Abstract
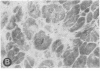
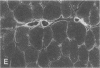
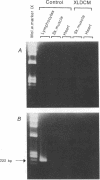
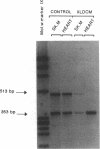
We have previously shown in a large X-linked pedigree that a deletion removing the dystrophin muscle promoter, the first muscle exon and part of intron 1 caused a severe dilated cardiomyopathy with no associated muscle weakness. Dystrophin expression was present in the muscle of affected males and transcription studies indicated that this dystrophin originated from the brain and Purkinje cell isoforms, upregulated in this skeletal muscle. We have now studied dystrophin transcription and expression in the heart of one member of this family. In contrast to the skeletal muscle, dystrophin transcription and expression were absent in the heart, with the exception of the distal Dp71 dystrophin isoform, normally present in the heart. The 43- and 50-kD dystrophin-associated proteins were severely reduced in the heart, despite the presence of Dp71, but not in skeletal muscle. The absence of dystrophin and the down-regulation of the dystrophin-associated proteins in the heart accounted for the severe cardiomyopathy in this family. The mutation present in these males selectively affects dystrophin expression in the heart; this could be secondary to the removal of cardiac-specific regulatory sequences. This family may represent the first example of a mutation specifically affecting the cardiac expression of a gene, present physiologically in both the skeletal and cardiac muscles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berko B. A., Swift M. X-linked dilated cardiomyopathy. N Engl J Med. 1987 May 7;316(19):1186–1191. doi: 10.1056/NEJM198705073161904. [DOI] [PubMed] [Google Scholar]
- Bies R. D., Friedman D., Roberts R., Perryman M. B., Caskey C. T. Expression and localization of dystrophin in human cardiac Purkinje fibers. Circulation. 1992 Jul;86(1):147–153. doi: 10.1161/01.cir.86.1.147. [DOI] [PubMed] [Google Scholar]
- Bies R. D., Phelps S. F., Cortez M. D., Roberts R., Caskey C. T., Chamberlain J. S. Human and murine dystrophin mRNA transcripts are differentially expressed during skeletal muscle, heart, and brain development. Nucleic Acids Res. 1992 Apr 11;20(7):1725–1731. doi: 10.1093/nar/20.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Codd M. B., Sugrue D. D., Gersh B. J., Melton L. J., 3rd Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation. 1989 Sep;80(3):564–572. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- Cox G. A., Sunada Y., Campbell K. P., Chamberlain J. S. Dp71 can restore the dystrophin-associated glycoprotein complex in muscle but fails to prevent dystrophy. Nat Genet. 1994 Dec;8(4):333–339. doi: 10.1038/ng1294-333. [DOI] [PubMed] [Google Scholar]
- Cserjesi P., Olson E. N. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991 Oct;11(10):4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe A., Kluyskens Y., Van Durme J. P., Naudts K., Claeys R., De Wagter X. Familial idiopathic dilated cardiomyopathy (IDC). Acta Cardiol. 1991;46(5):577–582. [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature. 1979 Apr 19;278(5706):714–718. doi: 10.1038/278714a0. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Cheng T. C., Cserjesi P., Chakraborty T., Olson E. N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992 Sep;12(9):3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Fabbrizio E., Nudel U., Hugon G., Robert A., Pons F., Mornet D. Characterization and localization of a 77 kDa protein related to the dystrophin gene family. Biochem J. 1994 Apr 15;299(Pt 2):359–365. doi: 10.1042/bj2990359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Kelvin D. J., Sternberg E. A., Olson E. N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989 Nov;9(11):5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber H. L., Unverferth D. V., Baker P. B., Ryan J. M., Baba N., Wooley C. F. Evolution of a hereditary cardiac conduction and muscle disorder: a study involving a family with six generations affected. Circulation. 1986 Jul;74(1):21–35. doi: 10.1161/01.cir.74.1.21. [DOI] [PubMed] [Google Scholar]
- Greenberg D. S., Sunada Y., Campbell K. P., Yaffe D., Nudel U. Exogenous Dp71 restores the levels of dystrophin associated proteins but does not alleviate muscle damage in mdx mice. Nat Genet. 1994 Dec;8(4):340–344. doi: 10.1038/ng1294-340. [DOI] [PubMed] [Google Scholar]
- Hugnot J. P., Gilgenkrantz H., Vincent N., Chafey P., Morris G. E., Monaco A. P., Berwald-Netter Y., Koulakoff A., Kaplan J. C., Kahn A. Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7506–7510. doi: 10.1073/pnas.89.16.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello R. C., Mar J. H., Ordahl C. P. Characterization of a promoter element required for transcription in myocardial cells. J Biol Chem. 1991 Feb 15;266(5):3309–3316. [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Kass S., MacRae C., Graber H. L., Sparks E. A., McNamara D., Boudoulas H., Basson C. T., Baker P. B., 3rd, Cody R. J., Fishman M. C. A gene defect that causes conduction system disease and dilated cardiomyopathy maps to chromosome 1p1-1q1. Nat Genet. 1994 Aug;7(4):546–551. doi: 10.1038/ng0894-546. [DOI] [PubMed] [Google Scholar]
- Klamut H. J., Gangopadhyay S. B., Worton R. G., Ray P. N. Molecular and functional analysis of the muscle-specific promoter region of the Duchenne muscular dystrophy gene. Mol Cell Biol. 1990 Jan;10(1):193–205. doi: 10.1128/mcb.10.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klietsch R., Ervasti J. M., Arnold W., Campbell K. P., Jorgensen A. O. Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ Res. 1993 Feb;72(2):349–360. doi: 10.1161/01.res.72.2.349. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lederfein D., Levy Z., Augier N., Mornet D., Morris G., Fuchs O., Yaffe D., Nudel U. A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscle tissues. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5346–5350. doi: 10.1073/pnas.89.12.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Burghes A. H., Mora M., Tomé F. M., Morandi L., Cornello F., Leturcq F., Jeanpierre M., Kaplan J. C., Reinert P. Immunohistochemical analysis of dystrophin-associated proteins in Becker/Duchenne muscular dystrophy with huge in-frame deletions in the NH2-terminal and rod domains of dystrophin. J Clin Invest. 1994 Jan;93(1):99–105. doi: 10.1172/JCI116989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Nonaka I., Tomé F. M., Arahata K., Collin H., Leturcq F., Récan D., Kaplan J. C., Fardeau M., Campbell K. P. Mild deficiency of dystrophin-associated proteins in Becker muscular dystrophy patients having in-frame deletions in the rod domain of dystrophin. Am J Hum Genet. 1993 Aug;53(2):409–416. [PMC free article] [PubMed] [Google Scholar]
- Michels V. V., Moll P. P., Miller F. A., Tajik A. J., Chu J. S., Driscoll D. J., Burnett J. C., Rodeheffer R. J., Chesebro J. H., Tazelaar H. D. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992 Jan 9;326(2):77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- Muntoni F., Cau M., Ganau A., Congiu R., Arvedi G., Mateddu A., Marrosu M. G., Cianchetti C., Realdi G., Cao A. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N Engl J Med. 1993 Sep 23;329(13):921–925. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- Muntoni F., Mateddu A., Cianchetti C., Marrosu M. G., Clerk A., Cau M., Congiu R., Cao A., Melis M. A. Dystrophin analysis using a panel of anti-dystrophin antibodies in Duchenne and Becker muscular dystrophy. J Neurol Neurosurg Psychiatry. 1993 Jan;56(1):26–31. doi: 10.1136/jnnp.56.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F., Melis M. A., Ganau A., Dubowitz V. Transcription of the dystrophin gene in normal tissues and in skeletal muscle of a family with X-linked dilated cardiomyopathy. Am J Hum Genet. 1995 Jan;56(1):151–157. [PMC free article] [PubMed] [Google Scholar]
- Muntoni F., Strong P. N. Transcription of the dystrophin gene in Duchenne muscular dystrophy muscle. FEBS Lett. 1989 Jul 31;252(1-2):95–98. doi: 10.1016/0014-5793(89)80896-8. [DOI] [PubMed] [Google Scholar]
- Navankasattusas S., Zhu H., Garcia A. V., Evans S. M., Chien K. R. A ubiquitous factor (HF-1a) and a distinct muscle factor (HF-1b/MEF-2) form an E-box-independent pathway for cardiac muscle gene expression. Mol Cell Biol. 1992 Apr;12(4):1469–1479. doi: 10.1128/mcb.12.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio H., Takeshima Y., Narita N., Yanagawa H., Suzuki Y., Ishikawa Y., Ishikawa Y., Minami R., Nakamura H., Matsuo M. Identification of a novel first exon in the human dystrophin gene and of a new promoter located more than 500 kb upstream of the nearest known promoter. J Clin Invest. 1994 Sep;94(3):1037–1042. doi: 10.1172/JCI117417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Campbell K. P. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991 Dec;115(6):1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Matsumura K., Ionasescu V. V., Towbin J. A., Bosch E. P., Weinstein S. L., Sernett S. W., Campbell K. P. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology. 1993 Apr;43(4):795–800. doi: 10.1212/wnl.43.4.795. [DOI] [PubMed] [Google Scholar]
- Parmacek M. S., Leiden J. M. Structure and expression of the murine slow/cardiac troponin C gene. J Biol Chem. 1989 Aug 5;264(22):13217–13225. [PubMed] [Google Scholar]
- Parmacek M. S., Vora A. J., Shen T., Barr E., Jung F., Leiden J. M. Identification and characterization of a cardiac-specific transcriptional regulatory element in the slow/cardiac troponin C gene. Mol Cell Biol. 1992 May;12(5):1967–1976. doi: 10.1128/mcb.12.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Greenberg D. S., Tal M., Yaffe D., Nudel U. Dp71, the nonmuscle product of the Duchenne muscular dystrophy gene is associated with the cell membrane. FEBS Lett. 1993 Aug 9;328(1-2):197–202. doi: 10.1016/0014-5793(93)80992-4. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Hong N. A., Bishopric N. H., Kedes L. Myocardial activation of the human cardiac alpha-actin promoter by helix-loop-helix proteins. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4047–4051. doi: 10.1073/pnas.89.9.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewry C. A., Sansome A., Matsumura K., Campbell K. P., Dubowitz V. Deficiency of the 50 kDa dystrophin-associated glycoprotein and abnormal expression of utrophin in two south Asian cousins with variable expression of severe childhood autosomal recessive muscular dystrophy. Neuromuscul Disord. 1994 Mar;4(2):121–129. doi: 10.1016/0960-8966(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Towbin J. A., Hejtmancik J. F., Brink P., Gelb B., Zhu X. M., Chamberlain J. S., McCabe E. R., Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993 Jun;87(6):1854–1865. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- Valantine H. A., Hunt S. A., Fowler M. B., Billingham M. E., Schroeder J. S. Frequency of familial nature of dilated cardiomyopathy and usefulness of cardiac transplantation in this subset. Am J Cardiol. 1989 Apr 15;63(13):959–963. doi: 10.1016/0002-9149(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Voit T., Haas K., Léger J. O., Pons F., Léger J. J. Xp21 dystrophin and 6q dystrophin-related protein. Comparative immunolocalization using multiple antibodies. Am J Pathol. 1991 Nov;139(5):969–976. [PMC free article] [PubMed] [Google Scholar]
- Vuolteenaho R., Nissinen M., Sainio K., Byers M., Eddy R., Hirvonen H., Shows T. B., Sariola H., Engvall E., Tryggvason K. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994 Feb;124(3):381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Ikeda S., Nakamura A., Kagoshima M., Takeda S., Shoji S., Yanagisawa N. Molecular analysis of the Duchenne muscular dystrophy gene in patients with Becker muscular dystrophy presenting with dilated cardiomyopathy. Muscle Nerve. 1993 Nov;16(11):1161–1166. doi: 10.1002/mus.880161104. [DOI] [PubMed] [Google Scholar]