Abstract
BACKGROUND
Several studies have recently suggested an association between body mass index (BMI) and disease progression after radical prostatectomy. In the current study, the authors examined this association and that between the reciprocal of BMI (INVBMI, 1/BMI) and progression-free probability in men treated with radical retropubic prostatectomy (RRP) for clinically localized prostate carcinoma.
METHODS
The authors retrospectively studied 2210 patients who underwent RRP at Memorial Sloan-Kettering Cancer Center between September 1986 and May 2003. Clinicopathologic variables analyzed included BMI (kg/m2), preoperative serum prostate-specific antigen level (ng/mL), clinical T classification, year of surgery, race, biopsy-derived primary and secondary Gleason grades, and INVBMI, known to better correlate with percent body fat than BMI. Cox regression analysis was used to examine the possible association between BMI or its reciprocal with disease progression after controlling for the effects of common prognostic factors. The areas under the receiver operating curve (AUC) for models with and without INVBMI were calculated
RESULTS
Of the 2210 patients analyzed, 251 experienced disease progression in a median follow-up time of 25.9 months (range, 0-143 months). After adjusting for all clinical variables, both BMI (P = 0.071; hazards ratio [HR] = 1.027) and INVBMI (P = 0.041; HR < 0.001) were associated with disease progression. However, the areas under AUC for models with and without INVBMI were similar (range, 0.794 - 0.798).
CONCLUSIONS
Although conflicting evidence has been reported regarding the link between obesity and an increased risk of developing prostate carcinoma, as well as an increased risk of developing aggressive disease and prostate carcinoma-related mortality, the authors found weak associations with disease progression for both BMI and INVBMI. These variables were of negligible prognostic value in men who received surgery. Studies with longer follow-up, that examine alternative end points, and that follow treatment(s) besides surgery are needed. Cancer 2005;103: 2030-4.
Keywords: prostate carcinoma, body mass index, obesity, disease progression, reciprocal of body mass index
Obesity has become increasingly prevalent in the United States over the past several decades. Recent data report the age-adjusted prevalence of obesity in the United States to have increased from 22.9% between 1988 and 1994 to approximately 30.5% between 1999 and 2000, with 27.7% of men between the ages of 20 and 74 classifiedas being obese (body mass index [BMI] ≥ 30).1 BMI is a reasonable, but not perfect, surrogate predictor of obesity.2 Its wide utility stems from the finding that it is calculated from simple and inexpensive measurements of height and weight (BMI = kg/m2). The reciprocal of BMI (INVBMI, 1/BMI) is known to improve the linearity of the relation between BMI and percent body fat (PBF).3-5
Obesity increases the risk of certain types of cancers, such as those involving the gastrointestinal tract,6 and hormone-dependent neoplasms, including endometrial carcinomas.7 However, its association with prostate carcinoma remains unclear. The incidence of prostate carcinoma has grown rapidly, with an estimated 230,110 newly diagnosed cases in the United States alone in 2004, making it the most commonly diagnosed neoplasm overall, and the second most common cause of cancer deaths among males.8 In general, studies analyzing a link between obesity (or BMI) and prostate carcinoma risk and/or disease progression have yielded inconsistent results.7,9-16
Radical retropubic prostatectomy (RRP) is the most common primary definitive therapy for treating clinically localized prostate carcinoma.17 Considering the increasing trend of obesity among men in the United States and the likely association between prostate carcinoma risk and obesity, it is of paramount importance to identify risk factors for disease progression for men diagnosed with prostate carcinoma and treated with RRP.
MATERIALS AND METHODS
Patient Selection
A prospective database was queried for men with clinically localized prostate carcinoma treated with RRP as the initial definitive treatment for prostate carcinoma between September 1986 and May 2003 performed by all surgeons at the Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY). Because androgen deprivation therapy is known to influence body fat,18 747 of 3796 consecutive patients were excluded due to previous neoadjuvant therapy. In addition, 839 patients were excluded due to missing preoperative serum prostate-specific antigen (PSA) values (n = 34), race information (n = 48), biopsy-derived Gleason grade data (n = 436), clinical stage values (n = 25), and height and/or weight information (n = 296).This left 2210 consecutive men with complete clinical information.
Serum PSA levels were measured either at MSKCC using the Hybritech Tandem R assay (Hybritech Inc., San Diego, CA) or at an outside institution. Clinical stage was obtained solely from digital rectal examination using the 2002 American Joint Committee on Cancer TNM classification. Neoadjuvant therapy was defined as receipt of hormonal or chemical treatment before RRP. Height and weight information was obtained from objective measurements at the time of initial consultation or at the time of hospital admission for surgical management. BMI was calculated by dividing the weight (kg) by the square of the height (m2), and INVBMI was calculated as 1/BMI.
Patients were stratified into the following BMI categories according to the World Health Organization (WHO) classification19: Category 1, acceptable (BMI < 25 kg/m2); Category 2, overweight (BMI ≥ 25.0 but < 30 kg/m2); Category 3, obese (BMI ≥ 30.0 but < 40 kg/m2); and Category 4, morbidly obese (BMI ≥ 40.0 kg/m2). Disease progression was defined as the first occurrence of the following states: biochemical recurrence (BCR), receipt of secondary therapy in the form of radiotherapy, antineoplastic or hormonal agents, biopsy-proven local disease recurrence, evidence of distant metastatic progression by bone scan or other tests, or prostate carcinoma-related death. BCR was defined as a serum PSA level ≥ 0.2 ng/mL followed by an increase.
Statistical Methods
Freedom from disease progression was calculated with the Kaplan-Meier method, and the log-rank test was used to compare BMI strata. A Cox proportional hazards regression analysis was conducted. The areas under the receiver operating characteristic (ROC) curve for models with and without INVBMI were calculated. Prognostic factors included biopsy-derived primary and secondary Gleason grades, pretreatment serum PSA level (ng/mL), clinical T classification, race, BMI, and INVBMI. The clinical stage and race were treated as categorical variables, whereas the other factors were treated as ordinal variables. PSA level was analyzed as its natural log. Statistical analysis was performed using S-PLUS (Insightful Corp., Seattle, WA) and SPSS (SPSS, Inc., Chicago, IL) computer software packages.
RESULTS
In the current study, 2210 patients with a median follow-up of 25.9 months (range, 0-143 months) were analyzed. Table 1 shows the clinicopathologic characteristics of the patients. Of the 2210 patients, 251 patients demonstrated disease progression after RRP, 206 demonstrated BCR as the first evidence of disease progression, 1 developed local disease progression, 2 developed distant metastatic disease, and 42 received secondary treatment (radiotherapy, n = 29; antineoplastic agents, n = 5; and hormones, n = 8). No patients demonstrated disease-specific mortality as the first evidence of disease progression. The 2 and 5-year progression-free probabilities for the WHO-derived BMI categories are shown in Table 2. There was no difference among the WHO-derived BMI categories in predicting disease progression (P = 0.143) (Figure 1).
TABLE 1.
Patient Cohort (n = 2210)
| Patient characteristics | No. of patients (%) |
|---|---|
| Clinical stage | |
| T1C | 1205 (54.5) |
| T2A | 459 (20.8) |
| T2B | 363 (16.4) |
| T2C | 146 (6.6) |
| T3 | 37 (1.7) |
| Race | |
| Asian | 36 (1.6) |
| Black | 132 (6.0) |
| White Hispanic | 92 (4.2) |
| White non-Hispanic | 1950 (88.2) |
| BMI (kg/m2) | |
| Minimum | 17.7 |
| First quartile | 25.2 |
| Median | 27.4 |
| Mean | 27.8 |
| Third quartile | 29.9 |
| Maximum | 65.6 |
| Preoperative PSA (ng/mL) | |
| Minimum | 0 |
| First quartile | 4.5 |
| Median | 6.0 |
| Mean | 7.6 |
| Third quartile | 8.6 |
| Maximum | 78.4 |
| Biopsy primary Gleason grade | |
| Minimum | 1 |
| First quartile | 3 |
| Median | 3 |
| Mean | 3.1 |
| Third quartile | 3 |
| Maximum | 5 |
| Biopsy secondary Gleason grade | |
| Minimum | 1 |
| First quartile | 3 |
| Median | 3 |
| Mean | 3.2 |
| Third quartile | 3 |
| Maximum | 5 |
BMI: body mass index; PSA: prostate-specific antigen.
TABLE 2.
The 2- and 5-Year Progression-Free Probabilities (PFP) for WHODerived BMI Categories
| Patient cohort (n = 2210) | 2-yr PFP (95% CI) | 5-yr PFP (95% CI) |
|---|---|---|
| BMI < 25 (n = 512) | 92.1% (89.5-94.7) | 83.8% (79.5-88.4) |
| 25 ≤ BMI < 30 (n = 1158) | 93.7% (92.1-95.3) | 82.6% (79.3-86.0) |
| 30 ≤ BMI < 40 (n = 519) | 90.3% (87.3-93.3) | 78.0% (72.3-84.0) |
| BMI ≥ 40 (n = 21) | 81.0% (62.9-100) |
FIGURE 1.
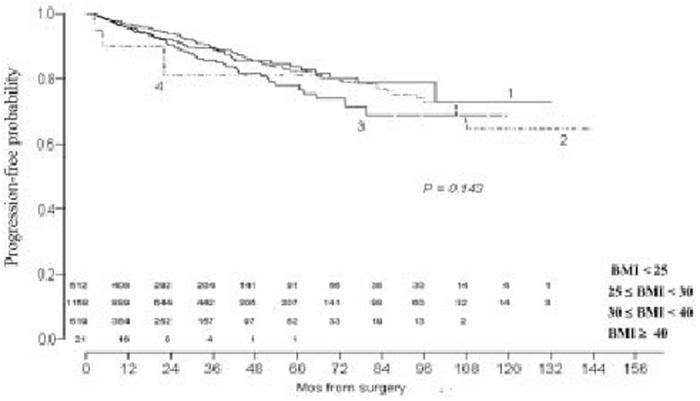
Progression-free probability is stratified by World Health Organization body mass index categories. Numerals above months indicate number of patients at risk for disease progression.
In the univariate analysis of our cohort, BMI and INVBMI as continuous variables were associated with disease progression (P = 0.027 and P = 0.044, respectively). Similarly, all other risk factors were significant. In the multivariate analysis, and after adjusting for all clinical variables, BMI as a continuous variable demonstrated a trend towards significance (P = 0.071) and INVBMI was significant (P = 0.041). These findings as well as the hazard ratios (HR) for the variables are summarized in Table 3.
TABLE 3.
| Factor | P Value | Hazard ratio (CI) |
|---|---|---|
| Multivariate with BMI | ||
| BMI (continuous variable) | 0.071 | 1.027 (0.998-1.058) |
| Pretreatment serum PSA level | < 0.001 | 2.198 (1.793-2.696) |
| Biopsy-derived primary Gleason grade | < 0.001 | 2.478 (1.973-3.109) |
| Biopsy-derived secondary Gleason grade | < 0.001 | 1.546 (1.263-1.891) |
| Clinical T stage | < 0.001 | |
| T1c versus T3 | < 0.001 | 0.245 (0.140-0.430) |
| T2a versus T3 | < 0.001 | 0.250 (0.136-0.460) |
| T2b versus T3 | 0.011 | 0.480 (0.272-0.848) |
| T2c versus T3 | 0.001 | 0.347 (0.106-0.666) |
| Yr of surgery | 0.134 | 0.963 (0.918-1.012) |
| Race | 0.042 | |
| Asian versus White non-Hispanic | 0.327 | 1.568 (0.638-3.856) |
| Blacks versus White non-Hispanic | 0.01 | 1.861 (1.163-2.978) |
| White Hispanics versus White non-Hispanics | 0.248 | 1.383 (0.797-2.398) |
| Multivariate with (1/BMI) Instead of BMI | ||
| 1/BMI (continuous variable) | 0.041 | < 0.001 (< 0.001-0.343) |
| Pretreatment serum PSA level | < 0.001 | 2.204 (1.797-2.702) |
| Biopsy-derived primary Gleason grade | < 0.001 | 2.503 (1.991-3.150) |
| Biopsy-derived secondary Gleason grade | < 0.001 | 1.549 (1.263-1.898) |
| Clinical T Stage | < 0.001 | |
| T1c versus T3 | < 0.001 | 0.248 (0.141-0.435) |
| T2a versus T3 | <0.001 | 0.254 (0.138-0.468) |
| T2b versus T3 | 0.012 | 0.484 (0.275-0.853) |
| T2c versus T3 | 0.002 | 0.348 (0.181-0.669) |
| Yr of surgery | 0.110 | 0.961 (0.915-1.009) |
| Race | 0.041 | |
| Asian versus White non-Hispanic | 0.306 | 1.600 (0.650-3.930) |
| Blacks versus White non-Hispanic | 0.010 | 1.856 (1.159-2.970) |
| White Hispanics versus White non-Hispanics | 0.246 | 1.385 (0.799-2.403) |
CI: Confidence interval.
After further analysis, the areas under the receiver operating curve for models with and without INVBMI were similar (range, 0.794 - 0.798).
DISCUSSION
Obesity has been linked to the increased risk of cancer-related death for many cancers, with ≤ 14% of all cancer deaths due to obesity in men > 50 years.6 Data examining the association between prostate carcinoma risk and obesity have been inconsistent. Although several series have refuted a link between obesity and the increased risk of developing prostate carcinoma,7,10-12 much data also exist that provide evidence in support of this association.9,20,21 Subsequently, several studies have assessed the association between obesity and the development of more aggressive tumors.9,13,15,22
Andersson et al.20 performed a retrospective analysis of 135,006 Swedish construction workers to evaluate the relation between prostate carcinoma-specific death and obesity. Of 2368 men diagnosed with prostate carcinoma, they observed 708 cancer-related deaths during a mean follow-up period of 18 years. They found a positive association between increasing BMI and mortality rates when compared with the reference BMI group (BMI < 22.1). However, they used a nonstandard BMI grouping.19
Chang et al15 analyzed the association between leptin, an adiposity-related hormone, and prostate carcinoma and found that the former was involved in clinically significant prostate carcinoma. Although leptin levels are usually increased in obese subjects, a considerable 5-10% of obese humans have relatively low levels of leptin.23 The effect of leptin on disease progression or prostate carcinoma-related mortality after RRP is yet to be determined.
Amling et al.14 studied the association between BMI and prostate carcinoma recurrence after RRP. In univariate analysis, BMI ≥ 30 was associated with BCR (P = 0.028). It is unclear whether the particular cutoff point used would affect the findings. In multivariate analysis, which included race, BMI was no longer associated with BCR (P = 0.146).
Freedland et al.16 analyzed the impact of obesity on disease progression after RRP. In their multivariable analyses, BMI, whether categorical (BMI ≥ 35 relative to normal weight, P = 0.002) or continuous (P = 0.05), was a significant predictor of BCR. However, the analysis of the categorical BMI variable did not control for the testing of multiple cutoff points in univariable analysis,24 in addition to categorizing patients with BMI < 35 as normal weight. Furthermore, both multivariable analyses were performed using a stepwise approach, which results in P values that are biased low.25
In our multivariate analysis, BMI was weakly associated with disease progression. This finding is consistent in the literature. None of the previous studies to date has shown BMI to be strongly associated with disease progression after controlling for all common pretreatment predictors without the use of stepwise variable selection. Our study is unique in that it utilizes INVBMI, which has been shown to improve the linearity of BMI to PBF. However, the effects of BMI and INVBMI are very slight, as judged by their trivial effects on the areas under the ROC curves of the models. To complement previous studies,14,16 we tested an interaction between INVBMI and race, but found no such effect (P = 0.718).
Our results should not be interpreted as condemning weight loss. Clearly, obesity is associated with a variety of health problems, which are not the focus of the current study. Our results only suggest that obesity is not an incremental prognostic factor when determining whether prostate carcinoma will recur after surgery. Clearly, this does not suggest that weight loss for somone who is obese would not be valuable.
Our study has several limitations. Our patient group was composed only of patients who elected to undergo surgical treatment for prostate carcinoma. Because morbidly obese patients with obesity-related complications (e.g., diabetes mellitus, cardiac illness, pulmonary disease, etc.) may have been excluded, as they were not deemed appropriate surgical candidates, our spectrum of BMI patients can be constrained relative to the general population of all patients diagnosed with prostate carcinoma.
Another limitation pertains to our chosen end point. BCR, as a measure of disease progression, is of uncertain survival significance, although D’Amico et al.26 reported that patients with a higher risk of PSA recurrence are more likely to die of prostate carcinoma.26 Studies that examine subsequent end points, such as metastasis and death, and have a longer follow-up, are needed.
Although obtained from a prospective database, our study is retrospective in nature and, in addition, was unable to adjust for other risk factors that may play a role in the progression of prostate carcinoma (e.g., family history of prostate carcinoma), or the correlation of INVBMI to PBF (e.g., history of smoking and level of exercise).5 The use of dual-energy X-ray absorptiometry is reported to be more accurate than INVBMI in assessing PBF.5 However, we did not have this information available. Moreover, the finding that these patients were not on a specific treatment protocol suggests the potential for bias for the patients for whom we have data. Thus, our results may not generalize to patients followed in cohort protocols.
Several series have attempted to demonstrate a positive association between BMI and an increased risk of developing prostate carcinoma, as well as an increased probability of higher-grade disease and disease progression after RRP. Our study found that INVBMI and BMI were weakly associated with disease progression and were of trivial prognostic value.
Footnotes
Supported, in part, by NIH P50-CA92629 from SPORE in Prostate Cancer and by a gift from the Leon Lowenstein Foundation.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. University of Michigan Press; Ann Arbor: 1990. [Google Scholar]
- 3.Flegal KM. Anthropometric evaluation of obesity in epidemiologic research on risk factors: blood pressure and obesity in the health examination survey. Cornell University; 1982. PhD dissertation (UMI:8228449) [Google Scholar]
- 4.Flegal KM. Is an inverted weight-height index a better index of body fatness [abstract]? Obes Res. 1997;5:93S. [Google Scholar]
- 5.Fernandez JR, Heo M, Heymsfield SB, et al. Is percentage body fat differentially related to body mass index in Hispanic Americans, African Americans, and European Americans? Am J Clin Nutr. 2003;77:71–75. doi: 10.1093/ajcn/77.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson F, Wolk A, Pedersen NL, et al. Obesity and hormone-dependent tumors: cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer. 2003;106:594–599. doi: 10.1002/ijc.11266. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Mydlo JH, Tieng NL, Volpe MA, Chaiken R, Kral JG. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4:101–105. doi: 10.1038/sj.pcan.4500514. [DOI] [PubMed] [Google Scholar]
- 10.Hsing AW, Deng J, Sesterhenn IA, et al. Body size and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev. 2000;9:1335–1341. [PubMed] [Google Scholar]
- 11.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–549. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 12.Irani J, Lefebvre O, Murat F, Dahmani L, Dore B. Obesity in relation to prostate cancer risk: comparison with a population having benign prostatic hyperplasia. BJU Int. 2003;91:482–484. doi: 10.1046/j.1464-410x.2003.04133.x. [DOI] [PubMed] [Google Scholar]
- 13.Amling CL, Kane CJ, Riffenburgh RH, et al. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58:723–728. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 14.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 15.Chang S, Hursting SD, Contois JH, et al. Leptin and prostate cancer. Prostate. 2001;46:62–67. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, Carvalhal GF, Catalona WJ, Young JD. Primary treatment choices for men with clinically localized prostate carcinoma detected by screening. Cancer. 2000;88:1122–1130. [PubMed] [Google Scholar]
- 18.Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, Dalkin BL. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95:2136–2144. doi: 10.1002/cncr.10967. [DOI] [PubMed] [Google Scholar]
- 19.Physical status: the use and interpretation of anthropometry Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 20.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 21.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 22.Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55:140–146. doi: 10.1002/pros.10211. [DOI] [PubMed] [Google Scholar]
- 23.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829–835. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]
- 25.Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994;69:979–985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Amico AV, Cote K, Loffredo M, Renshaw AA, Chen MH. Pretreatment predictors of time to cancer specific death after prostate specific antigen failure. J Urol. 2003;169:1320–1324. doi: 10.1097/01.ju.0000049200.30192.d1. [DOI] [PubMed] [Google Scholar]


