg and 25 mg capsule had similar bioavailability in dogs [10]. Subjects were instructed to fast for one hour before and two hours after each dose, and were not routinely premedicated. At the final dose level evaluated, 150mg, concomitant use of CYP3A4 inhibitor/inducers and CYP2D6 inhibitors was prohibited after in vitro metabolism data became available [15]. Doses were administered under direct supervision in the outpatient clinic for the first 8 weekly doses, after which patients were given a treatment diary and evaluated every 4 weeks. Treatment with growth factors was not permitted during the first 2 cycles of study drug administration. Study drug was not administered on scheduled days if there were dose-limiting toxicities (DLT), platelets < 100,000/mm3, or ANC < 1,500/mm3. Missed doses were not substituted. Treatment was continued until unacceptable toxicity, disease progression, or withdrawal of consent.
Toxicity assessment
Toxicity was assessed weekly using the NCI CTCv2. DLT was defined as dose delays > 2 weeks, grade 4 haematologic toxicity (except grade 4 neutropenia lasting <5 days), or grade 3 non-haematologic toxicity. The MTD was considered to be the dose level where ≤1 of 6 subjects experienced DLT.
Pretreatment and Follow-Up Studies
Baseline evaluations were conducted within two weeks before study entry and included: history and physical exam, review of concurrent medications, complete blood counts (CBC), serum chemistries, liver function tests, and urinalysis. Radiologic evaluations (including chest xray) and baseline ECG were performed within 4 weeks of study entry. Serum chemistries and liver function tests were performed weekly during the first 2 cycles before treatment and before each cycle thereafter. A CBC with differential was performed twice weekly during cycle 1, and weekly thereafter. Physical exams were repeated at the beginning of each cycle. Response of measurable lesions was assessed using RECIST criteria every 2 cycles [16]. A 30-day off-study follow-up evaluation of toxicities and blood tests was performed.
Drug Assay and Pharmacokinetic Analysis
BPU
PK studies were performed during the first 2 cycles of study drug administration for all subjects, and throughout treatment for the subjects enrolled at the last dose level. Serial sampling of venous blood was performed pre-treatment and post-treatment to 168 hours during weeks 1 and 4. Weekly pre-treatment samples were obtained during the first 8 weeks on treatment and for the duration of study and for 4 weeks following discontinuation at the final dose level. Blood samples were collected in heparinised tubes and were processed by centrifugation at 1,000 g at 4°C for 10 minutes. Plasma was divided into multiple aliquots and was stored at −70°C until analysis. Urine was collected, and stored in the refrigerator, during weeks 1 and 4 from 0 to 72 hours. Urine was divided into multiple aliquots and was stored at −70°C until analysis.
BPU concentrations in plasma were measured in plasma using validated analytical methods: LC-MS-MS [17] and LC/UV [18]. A third validated method, LC-MS-MS, was used for the final two dose levels to quantitate BPU, mmBPU, and aminoBPU in plasma and urine, and three non-cytotoxic metabolites of BPU (G280, G308, and G322) in urine (for chemical structures see Ref 11) [19]. A fourth non-cytotoxic metabolite, G373, was qualitatively assessed in the urine [19]. The LC/UV method was used as a rapid technique in order to perform real-time PK monitoring of plasma concentrations every 2-4 weeks in the final dose level after a grade 5 event and characterization of long half-life of cytotoxic metabolites. Subjects would be removed from the trial for safety reasons if the concentrations of BPU or mmBPU exceeded predetermined thresholds based on PK and toxicity data from previous patients treated on the trial (see Table 1). All results reported below utilise the LC-MS-MS method.
Table 1.
Threshold concentrations of BPU and metabolites for continuation of therapy on 150 mg dose level. Patients would have study drug held for >3 weeks, and then re-started at 50% (75 mg) if any of the following were exceeded a:
| Week | BPU | mmBPU | ||
|---|---|---|---|---|
|
Cmin (nM) |
AUC (μg*h/mL) |
Cmin (nM) |
AUC (μg*h/mL) |
|
| 2-4 | >60 | >400 | ||
| 4 | >15 | >100 | ||
| 5-12 | >60 | >600 | ||
| >12b | - | - | - | - |
IRB-approved thresholds were empirically chosen based on visual inspection of figures 2-3 for the protocol amendment subsequent to the grade 5 event at the 320 mg dose level.
Patients would not be discontinued from the study for PK thresholds after 12 weeks since by definition some type of clinical benefit (even stable disease) would have been achieved.
Individual PK parameters were estimated by standard noncompartmental analysis, which was performed using WinNonlin version 5.0 (Pharsight Corporation, Mountain View, CA) [20].
Midazolam
The oral midazolam test was performed during the first 2 cycles of study drug administration for the subjects enrolled at the final dose level. Within 72 hours prior to BPU treatment, patients were given a single 3mg dose of oral midazolam (Versed· Syrup, Roche). Serial sampling of venous blood was performed pre-treatment and post-treatment to 7 hours during week 1 and 8. Blood samples were processed to plasma and quantitated using a validated LC-MS-MS assay as previously described [21]. Individual PK parameters were estimated by standard noncompartmental analysis using WinNonlin [20].
Statistical Considerations
All study subjects who received at least one dose of study drug are included in the toxicity and efficacy analysis. For PK analysis, parameters were summarised using descriptive statistics. Differences between PK parameters were compared by a paired Student's t test or repeated measures ANOVA. Associations between CYP3A phenotype as determined by the oral midazolam test at baseline and at week 8 and BPU PKs obtained during weeks 1 and 8 were evaluated by use of Pearson's correlation coefficient. Statistical calculations were performed with the software package JMP version 3.1 (SAS Institute, Carey, NC). The a priori level of significance was set at P<0.05.
Results
Patient Characteristics
Nineteen patients were enrolled between August 2001 and October 2004. The subjects had a variety of solid tumour types, including head and neck, non-small cell lung, neuroendocrine, and renal cancer (Table 2). In all, 54 four-week cycles of BPU were administered over a dose range of 5 to 320mg (Table 3). The median number of cycles was 2 (range, 1.5-15). One patient at the 150mg dose level did not complete cycle two due to rapid disease progression. One patient at the 20mg dose level was escalated to 40mg for cycles 6-8, but subsequently returned to the original dose level.
Table 2.
Patient Characteristics
| Characteristic | No. of Patients (N=19) |
|---|---|
| Sex | |
| Male | 11 |
| Female | 8 |
| Age, years | |
| Median | 60 |
| Range | 41-77 |
| ECOG Performance Status | |
| 0 | 6 |
| 1 | 12 |
| 2 | 1 |
| Diagnosis | |
| Head and Neck | 3 |
| Lung (NSC) | 2 |
| Renal | 2 |
| Neuroendocrine | 2 |
| Other* | 10 |
| Prior Treatment | |
| Chemotherapy | 17 |
| Radiotherapy | 14 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group
Includes one case each of cancer of the gallbladder, rectum, pancreas, esophagus, stomach, breast, liver, and thyroid; adenocarcinoma of unknown primary; and one sarcoma
Table 3.
Dose Escalation
| Dose level |
BPU dose (mg) |
No. of patients |
No. of cyclesa |
No. of DLT |
|---|---|---|---|---|
| 1 | 5 | 1 | 2 | 0 |
| 2 | 10 | 1 | 2 | 0 |
| 3 | 20b | 1 | 15 | 0 |
| 4 | 40 | 1 | 2 | 0 |
| 5 | 80 | 1 | 2 | 0 |
| 6 | 160 | 1 | 2 | 0 |
| 7 | 320c | 6 | 14 | 2 |
| 8 | 150d | 7e | 15 | 0 |
Each cycle consisted of four weekly doses of oral BPU (one month)
This patient received three cycles (cycles 6-8) of drug administration at 40 mg, and was subsequently returned to 20 mg due to grade 3 syncope. This was the only subject who was dose-escalated.
Dose level expanded due to decline in apparent clearance
For this dose level, a 25 mg capsule was used. At all previous dose levels, a 5 mg capsule was administered.
One patient not evaluable for toxicity due to early progression
Although few toxicities >grade 1 were seen at the first 6 dose levels, the 320mg dose level was expanded to six subjects to obtain more PK data due to: 1) an approximately 8-fold decrease in clearance seen between 40mg and 80mg, and 2) the excessive number of 5mg capsules to be swallowed at the subsequent dose level (128 capsules at 640mg). Two among six subjects at the 320mg dose level experienced delayed haematologic toxicities (see below). The study was subsequently re-opened and completed at a reduced dose of 150mg using 25mg capsules. Further dose levels were not explored due to safety concerns.
Toxicity
Most subjects reported little or no toxicities (Table 4). The most common adverse events were fatigue (42%), nausea (21%), and cytopenias (11-16%). The patient at 20mg experienced a grade 3 syncopal event after escalation to 40mg that was felt to be possibly related to study drug. He was also on antihypertensives and was orthostatic at the time, and no further episodes occurred after returning to the 20mg dose level. Mild (grade 1) diarrhoea and headache were also each reported in 2 subjects. Even the most serious adverse event, neutropenia, was seen in only 2 subjects.
Table 4.
Treatment-Related Side Effects per Dose Level
| Dose Level (mg) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity | 5 (n=1) |
10 (n=1) |
20 (n=1)a |
40 (n=1) |
80 (n=1) |
160 (n=1) |
320 (n=6) |
150 (n=7) |
| Neutropenia | G4:1 | |||||||
| Neutropenic infection | G5:1 | |||||||
| Anaemia | G3:1 | G2:1 | ||||||
| G1:1 | ||||||||
| Thrombocytopenia | G3:1 | |||||||
| G1:1 | ||||||||
| Fatigue | G2:1 | G1:4 | G1:3 | |||||
| Headache | G1:1 | G1:1 | ||||||
| Diarrhoea | G1:1 | G1:1 | ||||||
| Nausea | G1:1 | G1:1 | G1:1 | G1:1 | ||||
| Vomiting | G1:1 | |||||||
| Alopecia | G1:2 | |||||||
| Rhinitis | G1:1 | |||||||
| Cough | G1:1 | |||||||
| Myalgia | G1:1 | |||||||
| Arthralgia | G1:1 | |||||||
| Syncope | G3:1 | |||||||
NOTE: Number of worst grade adverse events possibly, probably, or definitely attributed to BPU during study drug administration. Toxicities are graded per the NCI CTC version 2 criteria. “G1:2” denotes 2 patients had grade 1 toxicity. Dose-limiting toxicities are indicated in bold.
This subject was the only patient dose-escalated; after receiving five cycles (cycle 1-5) at 20 mg and three cycles (cycles 6-8) at 40 mg, the patient was returned to 20 mg (cycles 9-15) due to grade 3 syncope during cycle 8. No further syncopal events occurred after the subject's antihypertensive medication was discontinued
DLT was observed in two patients at the 320mg dose level. One subject, a 67-year old female with adenocarcinoma of unknown primary (metastatic to liver and lung, previously treated with 2 lines of cytotoxic chemotherapy over 15 months) developed sudden, prolonged neutropenia beginning at week 8, when her absolute neutrophil count (ANC) dropped within one week from 5460 to 21 cells/mm3. She was admitted and died after 24 days from complications of Pseudomonas aeruginosa bacteremia and Candida albicans fungemia. She also experienced grade 3 anaemia and thrombocytopenia and her bone marrow never recovered despite growth factor support (bone marrow biopsy was aplastic). A second subject, a 53-year old male with hepatocellular carcinoma, experienced an ANC nadir of 320 cells/mm3 after 12 weeks of study drug administration. This was a more gradual decline which began on week 13 (ANC = 1140) with a nadir on week 16 and full recovery by week 18. He also experienced grade 1 thrombocytopenia. He was considered a DLT because study drug was held >2 weeks.
After a full review of the clinical and PK data and assessment of drug exposure-toxicity relationships (see below), the study was subsequently amended to include real-time PK monitoring in which concentrations of BPU and mmBPU were measured every 2 weeks (twice) then every 4 weeks (Table 1). The study was re-opened and seven patients (six evaluable) received a dose of 150mg (<50% of the previous dose) using a 25mg capsule. None of these patients exceeded the PK thresholds during the first 2 cycles and only one grade > 1 toxicity episode (grade 2 anaemia) was observed. The dose of 150mg PO weekly was defined as the MTD for safety reasons despite the lack of DLT's.
Response
No responses were seen in this pre-treated patient population. One patient with adenoid cystic carcinoma experienced stable disease for 13 months and came off study because of progression. Two patients with disease stabilization after 2 cycles subsequently progressed.
Pharmacokinetic Studies
BPU in plasma
PK data were obtained and evaluable for 19 patients. Representative BPU, mmBPU, and aminoBPU plasma concentration-time profiles are illustrated in Figure 1 and plasma PK parameters are listed in Table 5 and 6.
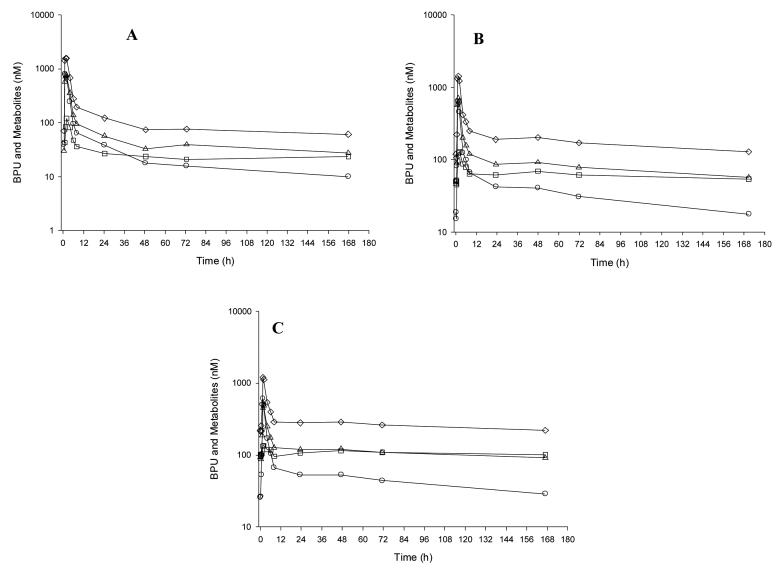
Figure 1.
Plasma concentration time curve in a single patient for BPU administered orally at a dose of 150 mg during week 1 (A), 4 (B), and 8 (C). The open circle (○), open triangle (△), open square (□) and open diamond (◇) represent BPU, mmBPU, aminoBPU, and cumulative exposure to BPU and the cytotoxic metabolites concentrations, respectively.
Table 5.
Pharmacokinetic parameters for BPU in plasma
| Dose (mg) |
Week | Number of Patients |
Pharmacokinetic Parametersa |
|||||
|---|---|---|---|---|---|---|---|---|
| Tmax (h) |
Cmax (nM) |
Vz/F (L) |
Cls/F (L/h) |
t1/2,z (h) |
AUCb (μM*h) |
|||
| 5 | 1 | 1 | 1.0 | 13.8 | 9667 | 121 | 56 | 0.09 |
| 5 | 4 | 1 | 1.0 | 9.6 | NR | 142 | NR | 0.08 |
| 10 | 1 | 1 | 4.2 | 9.1 | 16282 | 134 | 84 | 0.16 |
| 10 | 4 | 1 | 1.0 | 14.3 | NR | 101 | 27 | 0.21 |
| 20 | 1 | 1 | 1.5 | 115 | 4165 | 97 | 30 | 0.44 |
| 20 | 4 | 1 | 2.0 | 36.2 | NR | 160 | 26 | 0.27 |
| 40 | 1 | 1 | 1.0 | 192 | 11992 | 158 | 52 | 0.54 |
| 40 | 4 | 1 | 1.5 | 130 | NR | 114 | 27 | 0.75 |
| 80 | 1 | 1 | 6.0 | 579 | 1657 | 14 | 81 | 12.0 |
| 80 | 4 | 1 | 2.0 | 994 | NR | 15 | 111 | 11.3 |
| 160 | 1 | 1 | 1.0 | 2040 | 5802 | 22 | 182 | 15.4 |
| 160 | 4 | 1 | 1.5 | 838 | NR | 53 | 138 | 6.4 |
| 320 | 1 | 6 | 1.8 ± 0.4 | 3484 ± 799 | 3305 ± 2018 | 17 ± 6 | 161 ± 133 | 46.2 ± 22.6 |
| 320 | 4 | 6 | 1.7 ± 0.4 | 3129 ± 1366 | NR | 21 ± 9 | 123 ± 65 | 40.4 ± 24.1 |
| 150 | 1 | 7 | 2.7 ± 2.0 | 630 ± 349 | 6056 ± 2592 | 50 ± 19 | 95 ± 48 | 7.4 ± 3.0 |
| 150 | 4 | 7 | 2.0 ± 2.0 | 774 ± 278 | NR | 47 ± 28 | 186 ± 161 | 9.0 ± 5.1 |
| 150 | 8 | 6 | 3.3 ± 2.2 | 349 ± 173 | NR | 51 ± 35 | 198 ± 79 | 9.0 ± 6.1 |
Values are reported as the mean ± standard deviation for the 150 and 320 mg dose level.
AUCinf is reported for all dose levels for week 1; AUClast is reported for all dose levels for week 4; AUC0-168 h is reported for all dose levels for week 8.
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximal plasma concentration; NR = not reportable; Tmax, time of the maximal plasma concentration; T1/2, z, terminal half-life; Vz/F, apparent volume of distribution.
Table 6.
Pharmacokinetic parameters for mmBPU and amino-BPU in plasma
| Dose (mg) |
Week | Number of Patients |
Pharmacokinetic Parametersa |
|||||
|---|---|---|---|---|---|---|---|---|
| Tmax (h) |
Cmax (nM) |
t1/2,z (h) |
AUCb (μM*h) |
Metabolite:BPU AUC ratio |
||||
| mmBPU | 320 | 1 | 6 | 1.93 ± 0.21 | 2858 ± 742 | 279 ± 170 | 130 ± 73.0 | 3.5 ± 1.4 |
| mmBPU | 320 | 4 | 6 | 2.26 ± 0.88 | 3279 ± 996 | 483 ± 301 | 122 ± 56.2 | 3.2 ± 0.9 |
| mmBPU | 150 | 1 | 7 | 3.51 ± 2.63 | 517 ± 306 | 317 ± 89 | 29.2 ± 13.9 | 4.3 ± 2.2 |
| mmBPU | 150 | 4 | 7 | 2.51 ± 2.42 | 732 ± 338 | 283 ± 122 | 28.0 ± 16.0 | 4.1 ± 3.2 |
| mmBPU | 150 | 8 | 6 | 4.05 ± 1.81 | 349 ± 94 | 736 ± 420 | 29.8 ± 9.5 | 4.7 ± 3.9 |
| amino-BPU | 320 | 1 | 6 | 2.59 ± 1.10 | 251 ± 131 | 734 ± 101 | 15.7 ± 8.9 | 0.5 ± 0.2 |
| amino-BPU | 320 | 4 | 6 | 3.67 ± 1.50 | 582 ± 228 | 415 ± 158 | 54.3 ± 33.7 | 1.4 ± 0.6 |
| amino-BPU | 150 | 1 | 7 | 31.20 ± 67.88 | 46 ± 40 | 255 ± 276 | 2.6 ± 1.7 | 0.4 ± 0.4 |
| amino-BPU | 150 | 4 | 7 | 32.27 ± 62.41 | 87 ± 56 | 623 ± 283 | 9.0 ± 6.5 | 1.4 ± 1.4 |
| amino-BPU | 150 | 8 | 6 | 23.33 ± 23.40 | 71 ± 37 | 986 ± 504 | 10.4 ± 4.7 | 1.8 ± 1.8 |
Values are reported as the mean ± standard deviation for the 150 and 320 mg dose level.
AUCinf is reported for all dose levels for week 1; AUClast is reported for all dose levels for week 4; AUC0-168 h is reported for all dose levels for week 8.
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximal plasma concentration;Tmax, time of the maximal plasma concentration; T1/2, z, terminal half-life.
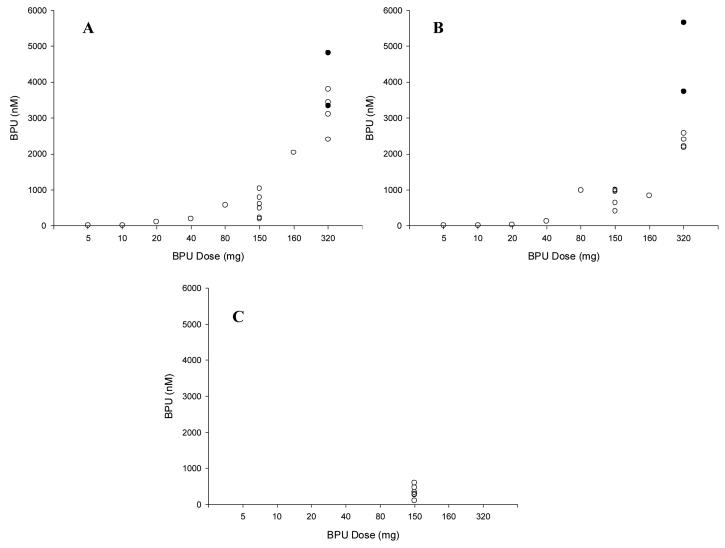
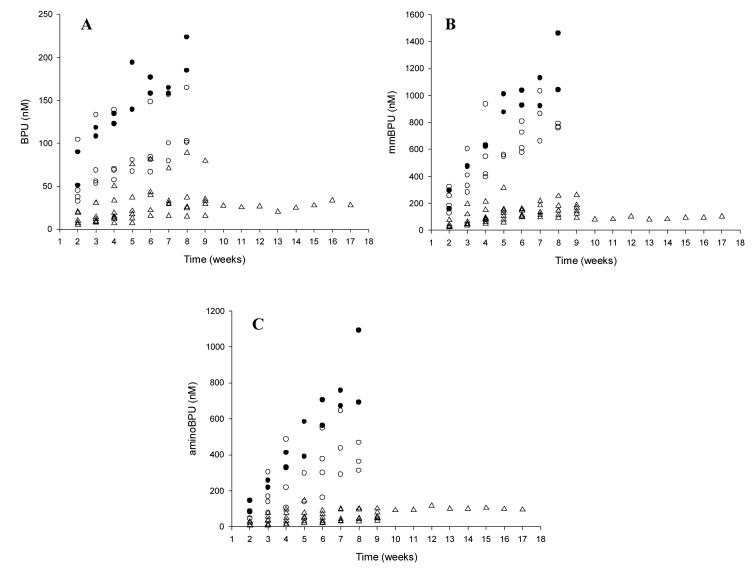
For BPU, Cmax values were reached on average at 2.3 hours, 1.7 hours, and 3.3 hours during weeks 1, 4, and 8, respectively. BPU concentrations were measurable at 1 week post-treatment in all patients treated at 80mg or higher. As the BPU dose was increased, a disproportionate increase in BPU exposure (Cmax and AUC) was observed (see Figure 2 and Table 5). This prompted, in part, an expansion of the patient cohort at 320mg. The mean terminal half-life of BPU ranged from 27 to 198 hours during weeks 1, 4, and 8 over all dose levels. Inspection of weekly pre-treatment concentrations indicates that BPU reached steady-state at approximately 8 weeks (Figure 3).
Figure 2.
BPU maximum plasma concentrations (Cmax) as a function of dose level and week 1 (A), 4 (B), and 8 (C). The closed circles (●) represent the two patients that experienced DLTs at the 320 mg dose level.
Figure 3.
BPU (A), mmBPU (B), and aminoBPU (C) minimal plasma concentrations (Cmin) over time. The open triangle (△) and open circle (○) represent the 150 mg dose level and 320 mg dose level, respectively. The closed circles (●) represent the two patients that experienced DLTs at the 320 mg dose level.
On average, mmBPU Cmax values were reached between 1 and 8 hours and always occurred at the same time or after the Tmax for BPU (Table 6). Mean terminal half-life values ranged from 103 to 1526 hours, which were longer than BPU. mmBPU reached steady-state at approximately 10 to 14 weeks (Figure 3). Average mmBPU:BPU AUC ratios were 3.9, 3.7, and 4.7 during weeks 1, 4, and 8, respectively.
Mean aminoBPU Tmax values were 3.1 hours with the 5mg capsule and 29.1 hours with the 25mg formulation and always occurred after the Tmax for BPU or mmBPU (Table 6). Because concentrations declined slowly during the 168- hour sampling period, the terminal half-life for aminoBPU could not be estimated for most patients. The mean terminal half-life was 607 hours (67% CV) when it could be estimated. AminoBPU reached steady-state at approximately 10 to14 weeks (Figure 3). Average aminoBPU:BPU AUC ratios were 0.5, 1.4, and 1.8 during weeks 1, 4, and 8, respectively, and were consistently lower for aminoBPU than for mmBPU.
The presence of the BPU metabolites G280, G308, G322, and G373 in plasma were monitored and quantitated on the BPU calibration curve; all concentrations were BLQ.
BPU in urine
The amount of BPU and metabolites excreted in urine was collected and analysed in 7 patients. On average, less than 1.5% of the BPU dose was excreted in urine as parent compound or metabolites, with a maximum of 3.6% observed in one patient during week 4. The most predominant metabolite in urine was G280, which is inactive [11].
Midazolam
The addition of the oral midazolam test as a phenotypic probe for CYP3A was added during week 1 and 8 at the 150mg dose level. CYP3A activity varied about 11-fold (midazolam apparent oral clearance mean, 40L/h; range = 10–111L/hr), which is consistent with previously published studies.[21] CYP3A activity at baseline was not correlated with BPU PK parameters during week 1 (p>0.05). However, CYP3A activity during week 8 was correlated with BPU clearance (p=0.007) and the relative extent of conversion to mmBPU (p=0.003) and aminoBPU (p<0.001) during the week 8 pharmacokinetic assessment.
Pharmacokinetic-Toxicity Relationship
Data from 19 patients receiving BPU are available to characterise drug exposure-toxicity relationships. The two patients who experienced a DLT had the highest BPU Cmax, BPU AUC, mmBPU Cmax, mmBPU AUC, aminoBPU Cmax (first and third highest), aminoBPU AUC values during week 4 and the highest pre-treatment concentrations (Figure 2 for BPU Cmax; Figure 3 for pre-treatment concentrations; AUC values not shown). BPU and metabolite exposure in the 7 subjects treated at 150mg were much lower than that observed at the 320mg dose level, potentially explaining the observed lack of neutropenia at 150mg (Figures 2-3).
Discussion
This first-in-human phase I study of BPU in patients with refractory, advanced solid tumours has defined 150mg PO weekly as the MTD for its administration in an uninterrupted weekly schedule. Sudden and life-threatening myelosuppression was observed at the 320mg dose level, and is likely related to the long half-life of both parent compound and cytotoxic metabolites. Although no DLTs and only a single grade 2 event were observed among the six patients treated at 150mg, the safety and PK schedule of this administration schedule discouraged us from exploring intermediate doses between 150 and 320 mg. Of note, it is possible that the toxicities at the 150 mg dose level are underestimated since only one subject out of seven received study drug for more than 8 weeks, when steady state levels are reached.
Alternative, interrupted schedules of BPU should be considered. A contemporary trial using a six-week on, two-week off schedule has successfully enrolled patients above the 320 mg dose schedule without excessive myelotoxicity [14]. As with this study, the 5 mg capsule formulation was changed to a 25mg capsule due to the need to swallow an excessive number of pills.
This trial shows several limitations of accelerated titration designs, which were developed as a method to reduce the number of patients treated at low doses of phase I agents and speed the completion of trials [13]. Although the average number of patients is reduced, accelerated titration does not necessarily decrease the average number of patients who experience grade 3/4 toxicities, and the built-in delays between dose levels do not speed up the completion of phase I trials as hoped. In this study, the toxicities observed in the fourth and fifth patients in dose level 320mg were delayed, sudden, and severe. The predetermined safety parameters to discontinue single-patient cohorts (grade 2 toxicity in any cycle or DLT in cycle 1) would have allowed dose escalation to 640mg after the one patient treated at 320mg, but the 320mg dose level was expanded due to the decreased clearance observed and number of pills required. This study also illustrates the utility of real-time access to clinical pharmacology resources in the early stages of human testing.
BPU has several interesting PK properties, including a long terminal half-life, which allows once weekly dosing, and extensive metabolism to cytotoxic metabolites which have longer half-lives and represent up to 1250% of the exposure to the parent compound. The reason for the marked decrease in clearance between low and higher dose levels is not clear. A possible explanation is saturation of the metabolic pathways. We have determined that BPU is metabolised predominantly by CYP3A4 and CYP1A1 and to a lesser extent by CYP2C8, CYP2D6, CYP3A5, and CYP3A7 [11, 19]. These studies will further guide the future development of this drug using alternative dosing schedules and possible combination trials with CYP3A inhibitors. Examination of the concomitant medications taken by our subjects for CYP3A4 inhibitors or inducers did not show any clear relationships.
Clinical investigators involved in early-phase testing of new drugs are constantly reminded of the need to minimise exposing human subjects to potentially subtherapeutic levels while obtaining critical safety and PK data. Several methods for early phase studies of cytotoxic agents in humans have been suggested as alternatives to the modified Fibonacci series [22]. Although accelerated titration schedules have been successfully employed in many studies, our study should serve as a reminder of its potential limitations [23-25].
Acknowledgement
We would like to thank Ping He and Yelena Zabelina for their technical support; Susan Davidson for her quality assurance of the data; Manuel Hidalgo and Saeed Khan for their scientific input.
Footnotes
Funding:
Funded by U01 CA70095. Correlative studies supported by National Institutes of Health grant P30CA069773, National Cancer Institute Cancer Therapy and Evaluation Program's Translational Research Fund, and the Commonwealth Foundation for Cancer Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors' Disclosure of Potential Conflicts of Interest
None declared.
References
- 1.Okada H, Koyanagi T, Yamada N, Haga T. Synthesis and antitumor activities of novel benzoylphenylurea derivatives. Chem Pharm Bull (Tokyo) 1991 Sep;39(9):2308–2315. doi: 10.1248/cpb.39.2308. [DOI] [PubMed] [Google Scholar]
- 2.Hollingshead MG, Sackett DL, Alley MC, et al. The anticancer activity of six benzoylphenylurea compounds and their interaction with tubulin [abstract 1126] Proc Amer Assoc Cancer Res. 1998;39:164. [Google Scholar]
- 3.Okada H, Koyanagi T, Yamada N. Synthesis and antitumor activities of prodrugs of benzoylphenylureas. Chem Pharm Bull (Tokyo) 1994 Jan;42(1):57–61. doi: 10.1248/cpb.42.57. [DOI] [PubMed] [Google Scholar]
- 4.Okada H, Kato M, Koyanagi T, Mizuno K. Synthesis and antitumor activities of water-soluble benzoylphenylureas. Chem Pharm Bull (Tokyo) 1999 Mar;47(3):430–433. doi: 10.1248/cpb.47.430. [DOI] [PubMed] [Google Scholar]
- 5.Jain N, Yang G, Tabibi SE, Yalkowsky SH. Solubilization of NSC-639829. Int J Pharm. 2001 Aug 28;225(12):41–47. doi: 10.1016/s0378-5173(01)00773-6. [DOI] [PubMed] [Google Scholar]
- 6.Ando N, Nakajima T, Masuda H, et al. Antimicrotubule effects of the novel antitumor benzoylphenylurea derivative HO-221. Cancer Chemother Pharmacol. 1995;37(12):63–69. doi: 10.1007/BF00685630. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Aziz W, Hickey R, Edelman M, Malkas L. Effect of novel benzoylphenylurea derivatives on DNA polymerase alpha activity using the synthesome-based in vitro model system. Invest New Drugs. 2003 Nov;21(4):421–428. doi: 10.1023/a:1026247101229. [DOI] [PubMed] [Google Scholar]
- 8.Alley MC, Covey JM, Pacula-Cox CM, et al. In vitro and in vivo pharmacologic evaluations of N,N-dimethyl-amino-benzoylphenylurea and a principal metabolite which exhibit multi-spectrum anticancer activities [abstract 2035] Proc Amer Assoc Cancer Res. 2001;42:378. [Google Scholar]
- 9.Nakajima T, Masuda H, Okamoto T, et al. Antitumor activity on murine tumors of a novel antitumor benzoylphenylurea derivative, HO-221. Cancer Chemother Pharmacol. 1991;28(5):351–356. doi: 10.1007/BF00685688. [DOI] [PubMed] [Google Scholar]
- 10.Noker PE, Weinberg DS, Page JG, Schweikart KM, Tomaszawski JE. Oral bioavailability of dimethyl amino benzoylohenyl urea (BPU) in dogs [abstract 1737] Proc Amer Assoc Cancer Res. 2003;44:394. [Google Scholar]
- 11.Rudek MA, Zhao M, Smith NF, et al. In vitro and in vivo clinical pharmacology of dimethyl benzoylphenylurea, a novel oral tubulin-interactive agent. Clin Cancer Res. 2005 Dec 1;11(23):8503–8511. doi: 10.1158/1078-0432.CCR-05-1037. [DOI] [PubMed] [Google Scholar]
- 12.Gurulingappa H, Amador ML, Zhao M, Rudek MA, Hidalgo M, Khan SR. Synthesis and antitumor evaluation of benzoylphenylurea analogs. Bioorg Med Chem Lett. 2004 May 3;14(9):2213–2216. doi: 10.1016/j.bmcl.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997 Aug 6;89(15):1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 14.Edelman MJBK, Meiller T, Porter N, Nemieboka N, Desanto J, Colevas D. Phase I, pharmacokinetic (PK) and pharmacodynamic study of benzoylphenylurea (BPU, NSC 639829), a novel antitubulin agent. Proc Amer Soc Clin Oncol 2003 (abstr #550) [Google Scholar]
- 15.Garimella TS, Edelman MJ, Horn JJ, Colevas AD, Bauer KS. In Vitro Metabolism Of The Novel Antimicrotubule Agent Benzoylphenylurea (bpu) Currently Under Phase I Investigation In Patients With Advanced Malignancy [abstract T3328] AAPS Pharm Sci. 2003;5(4) [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Zabelina Y, Rudek MA, Wolff AC, Baker SD. A rapid and sensitive method for determination of dimethyl benzoylphenyl urea in human plasma by using LC/MS/MS. J Pharm Biomed Anal. 2003 Nov 24;33(4):725–733. doi: 10.1016/s0731-7085(03)00424-2. [DOI] [PubMed] [Google Scholar]
- 18.Rudek MA, Zabelina Y, Zhao M, Wolff AC, Baker SD. A method for determination of dimethyl benzoylphenyl urea (BPU) in human plasma by using LC/UV. Biomed Chromatogr. 2004 Jun;18(5):282–287. doi: 10.1002/bmc.314. [DOI] [PubMed] [Google Scholar]
- 19.Rudek MA, Zhao M, He P, et al. Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate dimethyl benzoylphenylurea (BPU) and its five metabolites in human plasma and urine for clinical pharmacology studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Dec 15;828(12):41–54. doi: 10.1016/j.jchromb.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Gibaldi MPD. Pharmacokinetics. Marcel Dekker; New York, NY: 1982. Noncompartmental Analysis Based on Statistical Moment Theory; pp. 409–417. [Google Scholar]
- 21.Li JKM, Brahmer J, Spitz A, Zhao M, Hidalgo M, Baker SD. CYP3A4-phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. J Natl Cancer Inst. 2006 doi: 10.1093/jnci/djj466. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Bolognese JA. A Monte Carlo comparison of three up-and-down designs for dose ranging. Control Clin Trials. 1983;4:187–196. doi: 10.1016/0197-2456(83)90002-8. [DOI] [PubMed] [Google Scholar]
- 23.Grem JL, Morrison G, Guo XD, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005 Mar 20;23(9):1885–1893. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 24.Syed S, Takimoto C, Hidalgo M, et al. A phase I and pharmacokinetic study of Col-3 (Metastat), an oral tetracycline derivative with potent matrix metalloproteinase and antitumor properties. Clin Cancer Res. 2004 Oct 1;10(19):6512–6521. doi: 10.1158/1078-0432.CCR-04-0804. [DOI] [PubMed] [Google Scholar]
- 25.Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005 Feb 20;23(6):1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]