Introduction
Mechanical sensitivity plays an essential role in cells and higher organisms. Specialized exteroceptors transduce external stimuli such as sound, vibration, touch, and local gravity. Interoceptors regulate for the voluntary musculature and the filling of the hollow organs, such as blood pressure regulation. Cellular transducers provide local control of blood flow, regulation of cell volume, deposition of bone, etc (Sachs and Morris, 1998b;Hamill and Martinac, 2001). Hormonally coupled mechanical systems, such as renin-angiotensin and atrial naturietic peptide, regulate fluid volume. There are also autocrine and paracrine transducers that generate second messengers such as endothelin (Ostrow et al., 2000a;Ostrow and Sachs, 2005). Some of these processes are probably driven by mechanosensitive ion channels (MSCs) (Martinac, 2004).
Because mechanical transduction is ubiquitous, it is likely essential for cells of all phyla. In higher plants, mechanical transducers guide root, stem and leaf growth in response to gravity. Mechanosensitive ion channels (MSCs) can serve as sensory transducers in bacteria and other microorganisms, serving as sensors for volume regulation (Martinac, 2001;Sachs, 2002a). The fact that E. coli has as many as five different MSCs argues for their functional importance (Sachs, 2002a). Because of its universality, mechanical transduction is likely to have developed early in evolution(Martinac and Kloda, 2003), as a necessity for conducting metabolism in a membrane limited compartment. However, the variability of MSC structures also suggest repeated convergent evolution(Martinac, 2004).
Any physiological process is subject to a related pathology caused by malfunction of any of its components. While there are no known pathologies due to mutations of MSCs -except for osmosensitivity in mutated E. Coli (Levina et al., 1999;McLaggan et al., 2002) - the pervasive nature of MSCs indicates that we will find genetic and environmental factors that create human pathologies related to MSC malfunction. For example, studies on dystrophic muscle cells show that dystrophin mutations lead to a weakening of the membrane, thereby activating a Ca++ influx through MSCs (Yeung et al., 2003a;Patel et al., 2001). This influx can be blocked by Gd+3, streptomycin (Yeung et al., 2003b) and the peptide GsMTx4 (Yeung et al., 2005). In this case the pathology does not seem to arise from the channels themselves but from pathological change in the mechanical environment. Presumably we will discover much such pathology since all cells have a characteristic and elaborate mechanical environment.
Although mechanically sensitive of ion channels are widespread (Martinac and Kloda, 2003), no similarity in primary structure is evident in this channel family. For example, in E. Coli, the two dominant mechanosensitive channels MscL and MSCs (generically noted MSCs), differ fundamentally in sequence and structure. MscL is a pentamer(Chang et al., 1998)and MscS is a heptamer (Bass et al., 2003), and the primary sequences of the subunits have little homology. The only well characterized MSC cloned from eukaryotes are a subset of the K+ selective 2P family (Patel et al., 2001). These channels have no useful sequence homology to the bacterial channels. Thus, mechanosensitivity, while universal, does not obey the delightful homologies of many of the voltage and ligand gated channels, an example of convergent evolution. Moreover, from a mechanistic viewpoint, bacterial MSCs are almost certainly different from eukaryotic channels given the difference in cytoskeletal structure that influences the mechanics. What we learn from bacteria does not necessarily apply to eukaryotic MSCs. Within the phenotypic MSC families, however, there appears to be a useful discriminator – those channels that are stimulated by stress in the cytoskeleton and extracellular matrix (Corey, 2003b;Corey, 2003a) as in the cochlea, and those that are stimulated by stress in the bilayer, as with bacterial MSCs.
The intrinsic mechanosensitivity of channels depends on dimensional changes between the closed and open states (Sachs and Morris, 1998b;Hamill and Martinac, 2001;Sukharev et al., 1999;Markin and Sachs, 2004a;Markin and Sachs, 2006;Wiggins and Phillips, 2004). A detailed study of the kinetics of the prototype channel MscL has at least eight rate constants to characterize the gating reaction. Of these, however, only a single rate constant is significantly sensitive to tension (Sukharev et al., 1999). Conversely, channels that have been previously characterized as ligand gated (Vandorpe et al., 1994) or voltage sensitive (Gu et al., 2001) may turn out to be mechanically sensitive (Laitko and Morris, 2004;Morris, 2004;Calabrese et al., 2002). This poses an intriguing problem in evolution: how to design structures with the necessary flexibility to support large conformational changes (Jiang et al., 2003b;Jiang et al., 2003a) while avoiding unnecessary mechanical activation?
The physiological function of MSCs in non-specialized tissues has not been demonstrated in eukaryotes. Theoretically, one common ground (Sachs, 2002b) may be volume regulation (Christensen, 1987), although this has not been demonstrated. In fact, preliminary data from our lab suggests that stretch-activated cation channels, SACs, are not involved, since volume regulation is insensitive to the specific blocker, GsMTx-4. In general, to establish the physiological function of a protein, one needs to activate or inactivate it by controlled non-physiologic stimuli. Specific pharmacologic agents are one approach, and genetic knockouts (Corey, 2003b) another.
There is only one specific pharmacological agent for MSCs: the peptide GsMTx4 (and its mutants) (Suchyna et al., 2000b). The search has been hampered, in part, by technical difficulties in defining the stimulus (Besch et al., 2002;Hamill and McBride, Jr., 1995). While you can buy stimulators for electrically activated channels and for ligand-gated channels, until this year, none were available for mechanically gated channels. Posing additional problems in a drug assay, the stimulus that actually reaches a channel depends intimately upon the geometry and constitutive mechanical properties of the cell cortex (Sachs and Morris, 1998a), factors that are generally unknown. Applying a precise pressure to a patch pipette does not define the force reaching the channel. MSCs are embedded in a heterogeneous, non-Newtonian mechanical structure consisting of the extracellular matrix, the bilayer and its embedded proteins, and the cytoskeleton (Garcia-Anoveros and Corey, 1996;Gillespie and Walker, 2001). The stress that activates MSCs may come from the lipid bilayer (Akinlaja and Sachs, 1998), but that tension depends on the cytoskeleton, the geometry, and the boundary conditions (Suchyna and Sachs, 2004). Despite this complexity, it appears that MSCs from nonspecialized tissues are activated by tension in the lipid bilayer (Suchyna and Sachs, 2004). The difficulty of defining the stimulus is also true for global stimuli such as hypotonic or shear stress. At the current time, measurement of absolute sensitivity requires working in lipid bilayers where the stress is reasonably well defined (Sukharev et al., 1999;Suchyna et al., 2004;Moe and Blount, 2005).
Some History of Cell Mechanics
The field of biological mechanotransduction is rapidly maturing, and to gain perspective, it is worth examining some of the history of cell mechanics. Biologists have long poked, prodded, and spun cells, and sea urchin eggs are a popular preparation because of their size (~ 70 μm). One of the earliest estimates of tension at a cell’s surface was done in 1926 by Fred Vles who estimated a value between 10–20 dyne/cm in European sea urchin eggs (Vles, 1926). He calculated the tension by measuring the distention of the egg suspended in a liquid drop. The same approach was tried for American sea urchin eggs, but, unlike their European counterparts, the New World sea urchin eggs did not flatten under 1G gravity. So E. N. Harvey used a different approach. He built a centrifuge on a microscope stage, spun the sea urchin egg (which is a single cell) and determined the force required to separate the egg in two (Harvey, 1931). He estimated the tension to be ~ 0.2 dyne/cm.
At the time, the existence of the cell membrane was not firmly established, and, as Harvey noted, he couldn’t tell if the tension arose due to a liquid-liquid interface or to a membrane tension because he could not discern ‘…whether or not the strain is independent of the stress’, as it would be for tension at a liquid-liquid interface. Surface tension at a liquid-liquid interface is a characteristic of the molecules present and independent of size of the interface (see Figure 1A, Laplace’s law).
Figure 1.
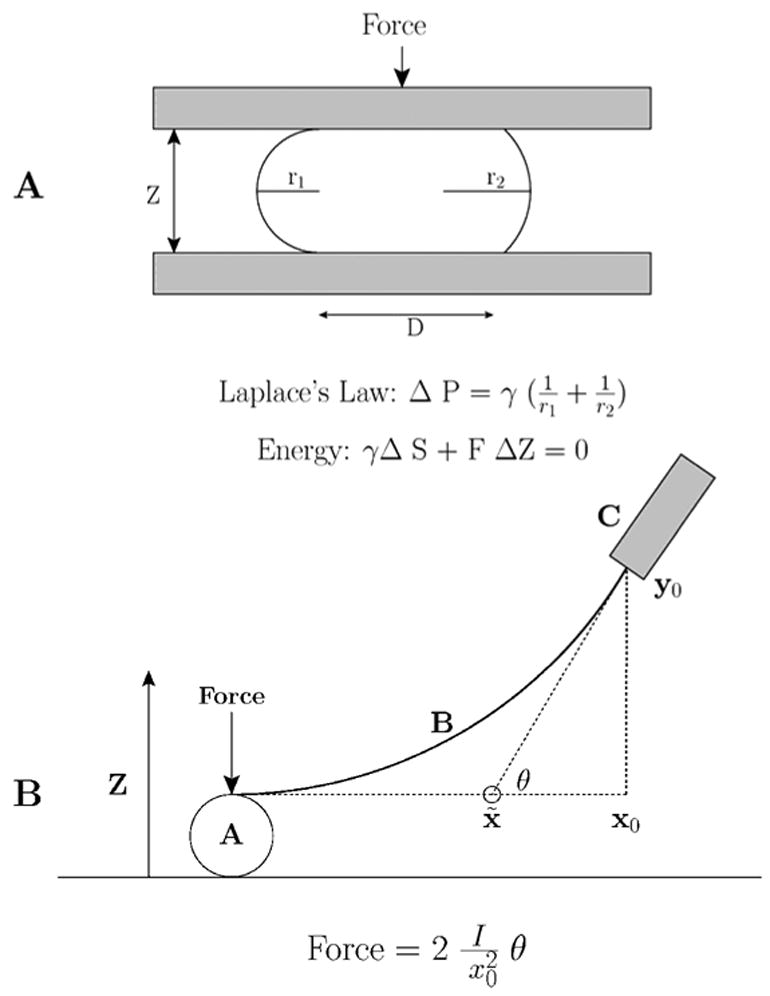
K. S. Cole’s ’flexure balance’. A. Applying force to the Arbacia egg. B. The parameters used to calculate the force (after Cole 1932a).
In 1932 Kenneth Cole understood this and argued that if the tension was at the cell’s membrane, tension should increase ‘…as the surface increases’ (Cole, 1932b). Cole decided to measure the force required to squish the Arbacia egg, and invented an extremely sensitive ‘flexure balance’ (Cole, 1932a), which resembles in some ways today’s atomic force microscope (Figure 1). Judging from Cole’s figure 4, plotting Z distance vs force (Cole, 1932b), the minimum force he could detect was ~ 0.7 pN, more sensitive than many present-day commercially available atomic force microscopes. Cole computed the area from the circular portion of the egg flattened against the substrate, and r1 and r2 were measured from photographs of the compressed egg. The force was measured with his balance (Figure 1B). From the pressure and curvature, Cole calculated the tension using Laplace’s law. As the force increased, the pressure increased, the principle radii decreased and the computed tension increased. He found that tension increased with applied force from 0.09 to 0.15 dynes/cm. Extrapolation to zero force resulted in a resting membrane tension of 0.08 dyne/cm, corresponding to a resting pressure of 40 dynes/cm2. Whatever was supporting the tension was acting as a stretched film, consistent with a membrane, and not a ‘liquid-liquid’ interface. Cole was the first to measure directly the amount of force required to compress a single cell.
Figure 4.
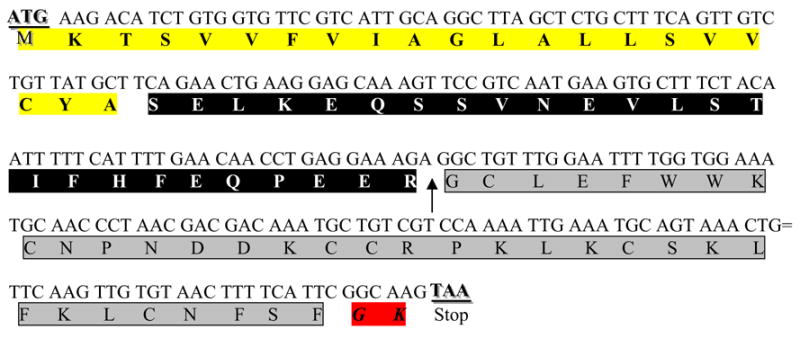
cDNA of the gene encoding GsMTx4 with the open reading frame. The first 21 amino acids are removed as a signal sequence (yellow). The protein is cleaved at an arginine (arrow) and the last two amino acids (red) are removed during amidation. The mature peptide 34 amino acid peptide is outlined in gray.
Cole’s estimate of the minimal surface tension was 2.5 fold less than Harvey’s determination of lytic tension in Arbacia, and Cole missed an opportunity to suggest that membranes cannot sustain much tension without breaking. But his results are essentially reproduced in other cells. Arbacia’s resting pressure is about 6-fold less than the pressure found in membrane blebs formed in human melanoma cell lines ( 270 dynes/cm2, (Dai and Sheetz, 1999a). In these same melanoma cells, the membrane tension in tethers is ~ 0.03 dyne/cm, and membrane tension in tethers in snail neurons is 0.04 dyne/cm (Dai et al., 1998). The formation of membrane blebs indicates cells normally have a resting pressure, and the cytoskeleton balances that outwardly directed pressure (Dai and Sheetz, 1999b). There must be load bearing contacts between the lipid plasmalemma and cytoskeleton. Being a polymath, Cole went on to invent the voltage clamp (Cole, 1949), enabling the quantitative study of voltage dependent processes in membranes.
Cole assumed that the cytoplasm played no role in his measurements. So the question was raised, how stiff is the cytoplasm? In 1950, Francis Crick and A. Hughes published a study of the mechanical properties of cytoplasm in chick fibroblasts in culture (Crick and Hughes, 1950). These cells phagocytose micron-size magnetic particles, whose motion could be recorded photographically. The magnetic particles could be rotated or dragged through the cytoplasm and the position of the particles sometimes showed a partial return to their original position (a recoil) after the magnetic field was turned off. Giving repeated stimuli resulted in either smaller motions or larger motions of the particle, indicating the stiffness of the cytoplasm was increasing or decreasing. Under conditions where particle motions became larger, the recoil decreased. Particles did not move when weak magnetic fields were applied for a long time. These results demonstrated that the mechanical properties of cytoplasm change with stress, a complex thixotropic. They estimated a modulus of rigidity ~ 100 dynes/cm2, and a viscosity ~ 10 poise, about 1000 times more viscous than water. Perhaps this was the first demonstration that repeated mechanical force changed the mechanical properties of the cytoplasm, and the first estimate of the rigidity of the cytoplasm. Also being a polymath, Crick went off to solve the structure of DNA with Jim Watson.
If the cytoplasm was a viscous goo, there is a problem applying Laplace’s law to determine membrane surface tension – the mechanical membrane is the cell cortex, and the bilayer is only part of the structure(Akinlaja and Sachs, 1998). The study of the mechanical properties of cells advanced when J.M Mitichison and M.M. Swann thought of aspirating a portion of the cell into a pipette to characterize the cell’s mechanical properties. They called their device the ’cell elastomer’ (Figure 2) (Mitchison and Swann, 1954a). They found a resting pressure of about 10 dynes/cm2 in Arbacia eggs, and this pressure increased with hypoosmolality. They compared the aspiration characteristics of Arbacia with various models, such as rubber balls of varying wall thickness, and found that Arbacia had a linear relationship of pipette suction vs aspiration length, (P vs x) while other models predicted a nonlinearity at high pressures. They concluded that application of Laplace’s law was inappropriate, and that Arbacia’s cortex does not behave like a bilayer, and the cytoskeleton dominated the deformation. In support of Crick and Hughes’ finding of mechanically induced changes in stiffness, they found hysteresis in the P vs x curves: the stiffness was larger when sucking membrane in than when pushing it out. This also supported the conclusion that a cell surface is not just a thin membrane. Akinlaja and Sachs(Akinlaja and Sachs, 1998) found the same result when examining patches of HEK293 cells: the membrane was stronger when pushed toward the cell interior than when pulled away. Mitchison and Swann estimated the mean mechanical properties of the cortex with a Young’s modulus of about 104 dynes/cm2 and concluded the sea urchin egg had ‘…a consistency of a weak table jelly…’ (Mitchison and Swann, 1954b).
Figure 2.
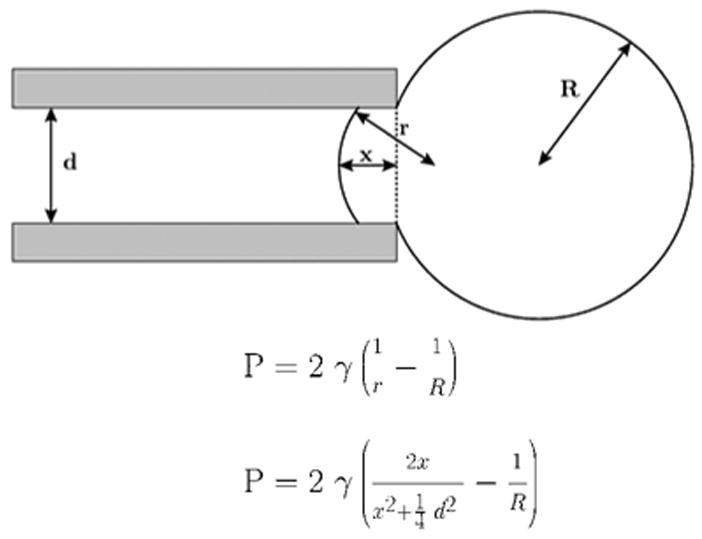
Aspiration of membranes into a micropipette (after (Mitchison and Swann, 1954a)). The top equation is Laplace’s law, and the bottom equation is a transformation relating the aspiration distance to the tension, γ.
In the late 1970’s, Neher and Sakmann’s group discovered that cell membranes aspirated into micropipettes can form an electrically tight plug – called the gigaseal (Hamill et al., 1981). This enabled the study of single ion channels. The stage was set for the discovery of membrane elements that transduce changes in membrane tension. During a study of nicotinic receptors in muscle cells, Guharay and Sachs and Brehm and colleagues found that applying suction to a pipette elicited ion channel activity (Guharay and Sachs, 1984;Brehm et al., 1984). Guharay and Sachs presented the first detailed analysis of these channels, terming them SACs (Stretch-Activated ion Channels, not an abbreviation of “Sachs”! Note: the term MSC refers to any kind of channel that is mechanosensitive). Pipette suction produced no change in activity for other channels, such as CaK or nicotinic acetylcholine receptors. No second messengers including Ca2+ were required for activation, and the gating was nearly independent of membrane potential. The response was a function of tension, not the hydrostatic pressure, since the channels activated with positive or negative pressure. The SACs formed a new class of channels. A more recent example of SAC activity is shown in Figure 3. In 1991, Sokabe et al. imaged the patch and estimated the relationship between membrane tension and channel activity (Sokabe et al., 1991).
Figure 3.
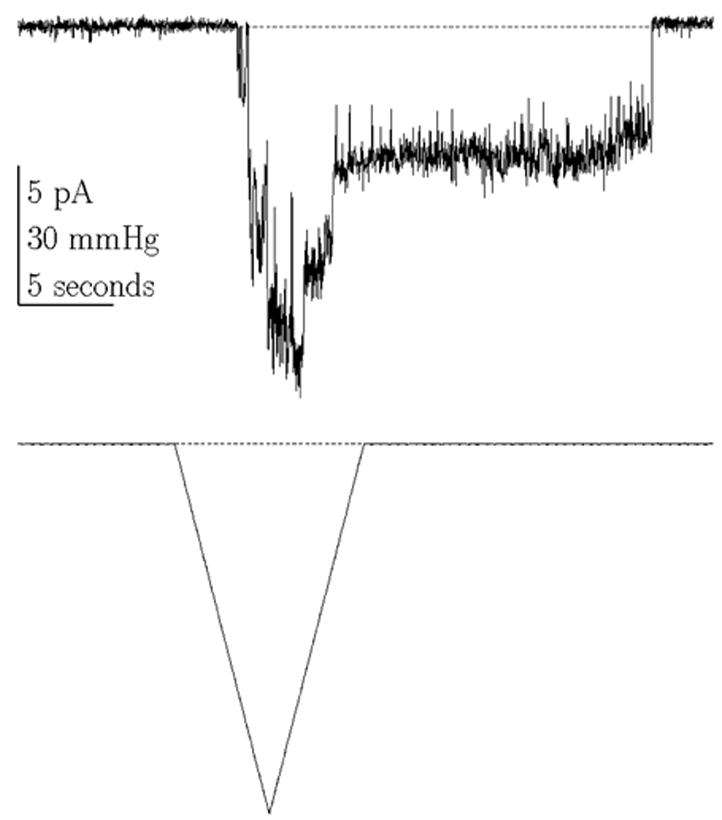
Endogenous mechanosensitive ion channel currents from COS 7L cells activated by a ramp of pressure. Notice the latencies in the on and off responses. (Vm = −75 mV, 100 Hz bandwidth, cell attached patch).
Sources of Tension
Two issues of MSC mechanics have received considerable attention: how is cortical tension transferred to the channels, and why can’t whole cell MSC currents be easily observed? These questions are related. There are two ways tension can be delivered to the channel (which clearly is a bilayer bound structure): one is through the bilayer and the second is through attachments to the cytoskeleton. Which source of stress gates the channel? This must first be resolved into the type of channel under consideration. The specialized receptors such as the stereocilia of hair cells in the cochlea(Corey, 2003a) and the touch receptors(Walker, 2000) appear to be directly activated by stress from direct attachments of polymers strings (e.g., collagen and tubulin) to the channel. The channels in non-specialized cells appear to be gated from tension in the bilayer, and this is the type of channel that we discuss in this review.
The unequivocal evidence that at least some MSCs are activated by stress in the bilayer comes from reconstitution experiments in purified lipid membranes (Chiang et al., 2004). In eukaryotes, MSCs in the frog oocyte are active in blebs that lack cytoskeleton (Zhang et al., 2000). Cloned TrpC1 appears to be an MSC (Maroto et al., 2005) which can be reconstituted in lipid vesicles. However, TRPC1 has two ankyrin binding sequences suggesting that the in situ situation may be more complicated (Falsig et al., 2005). Despite the evidence for activation via bilayer stress, the bilayer stress is affected by the cytoskeleton, and this is particularly visible when the gating displays curvature sensitivity(Bowman et al., 1992), or substantial latency in activation and inactivation (c.f., Figure 3). The difficulty in eliciting MSC currents in whole-cell recordings probably reflects the ability of the cytoskeleton to bear most of the mean stress.
Using a variety of mechanical stimuli, Morris and Horn failed to elicit (substantial) MSC currents in whole-cell recordings from snail neurons (Morris and Horn, 1991). They suggested that forming a patch leads to disruption of the sub-membrane cortex, only then exposing the bilayer to the applied stress. Bett and Sachs confirmed that whole-cell currents were difficult to elicit in ventricular cells from adult rats, but once activated, the current remained active and sensitive to a mechanical stimulus (Bett and Sachs, 2000). Hamill and McBride (Hamill and McBride, Jr., 1992) showed that inactivation of MSCs in cell-attached patches from oocytes is lost with following repeated stimulation. They suggested that stress damaged the cortical cytoskeleton and that is somehow connected to the process of inactivation (Hamill and McBride, Jr., 1992). Suchyna and Sachs showed similar behavior in astrocytes (Suchyna and Sachs, 2004). The bulk of the evidence suggests that MSCs sense tension within the bilayer, and that cytoskeletal elements modify that tension, and in a viscoelastic manner. There is another source of stress that arises in patch clamp experiments. Because the patch adheres to the pipette, the patch is always under tension and that tension is a significant fraction of the lytic tension (Mukhin et al., 2004;Akinlaja and Sachs, 1998;Honore et al., 2006).
Gating models
Channel opening must involve conformational changes, probably from the bilayer as discussed above, and the work of many groups, has begun to dissect the forces involved in gating (Sukharev and Anishkin, 2004;Wiggins and Phillips, 2004;Perozo et al., 2002;Sachs, 2002b;Markin and Sukharev, 2000;Blount et al., 1999;Sukharev et al., 1997;Moe and Blount, 2005). The simplest model is that the closed and open channels differ in size, so that in a tension field T, the two conformations differ in energy by T A, where A is the difference of in-plane area between closed and open conformations. This change in dimension does not need to be large; for the 2P type K+ channel TREK-1, it has been estimated to correspond to a change in radius of ~ 1Å (Honore et al., 2006).
There are other ways in which far field tension can modulate channel activity. If the channel changes shape, even at constant area, the torque on the channel can change the balance of energy between closed and open states. The boundary lipids might thin under tension coupling gating to hydrophobic mismatch(Dzikovski et al., 2004). Drugs may act on any of these properties to alter channel gating.
GsMTx4, a Peptide Blocker of SACs
Isolation and Purification
In a desperate attempt to find a pharmacological agent to inhibit SACs, we indulged in the most antithetical approach of the funding agencies: a blind search. We screened (diluted) invertebrate venoms since they don’t have lipases. Screening required an outside-out patch preparation that retained mechanical sensitivity, and only by chance, with motivation of an MD/PhD student, Lyle Ostrow, did we find that adult astrocytes were an acceptable preparation (Suchyna et al., 2000a). None of the scorpion venoms we tried had an effect, and only two of the spiders, one of which was a tarantula, Grammostola spatulata, with more venom than the smaller spider, so we focused upon that. The experiments required good stimulus control so we designd and build a pressure clamp (Besch et al., 2002). The screen led to the identification and isolation of the peptide GsMTx4, which is the only drug known to specifically affect cationic SACs (Figure 4).
The amino acid sequence for the peptide GsMTx4 was initially done with protein sequencing, and although most of that was correct, there proved to be a small error at the C terminus. The correct sequence was deduced from the gene isolated from the venom gland. A cDNA copy of the RNA was sequenced (Ostrow et al., 2003) revealing that, as anticipated, the protein exists in a pre-proform (Figure 4), where the first 21 amino acids (yellow) are a predicted signal sequence, and are removed during protein translocation (bimas.dcrt.nih.gov/molbio/signal). The last two amino acids are glycine-lysine (red), a known site for amidation (Gomez et al., 1984). The arginine adjacent to the active peptide molecule (prosequence in black) is presumably the cleavage site (indicated by arrow) to release active GsMTx4 peptide (gray).
Using this sequence, we chemically synthesized GsMTx4 with a phenylalanine-amide at the C-terminal and determined the conditions for folding (Ostrow et al., 2003;Oswald et al., 2002). The synthetic peptide appeared indistinguishable from wild type peptide in all physical-chemical properties. MALDI-MS revealed a mass of 4093.9 [M+H+] while GsMTx4 from venom had a mass of 4094.0 [M+H+]. Reverse phase liquid chromatography shows identical retention times and co-injection of the two compounds produced a single peak. Circular dichroism for both peptides was similar, having minima at ~ 192 and 202 nm. NMR spectra of both peptides demonstrated that the structures were the same (Ostrow et al., 2003). Finally, the peptide produced the same physiological response.
Structure
GsMTx4 follows the Inhibitory Cysteine Knot (ICK) motif with six cysteines (Pallaghy et al., 1994;Oswald et al., 2002), and as with similar neuroactive peptides, it is amphipathic (Figure 5). The hydrophobic face is in green and the charged residues in red (negative) and blue (positive). The hydrophobic surface (at the bottom) is surrounded by mostly positive charges. This design suggests that the peptide binds to membranes using its hydrophobic face to penetrate the lipid bilayer. While the net charge of GsMTx4 is +5, the charge itself seems no not to be the key to activity since poly-lysines have no effect on MSCs and single site alanine scanning also has not revealed any significant effect. The charges probably play a role in setting the depth of penetration of the peptide into the lipid.
Figure 5.
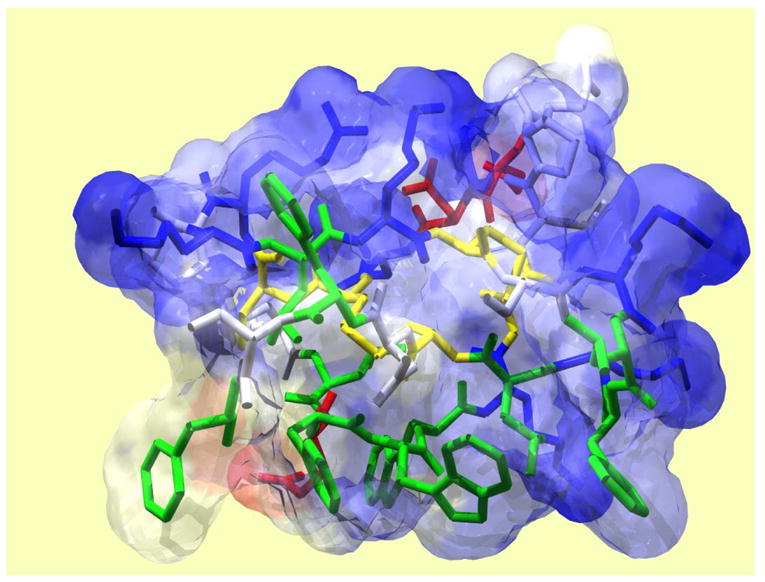
Solution structure of GsMTx4 determined by NMR spectroscopy. Disulfide bonds are shown in yellow, hydrophobic residues in green, acidic residues in red, and basic residues in blue. GsMTx4 has a predicted net charge of +5 at neutral pH.
GsMTx4 is homologous to other peptides derived from spider venoms. Figure 6 compares a number of peptides that have been isolated from various spiders. While their targets appear to be various voltage-gated channels, they all have features in common. All the peptides belong to the ICK family. All of these peptides, including GsMTx-4, appear to act as gating modifiers, and all of them have aromatic groups in the hydrophobic face of the C- and N- termini.
Figure 6.
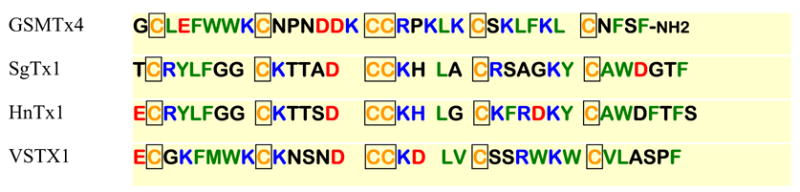
Sequence comparison of four channel active peptides derived from spider venoms. All peptides belong to the ICK family and are all gating modifiers. Hydrophobic residues are green, charged residues red for acidic, and blue for basic. Cysteines are yellow and boxed.
Interaction of GsMTx4 with lipids
The peptide’s ability to bind to membranes is probably a key to its mechanism of action (Suchyna et al., 2004). The MacKinnon group came to a similar conclusion regarding VsTx1, a peptide active on voltage dependent potassium channels (Lee and MacKinnon, 2004), as did Swartz’s group for the peptide SgTx1 (Lee et al., 2004;Wang et al., 2004). The ability to partition into membranes appears to be an essential feature for these gating modifiers.
We have examined GsMTx4 binding to lipid membranes using Trp quenching and surface plasmon resonance. Based on earlier work (White et al., 1998), Ladhokin’s group developed a sensitive method for measuring the partitioning of peptides into LUVs (Large Unilamellar Vesicles) using differential I− quenching (Posokhov et al., 2006). Partitioning into zwitterionic vesicles such as PC or PE involved an energy of transfer of ΔG=−6 kcal/mol(Ladokhin et al., 2000), and as expected, the affinity for anionic vesicles (75%PS) was higher, ΔG=−8 kcal/mol. Considering the positive charge of GsMTx-4, the modest increase in affinity with lipid charge argues for a low effective charge with a lack of additivity between the hydrophobic and charged interactions (Posokhov, Gottlieb, and Ladhokin, submitted). Preliminary data using surface plasmon resonance shows GsMTx4 binding to a variety of LUVs (Gottlieb, Ryan and Sachs, in preparation)
What is the orientation of GsMTx4 within the membrane? We examined the water accessibility of the Trps in the presence of LUVs. In solution, both Cs+ and I−quenched the fluorescence showing that the Trps were not shielded and the electrostatic interactions were negligible. This is similar to other soluble peptides containing tryptophan residues. However, in the presence of vesicles, the peptide’s Trps were protected from quenching, consistent with the hydrophobic face being buried in the lipids. The depth of the peptide penetration into the lipid was measured using brominated lipids(Powl et al., 2003;Powl et al., 2005). Bromine quenches Trp fluorescence, and it can be located at different distances along the acyl chains of the phospholipids. These measurements indicated that the Trps of GsMTx4 penetrate ~0.9nm from the center of the lipid bilayer (Posokhov, Gottlieb, and Ladhokin, submitted).
Physiological properties
The phenomenological dissociation rate constant was determined fitting the rate of washout of SACs currents (Suchyna et al., 2000d). The association rate constant was more difficult. The channels were activated with a 3 second pressure step, and one second into the stimulus, GsMTx4 was rapidly perfused (<10ms switching time). The exponential decay of this current was taken to be the association rate (Figure 7). We calculated the equilibrium constant from KD=kd/ka. This kinetic method of determining KD is important, since it is difficult to prevent run-down or run-up of the currents over time. The association rate was 3.3 x 105 M−1s−1, the dissociation rate ~ 0.2s−1, providing KD=0.5 μM. This is a lower affinity than many of the well known channel inhibiting peptides, but the association rate is similar.
Figure 7.
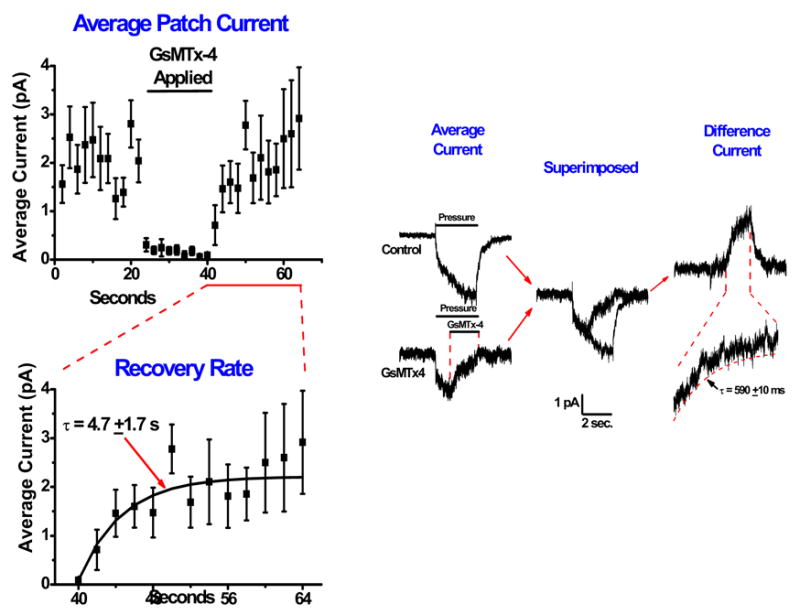
Determination of the association and dissociation rates of GsMTx4. Left panel, washout and fitting with recovery over ~5 seconds. The data were fitted with a single exponential. Right panel, association rates. Average currents with GsMTx4 applied are subtracted from the control currents to generate a difference current. The difference current was fit with a single exponential to determine the association rate.
We then tried to determine the mechanism of action. There are two basic types of channel inhibition: kinetic (gating modifiers) and pore blocking (Suchyna et al., 2004). Typically, pore blockers will reduce the current, but retain approximately the same time course and the same dose-response relationship (although the kinetics can be affected by the on and off rates of the blocker). Gating modifiers will change the time course and shift the dose response curve to stronger stimuli. Figure 8 shows the effect of GsMTx4 on astrocyte SACs. The activation curve is shifted to the right - the hallmark of a gating modifier. Note that seal breakage limits the maximum pressure used in this assay so that the channels could not be driven to saturation.
Figure 8.
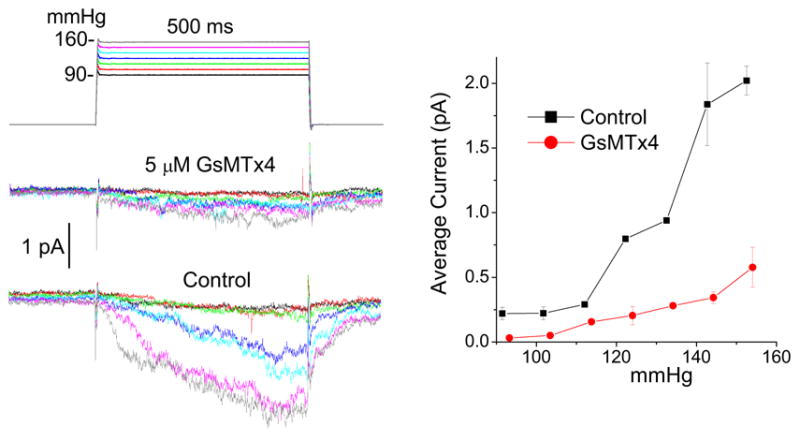
GsMTx4 is a gating modifier since the block can overcome with increasing suction. a) Average currents of an outside-out patch from rat astrocytes as a function of suction. b) The peak average currents as a function of suction showing a rightward shift for activation indicative of a gating modifier.
The mode of action
We looked for possible interactions between GsMTx4 and channels. There were two general modes of activity: a physical effect on the membrane(Hwang et al., 2003) or a specific effect on the channels. At the time we did these experiments, there were no known clones of the SACs, so that it was not possible to do experiments such as mutant cycle analysis. However, the one thing we did know about the channel was that it was made of L amino acids. Although phospholipids are modestly chiral, we expected that a physical mode of action would be insensitive to chirality of the peptide. We synthesized the D form of the peptide (enGsMTx-4) (Suchyna et al., 2004;Ostrow et al., 2003), and it was identical in all its chemical properties to the wild type peptide as measured by reverse phase chromatography and mass spectrometry. The only distinction was that the CD spectrum was the mirror image of the wild type peptide.
Remarkably, enGsMTx4 had nearly identical activity (Figure 9). This argues for a mechanism that doesn’t rely on direct stereo-chemical interactions. . GsMTx4 does not follow the standard lock and key model of traditional pharmacology.
Figure 9.
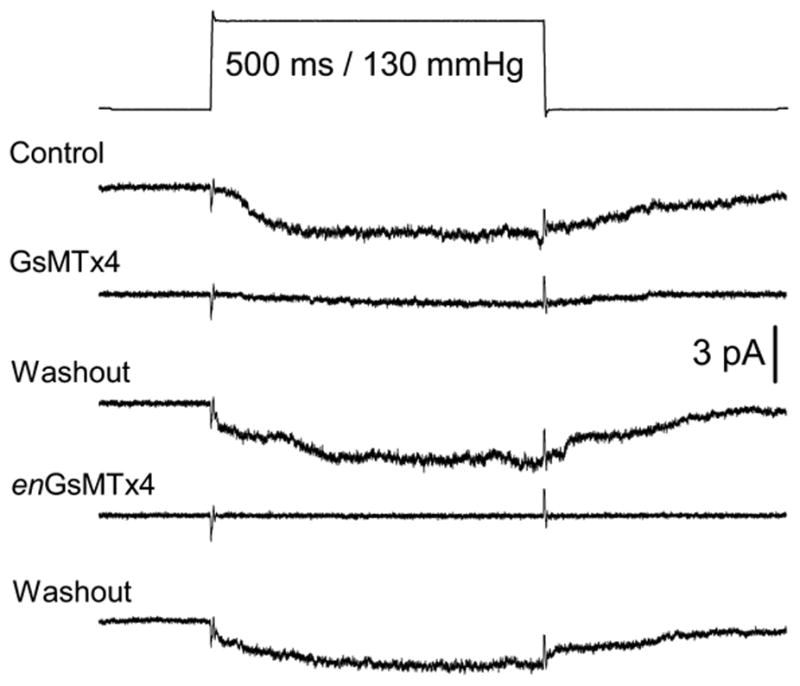
The equivalent effect of wt and enGsMTx4 on mechanosensitive ion channels. (Outside/Out patches, rat astrocytes)
How can a molecule globally affect the channel? Global effects on the membrane include such factors as alterations in surface charge(Bell and Miller, 1984;Winterhalter and Helfrich, 1988;Elinder and Arhem, 1994;Murray et al., 1999), or bending due to amphipath activity(Deuticke, 1968;Martinac et al., 1990;Perozo et al., 2002). For peptide surface charges to affect the channel, they have to reside within a Debye length of the channel, ~ 1nm. Bending, or changing the intrinsic curvature of the membrane will have a minor effect upon the membrane tension of a patch since the radius of patch curvature is so much larger than the channel dimensions(Markin and Sachs, 2004a;Markin and Sachs, 2004b;Markin and Sachs, 2006). Thus, GsMTx4 must be close to the channel. But, the interaction is not chiral, so where is it? Probably within the boundary lipids of the channel. Supporting the generic lack of amphipath activity, Iwasa (personal communication) showed that GsMTx-4, even at saturating doses, does not alter the shape of red blood cells (Sheetz et al., 1976;Sheetz and Singer, 1974). Presumably this is because there is an insufficient number density to change the intrinsic curvature.
If the peptide is close to the channel, and the peptide has a charge of +5e, binding of the peptide should affect channel conductance since the positive charges will repel incoming cations. Since the peptide is acting on the extracellular surface, it will affect inward currents more than outward currents. The results of these experiments are shown in Figure 10 (Suchyna et al., 2004). The outward current is shown at the left and inward at the right, with “typical” single channel currents at the top and the averages below. The bottom graph is the amplitude histogram of the single channel currents with the control data in black and the GsMTx4 data in red. The outward unitary current is unaffected, but the inward current is reduced by about 10%. Note that this experiment is only possible because GsMTx4 is a gating modifier not a pore blocker.
Figure 10.
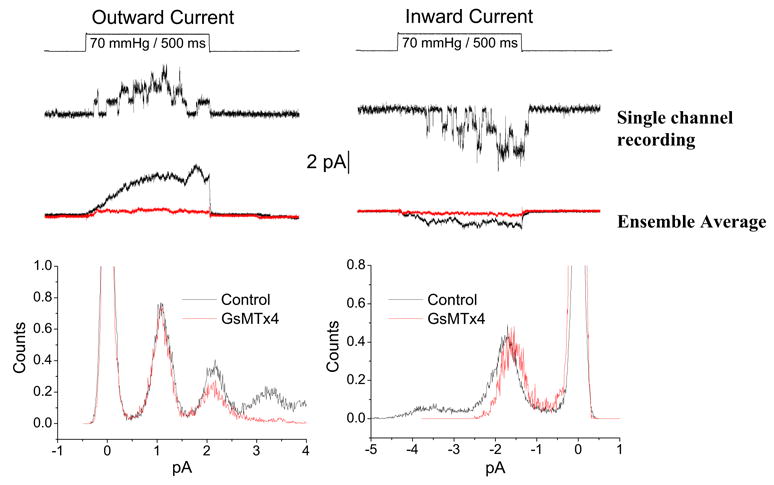
GsMTx4 affects the inward but not outward unitary currents of SACs from adult rat astrocytes. Outward currents at the left and inward currents at the right. Single channel recordings are shown underneath the pressure pulse. Ensemble averaged current in the presence (red) and absence (black) of GsMTx4. Single channel amplitude histograms show the change of unitary inward currents.
How does GsMTx4 modify gating? The channel kinetics are not significantly voltage sensitive, so the charge of the peptide is not a key. Andersen’s group has been able to show that gramicidin (gA), a dimeric channel, is a sensitive probe of the membrane mechanics around a channel(Lundb‘k and Andersen, 1994;Goulian et al., 1997;Goulian et al., 1998). They were also able to show that genistein, a presumed PKC inhibitor with wide ranging activities, alters the kinetics of gramicidin channels in pure lipid bilayers, obviously not acting as a PKC inhibitor(Hwang et al., 2003). This suggested that GsMTx4 might also affect gramicidin gating. gA is a cylindrical peptide half the thickness of a lipid monolayer (~ 1.5 nm). Two monomers, one from each leaflet of the bilayer, associate end-to-end forming a channel. The kinetics of channel creation and dissociation are affected by the thickness of the bilayer, and thus the local bending of the membrane (Andersen et al., 1999).
GsMTx4 did affect the gating of gA and a variety of its mutants including one of opposite chirality (Figure 11). This is expected if GsMTx4 bent the boundary lipids toward the channel, making the membrane appear thinner. Similar to our observations on the eukaryotic SACs, GsMTx4 decreased the unitary conductance for the gA analogs (Figure 11b). The fact that the gA amplitude histograms display a monomodal distribution suggests a uniform association of GsMTx4 with the channels. However, the concentration dependence of unitary current with concentration is linear, suggesting only single occupancy about the channel. These two observations can be reconciled if there is rapid exchange of GsMTx4 (faster than the response time of the electrophysiology) between the bulk membrane and the boundary lipids. This would appear to be true for the SACs since a close examination of the single channel currents does not show the fluctuations that might be a result of the coming and going of GsMTx-4.
Figure 11.
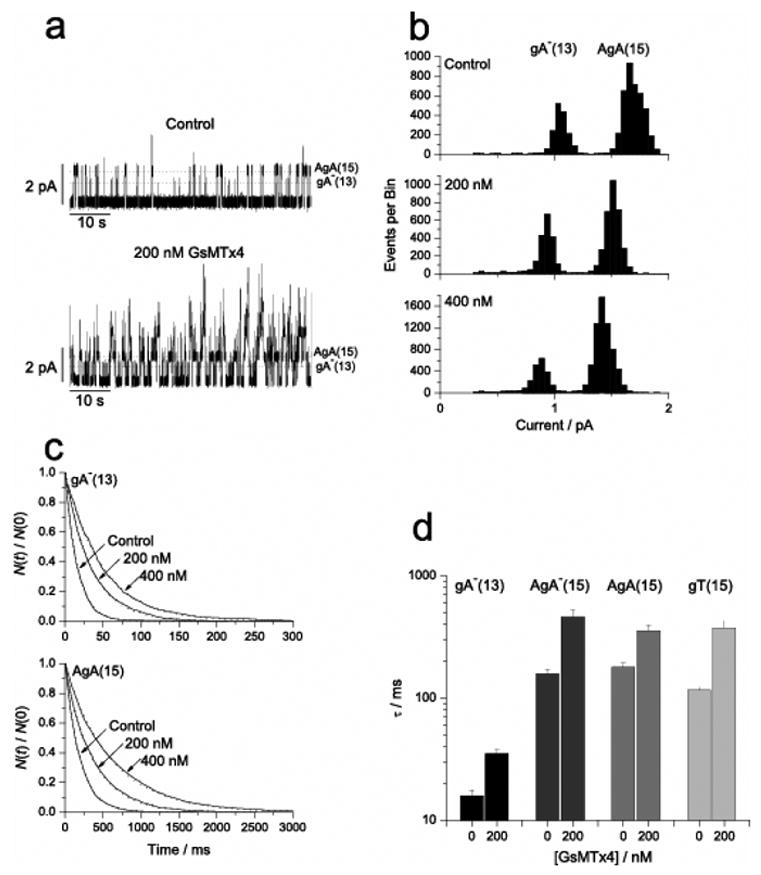
The effect of GsMTx4 on gramicidin gating. a) Raw channel data showing GsMTx4 increased activity. b) GsMTx4 decreased unitary conductance. c) Open channel life times increased with GsMTx4, i.e. decreased the closing rate. d) The GsMTx4 peptide decreased the closing rate of gA analogs varying in length and structure, including enantiomers.
Specificity of GsMTx-4
GsMTx4 is remarkably specific given its achiral site(s) of action
GsMTx4 does not affect voltage-gated channels in rat astrocytes (Suchyna et al., 2000c), or rabbit heart (Sachs, 2004b) It has no measurable effect on the action potential of resting isolated atrial myocytes from the rabbit(Sachs, 2004a), so that all of the channels and transporters responsible for the action potential are unaffected (Figure 12).
Figure 12.
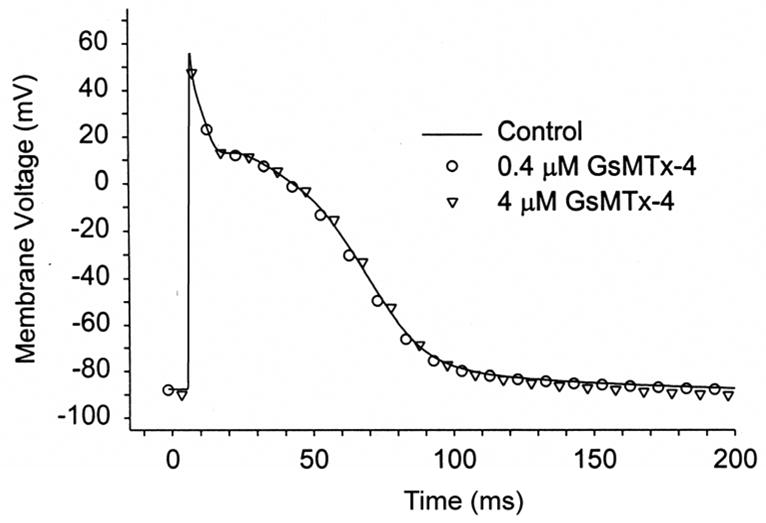
GsMtx-4 doesn’t affect the AP of resting rabbit atrial cells at 8X the KD. Perforated patch (amphotericin), n=5, 37 C° (Sachs, 2004b).
It has no significant effect on the electrophysiology or inotropy of the rabbit (Bode et al., 2001a)or sheep heart (Kalifa, Jalife, Gottlieb, Sachs, in progress) or human heart (Kockskamper et al, submitted), unless the heart is distended. Preliminary experiments have shown that injection of GsMTx4 into mice (at four times the Kd of MSCs assuming distribution in the blood volume) produces no behavioral change over 24 hours, except for a small reduction in water consumption, but a remarkable persistence of the heartbeat after sacrificing the animal (Suchyna et al, in preparation).
Even among MSCs, GsMTx4 is specific. It doesn’t affect auditory transduction(Marcotti et al., 2001) that may originate in TRPA1 channels (Lin et al., 2005), or the activity of 2P channels (E. Honore, personal communication). The basis of this specificity is unclear, but probably arises because only some channels respond to boundary layer stress.
A residual question in the literature has been background activity of MSCs in cells. This cannot be tested with a patch since adhesion of the membrane to the glass produces significant tension(Honore et al., 2006;Mukhin et al., 2004;Akinlaja and Sachs, 1998). Adding GsMTx4 to resting cells, in general, has little effect on cell electrophysiology (Figure 12), although it does have effects on the spontaneous contractions of mdx muscle cells (Suchyna and Sachs, submitted).
TRPC1 as a Potential Target of GsMTx4
One of the major issues still outstanding is the identity of the channels affected by GsMTx-4. Hamill, Martinac and collaborators recently suggested that TRPC1 channel is a mechanically activated cation channel(Maroto et al., 2005), making it an excellent candidate for inhibition by GsMTx-4. TRP (Transient Response Potential) channels were discovered in Drosophila when a mutation in the trp gene altered the organisms response to light(Minke, 1977;Montell et al., 1985). Human homologs were soon identified and approximately thirty genes have been isolated, representing six families of ion channels (Clapham, 2003a);(Beech et al., 2004a) One of these families is called the TRPC (Classical), and is a class of channels found predominately in smooth muscle. TRPC1 may form a homomeric cation channel, and it also form heteromers with different TRP subunits (Clapham, 2003b;Beech et al., 2004b).
Maroto et al(Maroto et al., 2005) introduced human TRPC into oocytes, isolated membrane proteins, fractionated the proteins, and after purifying them, functionally reconstituted an active protein fraction into liposomes. The mechanosensitivity was associated with a protein whose molecular weight is consistent with that of TRPC1. Activity of expression within CHO cells was inhibited by antibodies to TRPC1 and antisense RNA.
A natural question is whether GsMTx4 inhibits TRPC1. Based on Maroto et al’s work, we transfected CHO channels with TRPC1, and were able to evoke SAC activity in CHO cells after transfection with TRPC1 DNA. Channel activity in Outside/Out patches from transfected cells was inhibited by 5 μM of GsMTx4 acting as a gating modifier. Hamill also found that GsMTx4 inhibits TRPC1 (personal communication). However, more recent work in our lab showed that CHO and other cell lines all have a significant endogenous population of mechanosensitive channels with properties similar to that reported for TRPC1. These endogenous channels were not always visible in normal cells, but they could be revealed by treatment with cytochalasin. Apparently the channels are protected from stress by the cytoskeleton.
This raises critical issue as to what are the proper controls for expression of MSCs. Patel and collaborators (Lauritzen et al., 2005) have shown that transfecting cells with GFP is not a good control transfection for MSCs. Expression of DNA for active or inactive 2P channels causes extensive modification of the cytoskeleton, whereas transfection by GFP has no effect. To distinguish whether a channel under observation incorporates the subunit used for transfection requires that the candidate be modified in a way that makes it different from the endogenous channels. We have begun to test TRPC1 by making mutants of the pore domain of TRPC1 to produce different selectivity from the endogenous channel when expressed in COS7 cells. The activity of these channels can be blocked by GsMTx4 (Gottlieb et al, in preparation).
Recent data from Don Gill’s lab (Spassova et al, in preparation) shows that the channel TRPC6, found in smooth muscle, is stretch sensitive and activated by diacylglycerol. Activation by both of these stimuli is inhibited by GsMTx4 (Figure 13). This suggests that DAG is also probably acting as an amphipath and creating stress in the boundary layer.
Figure 13.
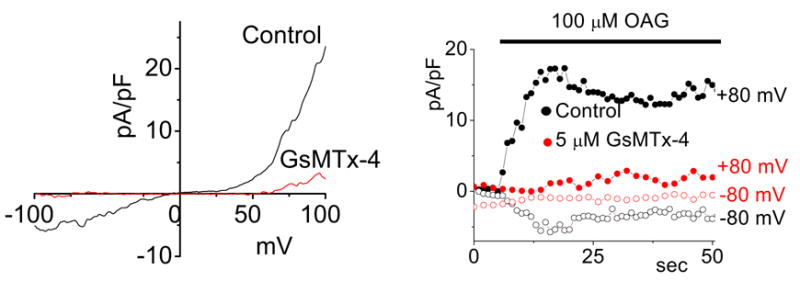
TRPC6 is a SAC and blocked by GsMTx4. CHO cells activated by the membrane permeable DAG derivative, OAG. Left panel, whole cell I/V properties showing inhibition by GsMTx4. Right panel, time course of activation by OAG (courtesy M. Spassova).
The spider venom gland
The spider venom gland
Spider venom contains hundreds of compounds, the targets of which are generally unknown. There are probably many compounds related to homeostasis of the gland, detritus from venom production, etc. There is no data to suggest that any more than a few compounds are toxic, despite the terminology. All data on GsMTx4 shows it to be non-toxic. Why might the spider make GsMTx-4? The venom is very viscous, with perhaps 50 mg/ml of protein. The tarantula, being a large and fast spider, has little difficulty in catching prey, so paralysis is not important. But it injects the prey with venom to dissolve the interior so it can be consumed. If one waited for an injection of viscous venom to diffuse throughout prey one cm in size, such as a cricket, it would take hours for the venom to diffuse. However if the heart kept beating, circulation would distribute the venom in minutes. This may be the physiological role of GsMTx4 in the venom.
How does the spider make venom? Little is known about the venom glands of spiders (Foil, 1979;Foil et al., 1979), and nothing about Grammostola spatulata in particular (Moon, M.J. 1992). Determining the structure of the gland is a first step. The gland of Grammostola follows the general architecture of spiders’ venom glands (Ridling and Phanuel, 1986) consisting of a tube of epithithelia wrapped in striated muscle, much like the heart, used for ejection of venom.
The venom gland of G. spatulata resembles that of Loxosceles intermedia based on electron and light microscopy. There are two layers of striated muscle fibers. The internal layer is in touch with an extracellular matrix, which is a basement membrane structure, and with a fibrillar collagen matrix separating the muscles from the epithelial cells(dos Santo et al., 2000) Figure 15 shows a light micrograph of the edge of the gland, and is similar to the description that was reported for L. reclusaby (Foil, 1979).
Figure 15.
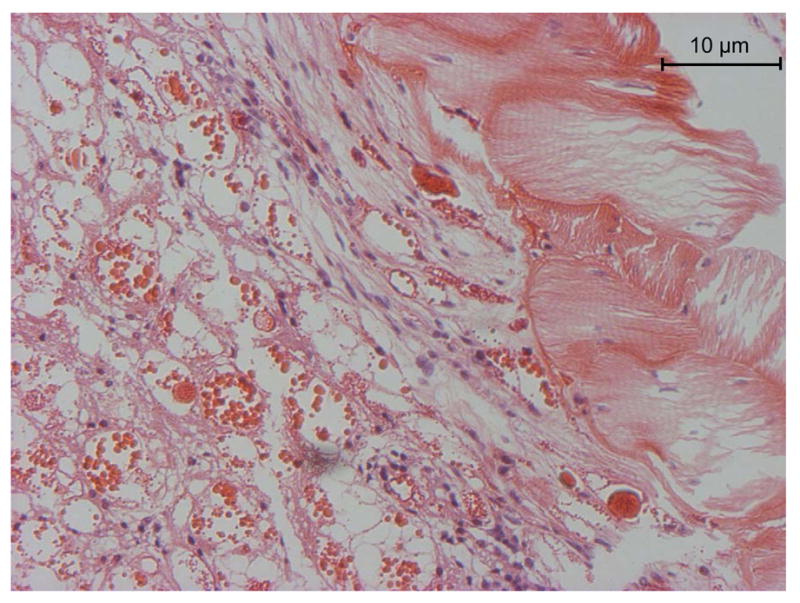
Hematoxylin-eosin stained section of flash frozen G. spatulata. The muscle fibers are sectioned diagonally at the right. The central duct is toward the left, but beyond the section. Beneath the muscle layer is connective tissue and then, with purple nuclei, the epithelia. The left part of the image contains the holocrine debris of the cells containing the venom. Large protein aggregates stain deep red.
Are there clones of cells that make each of the different peptides and other secreted organic molecules, or does every cell have the capacity to make everything? We cloned the DNA of a number of peptides from the venom, some of which are termed GsMTx-4, 2 and 1 (Ostrow et al., 2003), and thus had a number of peptide targets to explore. We did in situ hybridization of the RNA for several peptides and for housekeeping proteins. Figure 16 is an example of in situ hybridization as revealed by the expression of GsMTx4 (purple). The distribution of RNA within the cell is a gradient from the nucleus toward the membrane, presumably reflecting traffic through the rough ER.
Figure 16.
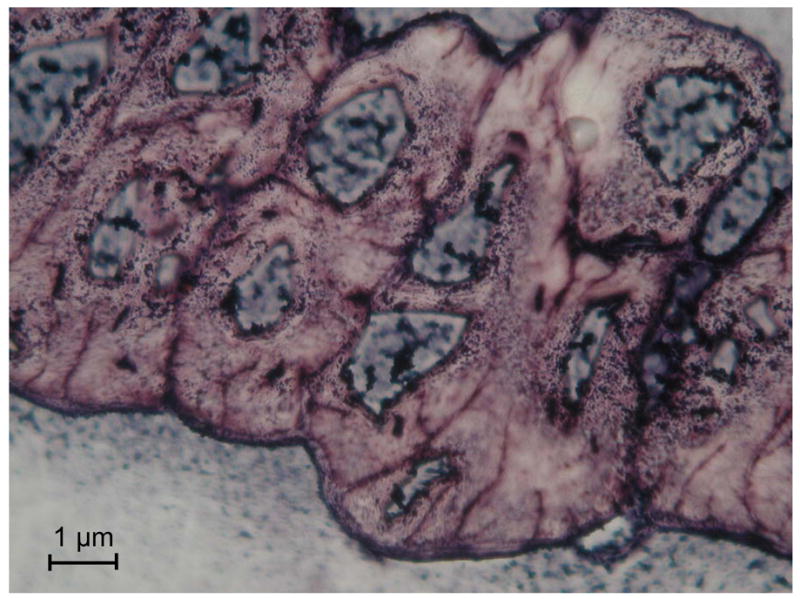
RT PCR in situ hybridization of RNA for GsMTx4 in the columnar epithelial layer. The lumen is toward the top and the muscle toward the bottom. The granular purple stain is the RNA coding for GsMTx4, the nuclei are bluish. All cells in the epithelia seem to express GsMTx4.
Potential Therapeutic Targets for GsMTx4
Cardiac Arrhythmias
Mechanical stress has a long history of increasing excitability in the heart (mechanoelectric feedback, MEF) (Kohl et al., 2003;Kohl and Sachs, 2002), and initiating arrhythmias (Sachs, 2004b;Baumgarten, 2004). The ventricles of an intact heart can even be be paced with inflation of an intraventricular balloon(Franz et al., 1992). The origin of this sensitivity is most likely via MSCs. MSCs have been observed in heart cells in many different laboratories (Bett and Sachs, 1997;Hu and Sachs, 1997;Niu and Sachs, 2003;Hoyer et al., 1997;Kohl et al., 1994;Tang et al., 2003). Furthermore, voltage sensitive ion channels found in the heart have also been shown to be mechanosensitive (Laitko and Morris, 2004;Morris, 2004;Lin et al., 2004;Gu et al., 2001;Tabarean et al., 1999). Bode et al (Bode et al., 2001b) showed that GsMTx4 at 170 nM can block atrial fibrillation induced by dilation (Figure 17). It provided complete protection to a diastolic pressure of 15mmH2O, and shortened the duration of fibrillation at all pressures.
Figure 17.
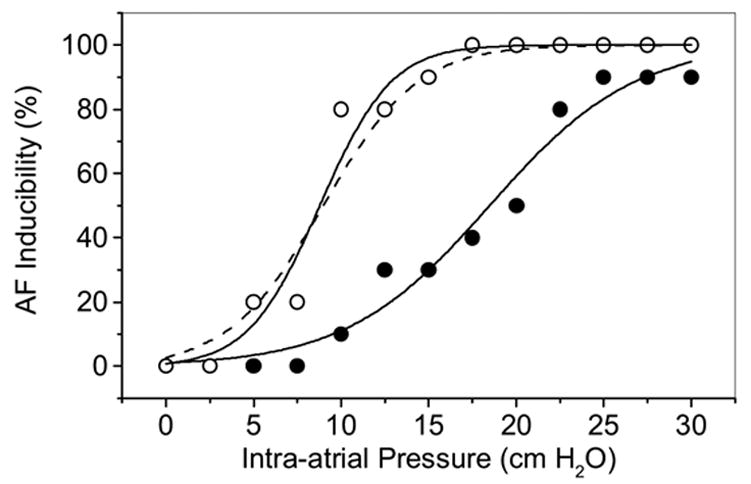
Block of dilation potentiated atrial fibrillation (AF) by 170nM GsMTx4 in the Langendorff perfused rabbit heart (Bode et al., 2001a). We have plotted how often burst stimulation resulted in AF lasting longer than 60s. Inflation increased the probability of AF (open symbols), but GsMTx4 shifted the dose-response curve to higher pressures (filled symbols).
Marban’s group described an arrhythmia where termination of high speed pacing produced spontaneous depolarizations (SDs) (Nuss et al., 1999). The SDs were not inhibited by any standard channel blockers, but by GsMTx4 (Sachs, 2004a). Perhaps high speed pacing overloads the SR resulting in spontaneous Ca2+ release and, causing MSC activation by local contraction and the resulting non-uniform stress.
Muscular Dystrophy
An intriguing connection between MSCs and pathology exists for the muscular dystrophies. Dystrophies arise from mutations in the dystroglycan complex (DGC), a protein complex that connects the actin cytoskeleton to the extracellular matrix (reviewed in (Blake and Martin-Rendon, 2002)). The DGC seems to provide structural support to the bilayer, in addition to providing various transmembrane functions. Mutations in the DGC lead to different forms of muscular dystrophy (Khurana and Davies, 2003). Duchenne Muscular Dystrophy (DMD), the most common of these disorders, is caused by mutations to the large cytoskeletal protein dystrophin. DMD muscle cells have elevated Na+ and Ca+2 levels, and activation of proteases by elevated Ca2+ may lead to the protein degradation associated with atrophy(Ruegg et al., 2002). A strategy for acute therapy is to improve Ca+2 homeostasis (Khurana and Davies, 2003).
Franco-Obregon and Lansman (Franco-Obregon and Lansman, 2002;Franco-Obregon, Jr. and Lansman, 1994) studied the MSCs in mouse mdx myoblasts (a mouse DMD model). SACs in the mdx cells become hyperactive with prolonged stimulation, as though a reinforcing structure was disrupted. While membrane stress can activate SACs, it also leads to channel inactivation (Suchyna and Sachs, 2004;Hamill et al., 1992;Niu and Sachs, 2003;Honore et al., 2006). If inactivation is disrupted, the channels will remain open contributing to excess Ca+2 uptake (Suchyna and Sachs, 2004), and inactivation requires cytoskeletal integrity (Suchyna and Sachs, 2004).
Yeung et al. (Yeung et al., 2003b) linked the pathology of mdx muscle fibers with the influx of cations through SACs. They showed that both Gd+3 and streptomycin, non-specific blockers of SACs, reduced elevated Na+ levels in stretched mdx fibers while having little effect on normal fibers. Allen‘s group also showed that GsMTx4 inhibits the Ca2+ elevation of stretched mdx muscle fibers(Yeung et al., 2005). As well as the muscle MSCs being permeant to Ca2+, Na+ influx can indirectly lead to Ca2+ elevation through the Na+/Ca2+ exchanger(Arnon et al., 2000;Bosteels et al., 1999). .
Astrocytes and Gliosis
Glia are the most abundant cells in the CNS. They secrete substances that act on themselves, on other types of glia, vascular endothelial cells, and neurons(Araque et al., 2001). SACs exit in fetal, neonatal and adult astrocytes(Sontheimer, 1994;Bowman et al., 1992) suggesting they may have a role in some brain pathologies including glial tumors (Ostrow and Sachs, 2005). Glial stretch not only activates SACs, but also releases Endothelin-1. Endothelin (ET) is a vasoactive peptide and potent autocrine mitogen produced by reactive and neoplastic astrocytes. The relationship between mechanical stretch and ET production combined with the observation that ET-1 positive reactive astrocytes are found in the mechanically deformed periphery of many CNS pathologies, led us to hypothesize that mechanical stress may regulate ET secretion in astrocytes through SACs. Ostrow and Sachs demonstrated that mechanical stimulation of adult rat astrocyte cultures caused a dramatic increase in ET-1 production and secretion (Ostrow et al., 2000b) (Figure 18).
Figure 18.
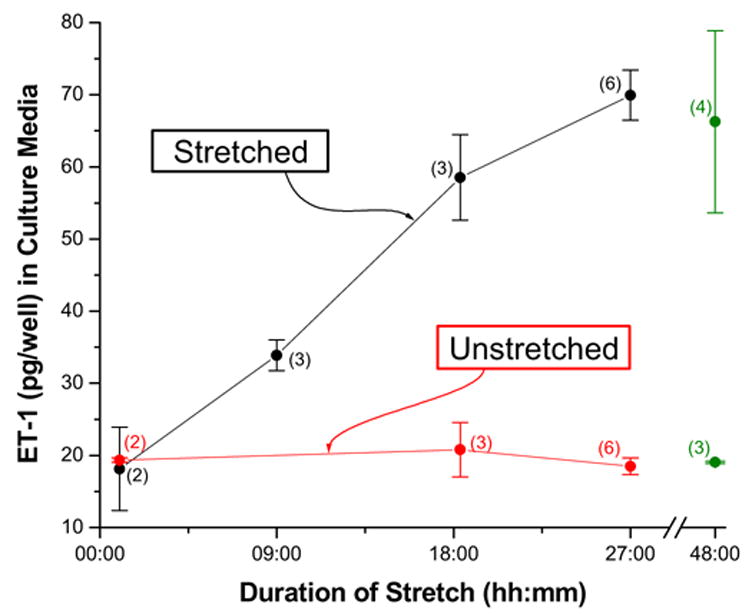
Cyclic stretch increases ET-1 secretion (Ostrow et al., 2000a).
Since brain pathology is usually associated with mechanical deformation, and ET induces the proliferation of astrocytes (and glioma cells), this may be a general pathway for activating/augmenting astroglial proliferation. Regardless of the stimulus, alterations in cell Ca2+ seems to be the common link in regulating the ET (Corder et al., 1993;Kitazumi and Tasaka, 1992;Yanagisawa et al., 1989;Brunner et al., 1994;Brunner et al., 1994;Brunner, 1995;Masaki et al., 1991;Morita et al., 1994). Vaz et al (Vaz et al., 1998) demonstrated that the edema following head trauma is reduced by the nonspecific SAC inhibitors Gd+3, amiloride and gentamicin, implying that SACs are involved in the swelling. GsMTx4 blocks SACs in astrocytes and also inhibits the stretch-induced ET-1 production by astrocytes, but it does not alter basal ET secretion. Glioma growth could be potentiated in a positive feedback cycle. Forces arising in the periphery of a lump growth could deform the surrounding tissue, activating SACs and increase secretion of ET-1. This, in turn, would stimulate further division, vascular invasion and growth.
Neurite gowth
Recently, GsMTx4 was shown to stimulate neurite growth (Jacques-Fricke et al., 2006). Gomez and colleagues used specific and nonspecific reagents including GsMTx4, to assay neurite extension. GsMTx4 was the most potent agent for stimulating neurite growth via a reduction of local Ca+2 influx (Neher, 1998) through channels that could also be activated by cell swelling. The potential for GsMTx4 as a therapy for spinal cord damage remains to be explored.
Anti-Microbial Activity
Recently, GsMTx4 was reported to affect the growth of Gram positive and Gram negative bacteria at mid-logarithmic-phase and promotes leakage of indicator dyes (Jung et al., 2006). GsMTx4 was more efficacious against Gram positive (0.5 to 8 μM) than Gram negative bacteria (8 to 64 μM). By contrast, other gating modifier peptide (VSTx1, SGTx, and GrTx) were ineffective at more than 32 μM.
We have also observed effects on bacterial growth when we expressed GsMTx4 as a fusion protein (Gottlieb, Besch, and Sachs, unpublished results). A plasmid containing the GsMTx4 gene linked to thioredoxin produced an initial growth phase followed by bacterial cell death, even without induction. The plasmid containing thioredoxin alone did not produce this effect and tighter control of gene expression of the fusion protein also prevented cell death, indicating that the presence of GsMTx4 was responsible for this phenomenon.
Conclusion
Mechanosensitive ion channels are widely distributed in non-specialized tissues and thus will be related to a wide variety of physiology and pathology. Because of the involvement of the cell cortex in SAC function, the physiological role can be altered by changes it the cytoskeleton as well as the channels themselves. The physiological role of the channels is just beginning to be explored with the availability of GsMTx4 as a specific reagent, and we look forward to new compounds to explore other MSCs.
Figure 14.
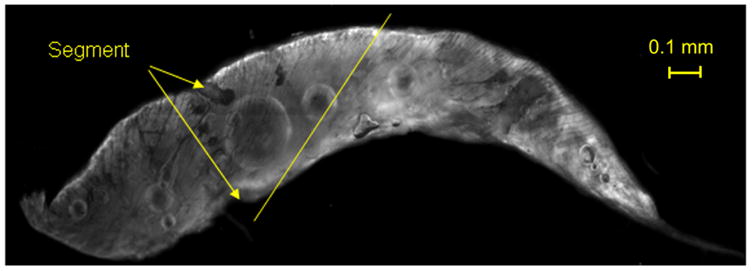
Venom gland of Grammostola spatulata. The duct to the fang is at the right. The striated muscle fibers are helically wrapped around the gland, and the angle at one position is indicated by the line. The gland is segmented into two compartments (marked segment), with contraction initiating at the left. The image is 2mm wide.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinlaja J, Sachs F. The breakdown of cell membranes by electrical and mechanical stress. Biophysical J. 1998;75:247–254. doi: 10.1016/S0006-3495(98)77511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OS, Nielsen C, Maer AM, Lundbæk JA, Goulian M, Koeppe RE. Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208–224. doi: 10.1016/s0076-6879(99)94013-2. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. American Journal Of Physiology-Cell Physiology. 2000;278:C163–C173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- Bass RB, Locher KP, Borths E, Poon Y, Strop P, Lee A, Rees DC. The structures of BtuCD and MscS and their implications for transporter and channel function. FEBS Lett. 2003;555:111–115. doi: 10.1016/s0014-5793(03)01126-8. [DOI] [PubMed] [Google Scholar]
- Baumgarten CM. Cell-volume activated ion channels in cardiac cells. In: Kohl P, Franz MR, Sachs F, editors. Cardiac Mechano-Electric Feedback and Arrhythmias: From Pipette to Patient. Elsevier; 2004. [Google Scholar]
- Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP (vol 559, pg 685, 2004) Journal Of Physiology-London. 2004b;560:949. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP (vol 559, pg 685, 2004) Journal Of Physiology-London. 2004a;560:949. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophysical J. 1984;45:279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Archiv-European Journal of Physiology. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- Bett GCL, Sachs F. Cardiac mechanosensitivity and stretch activated ion channels. Trends in Cardiovascular Medicine. 1997;7:4–9. doi: 10.1016/S1050-1738(96)00119-3. [DOI] [PubMed] [Google Scholar]
- Bett GCL, Sachs F. Whole-cell mechanosensitive currents in rat ventricular myocytes activated by direct stimulation. J Membr Biol. 2000;173:255–263. doi: 10.1007/s002320001024. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Martin-Rendon E. Intermediate filaments and the function of the dystrophin-protein complex. Trends Cardiovasc Med. 2002;12:224–228. doi: 10.1016/s1050-1738(02)00166-4. [DOI] [PubMed] [Google Scholar]
- Blount P, Sukharev SI, Moe PC, Martinac B, Kung C. Mechanosensitive channels of bacteria. Methods Enzymol. 1999;294:458–482. doi: 10.1016/s0076-6879(99)94027-2. [DOI] [PubMed] [Google Scholar]
- Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 2001a;409:35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- Bode F, Sachs F, Franz MR. Tarantula Peptide Inhibits Atrial Fibrillation During Stretch. Nature. 2001b;409:35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- Bosteels S, Matejovic P, Flameng W, Mubagwa K. Sodium influx via a non-selective pathway activated by the removal of extracellular divalent cations: possible role in the calcium paradox. Cardiovasc Res. 1999;43:417–425. doi: 10.1016/s0008-6363(99)00098-x. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Ding JP, Sachs F, Sokabe M. Mechanotransducing ion channels in astrocytes. Brain Res. 1992;584:272–286. doi: 10.1016/0006-8993(92)90906-p. [DOI] [PubMed] [Google Scholar]
- Brehm P, Kullberg R, Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus Laevis. J Physiol (London) 1984;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner F. Dependence of endothelin-1 secretion on Ca2+ Biochem Pharmacol. 1995;49:1785–1791. doi: 10.1016/0006-2952(95)00023-s. [DOI] [PubMed] [Google Scholar]
- Brunner F, Stessel H, Simecek S, Graier W, Kukovetz WR. Effect of intracellular Ca2+ concentration on endothelin-1 secretion. FEBS Lett. 1994;350:33–36. doi: 10.1016/0014-5793(94)00727-6. [DOI] [PubMed] [Google Scholar]
- Calabrese B, I, Tabarean V, Juranka P, Morris CE. Mechanosensitivity of N-type calcium channel currents. Biophys J. 2002;83:2560–2574. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Anishkin A, Sukharev S. Gating of the large mechanosensitive channel in situ: Estimation of the spatial scale of the transition from channel population responses. Biophysical J. 2004;86:2846–2861. doi: 10.1016/S0006-3495(04)74337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987;330:66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003b;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003a;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cole KS. A Flexure Balance. Review of Scientific Instruments. 1932a;3:121–122. Ref Type: Generic. [Google Scholar]
- Cole KS. Surface Forces of the Arbacia Egg. Journal of Cellular and Comparative Physiology. 1932b;1:1–9. Ref Type: Generic. [Google Scholar]
- Cole KS. Dynamic Electrical Characteristics of the Squid Axon Membrane. Archives des sciences physiologiques. 1949;3:253–258. [Google Scholar]
- Corder R, Khan N, Anggard EE, Vane JR. Calcium ionophores inhibit the release of endothelin-1 from endothelial cells. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S42–S45. doi: 10.1097/00005344-199322008-00013. [DOI] [PubMed] [Google Scholar]
- Corey D. Sensory transduction in the ear. J Cell Sci. 2003a;116:1–3. doi: 10.1242/jcs.00101. [DOI] [PubMed] [Google Scholar]
- Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003b;39:585–588. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- Crick F, Hughes A. THE PHYSICAL PROPERTIES OF CYTOPLASM A STUDY BY MEANS OF THE MAGNETIC PARTICLE METHOD. Exp Cell Res. 1950;1:37–80. [Google Scholar]
- Dai J, Sheetz MP. Membrane Tether Formation from Blebbing Cells. Biophysical J. 1999b;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sheetz MP. Membrane Tether Formation from Blebbing Cells. Biophysical J. 1999a;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JW, Sheetz MP, Wan XD, Morris CE. Membrane tension in swelling and shrinking molluscan neurons. Journal of Neuroscience. 1998;18:6681–6692. doi: 10.1523/JNEUROSCI.18-17-06681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B. Transformation and Restoration of Biconcave Shape of human Erythrocytes Induced by Amphiphilic Agents and Changes of Ionic Environment. Biochim Biophys Acta. 1968;163:494–500. doi: 10.1016/0005-2736(68)90078-3. [DOI] [PubMed] [Google Scholar]
- dos Santo V, Franco CR, Viggiano RL, da Silveira RB, Cantao MP, Mangili OC, Veiga SS, Gremski W. Structural and ultrastructural description of the venom gland of Loxosceles intermedia (brown spider) Toxicon. 2000;38:265–285. doi: 10.1016/s0041-0101(99)00155-5. [DOI] [PubMed] [Google Scholar]
- Dzikovski BG, Borbat PP, Freed JH. Spin-labeled gramicidin A: Channel formation and dissociation. Biophysical J. 2004;87:3504–3517. doi: 10.1529/biophysj.104.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder F, Arhem P. Effects of gadolinium on ion channels in the myelinated axon of Xenopus laevis: four sites of action. Biophysical J. 1994;67:71–83. doi: 10.1016/S0006-3495(94)80456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsig S, Pedersen B, Owsianik G, Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Foil LD. Ultrastructure of the venom gland of the brown recluse spider solider. International journal of insect morphology & embryology. International journal of insect morphology & embryology. 1979;8:325–334. [Google Scholar]
- Foil LD, Frazier JL, Norment BR. Partial characterization of lethal and neuroactive components of the brown recluse spider (Loxosceles reclusa) venom. Toxicon. 1979;17:347–354. doi: 10.1016/0041-0101(79)90262-9. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Jr, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol. 1994;481:299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregon A, Lansman JB. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J Physiol. 2002;539:391–407. doi: 10.1113/jphysiol.2001.013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias [published erratum appears in Cir. 86:1663 (1992)] Circulation. 1992;86:968–978. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Corey DP. Mechanosensation: touch at the molecular level. Curr Biol. 1996;6:541–543. doi: 10.1016/s0960-9822(02)00537-7. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- Gomez S, Dibello C, Hung LT, Genet R, Morgat JL, Fromageot P, Cohen P. C-Terminal Amidation of Neuropeptides - Gly-Lys-Arg Extension An Efficient Precursor of C-Terminal Amide. FEBS Lett. 1984;167:160–164. doi: 10.1016/0014-5793(84)80853-4. [DOI] [PubMed] [Google Scholar]
- Goulian M, Feygenson D, Mesquita ON, Moses E, Nielsen C, Andersen OS, Libchaber A. The effect of membrane tension on gramicidin A channel kinetics. Biophysical J. 1997;72:TU309. doi: 10.1016/S0006-3495(98)77790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M, Mesquita ON, Fygenson DK, Nielsen C, Andersen OS, Libchaber A. Gramicidin channel kinetics under tension. Biophysical J. 1998;74:328–337. doi: 10.1016/S0006-3495(98)77790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu CX, Juranka PF, Morris CE. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage- gated K+ channel. Biophys J. 2001;80:2678–2693. doi: 10.1016/S0006-3495(01)76237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol (London) 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Lane JW, McBride DW., Jr Amiloride: a molecular probe for mechanosensitive channels. TiPS. 1992;13:373–376. doi: 10.1016/0165-6147(92)90115-m. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv - European Journal of Physiology. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr Rapid adaptation of single mechanosensitive channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1992;89:7462–7466. doi: 10.1073/pnas.89.16.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr . Pressure/patch-clamp methods. In: Boulton A, Baker G, Walz W, editors. Neuromethods. Humana Press Inc; 1995. pp. 75–87. [Google Scholar]
- Harvey EN. The Tension at the Surface of marine Eggs, Especially Those of the Sea Urchin, Arbacia. Biological Bulletin. 1931;61:273–279. Ref Type: Generic. [Google Scholar]
- Honore E, Suchyna T, Patel AA, Sachs F. Desensitization of cloned 2P domain K channels. PNAS. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J, Kohler R, Distler A. Mechanosensitive cation channels in aortic endothelium of normotensive and hypertensive rats. Hypertension. 1997;30:112–119. doi: 10.1161/01.hyp.30.1.112. [DOI] [PubMed] [Google Scholar]
- Hu H, Sachs F. Stretch-activated ion channels in the heart. Cell and Molecular Cardiology. 1997;29:1511–1523. doi: 10.1006/jmcc.1997.0392. [DOI] [PubMed] [Google Scholar]
- Hwang TC, Koeppe RE, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb P, Sachs F, Gomez TM. Ca2+ influx through a mechanosensitive channel and release from intracellular stores have opposite effects on neurite outgrowth. 2006. Ref Type: Unpublished Work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003a;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 2003b;423:42–48. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Kim PI, Lee SK, Lee CW, Eu YJ, Lee DG, Earm YE, Kim JI. Lipid membrane interaction and antimicrobial activity of GsMTx-4, an inhibitor of mechanosensitive channel. Biochem Biophys Res Commun. 2006;340:633–638. doi: 10.1016/j.bbrc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- Kitazumi K, Tasaka K. Thrombin-Stimulated Phosphorylation of Myosin Light Chain and Its Possible Involvement in Endothelin-1 Secretion from Porcine Aortic Endothelial-Cells. Biochem Pharmcol. 1992;43:1701–1709. doi: 10.1016/0006-2952(92)90699-j. [DOI] [PubMed] [Google Scholar]
- Kohl P, Cooper PJ, Holloway H. Effects of acute ventricular volume manipulation on in situ cardiomyocyte cell membrane configuration. PROGRESS IN BIOPHYSICS & MOLECULAR BIOLOGY. 2003;82:221–227. doi: 10.1016/s0079-6107(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart - interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–956. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- Kohl P, Sachs F. Mechanoelectric feedback in cardiac cells. Proc R Soc Lond Math Phys Eng Sci. 2002;359:1173–1185. [Google Scholar]
- Ladokhin AS, Jayasinghe S, White SH. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- Laitko U, Morris CE. Membrane tension accelerates rate-limiting voltage-dependent activation and slow inactivation steps in a shaker channel. J Gen Physiol. 2004;123:135–154. doi: 10.1085/jgp.200308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Chemin J, Honore E, Jodar M, Guy N, Lazdunski M, Patel AJ. Cross-talk between the mechanogated K-2P channel TREK-1 and the actin cytoskeleton. Embo Reports. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Kim S, Roh SH, Endoh H, Kodera Y, Maeda T, Kohno T, Wang JM, Swartz KJ, Kim JI. Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry. 2004;43:890–897. doi: 10.1021/bi0353373. [DOI] [PubMed] [Google Scholar]
- Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Holt JR, Vollrath MA, Garcia-Anoveros J, Geleoc G, Kwan K, Hoffman MP, Zhang DS, Corey DP. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Biophysical J. 2005;88:287A–288A. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Lin W, Laitko U, Juranka PF, Morris CE. Is the pacemaker channel, HCN, like other voltage-gated channels, sensitive to membrane stretch? Biophysical J. 2004;86:292A. [Google Scholar]
- Lundb‘k JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol. 1994;104:645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Sachs F, Ashmore JF, Kros CJ. Effect of a peptide tarantula toxin on mechano-transduction in neonatal mouse cochlear hair cells. International Journal of Audiology. 2001;41:231. [Google Scholar]
- Markin V, Sachs F. Thermodynamics of mechanosensitivity. Curr Top Memb Trans. 2006 doi: 10.1088/1478-3967/1/2/007. [DOI] [PubMed] [Google Scholar]
- Markin V, Sukharev S. A physical model of the bacterial large-conductance mechanosensitive channel, MscL. Biophysical J. 2000;78:473A. [Google Scholar]
- Markin VS, Sachs F. Thermodynamics of mechanosensitivity. Physical Biology. 2004a;1:110–124. doi: 10.1088/1478-3967/1/2/007. [DOI] [PubMed] [Google Scholar]
- Markin VS, Sachs F. Thermodynamics of mechanosensitivity: lipid shape, membrane deformation and anesthesia. Biophysical J. 2004b;86:370A. [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nature Cell Biology. 2005 doi: 10.1038/ncb1218. Jan 23 2005 [epub] [DOI] [PubMed] [Google Scholar]
- Martinac B. Mechanosensitive channels in prokaryotes. Cell Physiol Biochem. 2001;11:61–76. doi: 10.1159/000047793. [DOI] [PubMed] [Google Scholar]
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Martinac B, Kloda A. Evolutionary origins of mechanosensitive ion channels. Prog Biophys Mol Biol. 2003;82:11–24. doi: 10.1016/s0079-6107(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. [Review] Circulation. 1991;84:1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- McLaggan D, Jones MA, Gouesbet G, Levina N, Lindey S, Epstein W, Booth IR. Analysis of the kefA2 mutation suggests that KefA is a cation-specific channel involved in osmotic adaptation in Escherichia coli. Mol Microbiol. 2002;43:521–536. doi: 10.1046/j.1365-2958.2002.02764.x. [DOI] [PubMed] [Google Scholar]
- Minke B. Drosophila Mutant with A Transducer Defect. Biophysics of Structure and Mechanism. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- Mitchison JM, Swann MM. The Mechanical Properties of the Cell Surface. I. The Cell Elastomer. J Exp Biol. 1954a;31:443–461. [Google Scholar]
- Mitchison JM, Swann MM. The Mechanical Properties of the Cell Surface. II. The Unfertilized egg of the sea - urchin. J Exp Biol. 1954b;31:461–472. [Google Scholar]
- Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila Phototransduction Mutation Trp by Germline Transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- Morita T, Kurihara H, Maemura K, Yoshizumi M, Nagai R, Yazaki Y. Role of Ca2+ and protein kinase C in shear stress-induced actin depolymerization and endothelin 1 gene expression. Circ Res. 1994;75:630–636. doi: 10.1161/01.res.75.4.630. [DOI] [PubMed] [Google Scholar]
- Morris CE. Stretch effects on voltage-gated channels in cardiac cells. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Feedback and Arrhythmias: From Pipette to Patient. Elsevier; 2004. [Google Scholar]
- Morris CE, Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991;251:1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- Mukhin SI, Baoukina SV. Inter-layer slide and stress relaxation in a bilayer lipid membrane in the patch-clamp setting. Biologicheskie Membrany. 2004;21:506–517. [Google Scholar]
- Murray D, Arbuzova A, Hangyas-Mihalyne G, Gambhir A, Ben Tal N, Honig B, McLaughlin S. Electrostatic properties of membranes containing acidic lipids and adsorbed basic peptides: theory and experiment. Biophys J. 1999;77:3176–3188. doi: 10.1016/S0006-3495(99)77148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 1998;24:345–357. doi: 10.1016/s0143-4160(98)90058-6. [DOI] [PubMed] [Google Scholar]
- Niu WZ, Sachs F. Dynamic properties of stretch-activated K+ channels in adult rat atrial myocytes. PROGRESS IN BIOPHYSICS & MOLECULAR BIOLOGY. 2003;82:121–135. doi: 10.1016/s0079-6107(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Nuss HB, Kaab S, Kass DA, Tomaselli GF, Marban E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. American Journal of Physiology-Heart and Circulatory Physiology. 1999;277:H80–H91. doi: 10.1152/ajpheart.1999.277.1.H80. [DOI] [PubMed] [Google Scholar]
- Ostrow KL, Mammoser A, Suchyna T, Sachs F, Oswald RE, Kubo S, Chino N, Gottlieb P. cDNA sequence and in vitro folding of GsMTx4, a specific peptide inhibitor of mechanosensitive channels. Toxicon. 2003;42:263–274. doi: 10.1016/s0041-0101(03)00141-7. [DOI] [PubMed] [Google Scholar]
- Ostrow L, Langan T, Sachs F. Stretch-induced Endothelin-1 Production by Astrocytes. The Journal of Cardiovascular Pharmacology. 2000a;36:S274–S277. doi: 10.1097/00005344-200036051-00081. [DOI] [PubMed] [Google Scholar]
- Ostrow L, Sachs F. Mechanosensation and Endothelin in Astrocytes - potential roles in reactive gliosis. Brain Research Reviews. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Ostrow LW, Langan TJ, Sachs F. Stretch-induced endothelin-1 production by astrocytes. J Cardiovasc Pharmacol. 2000b;36:S274–S277. doi: 10.1097/00005344-200036051-00081. [DOI] [PubMed] [Google Scholar]
- Oswald RE, Suchyna TM, McFeeters R, Gottlieb P, Sachs F. Solution structure of peptide toxins that block mechanosensitive ion channels. J Biol Chem. 2002;277:34443–34450. doi: 10.1074/jbc.M202715200. [DOI] [PubMed] [Google Scholar]
- Pallaghy PK, Nielsen KJ, Craik DJ, Norton RS. A common structural motif incorporating a cystine knot and a triple-stranded beta-sheet in toxic and inhibitory polypeptides. Protein Sci. 1994;3:1833–1839. doi: 10.1002/pro.5560031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honore E. Lipid and mechanogated 2P domain K(+) channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Structural Biology. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Posokhov YG, Gottlieb P, Morales MJ, Sachs F, Ladokhin AS. Quantitative Characterization of Membrane Interactions of the Cysteine Knot (CK) Family of Ion-Channel Blockers. Biophysical J. 2006:Pos145. doi: 10.1529/biophysj.107.112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powl AM, East JM, Lee AG. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry. 2003;42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- Powl AM, East JM, Lee AG. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry. 2005;44:5873–5883. doi: 10.1021/bi047439e. [DOI] [PubMed] [Google Scholar]
- Ridling MW, Phanuel GJ. Functional morphology of the poison apparatus and histology of the venom glands of three indian spiders. J Bombay Nat Hist Soc. 1986;86:334–354. [Google Scholar]
- Ruegg UT, Nicolas-Metral V, Challet C, Bernard-Helary K, Dorchies OM, Wagner S, Buetler TM. Pharmacological control of cellular calcium handling in dystrophic skeletal muscle. Neuromuscul Disord. 2002;12(Suppl 1):S155–S161. doi: 10.1016/s0960-8966(02)00095-0. [DOI] [PubMed] [Google Scholar]
- Sachs F. Retaining your identity under stress. Nat Struct Biol. 2002a;9:636–637. doi: 10.1038/nsb0902-636. [DOI] [PubMed] [Google Scholar]
- Sachs F. Retaining your identity under stress. Nature Structural Biology. 2002b;9:636–637. doi: 10.1038/nsb0902-636. [DOI] [PubMed] [Google Scholar]
- Sachs F. Heart Mechanoelectric Transduction. In: Jalife J, Zipes D, editors. Cardiac Electrophysiology; From Cell to Bedside. Saunders (Elsevier); Philadelphia: 2004a. pp. 96–102. [Google Scholar]
- Sachs F. Stretch activated channels in the heart. In: Kohl P, Franz MR, Sachs F, editors. Cardiac Mechano-Electric Feedback and Arrhythmias: From Pipette to Patient. Saunders (Elsevier); Philadelphia: 2004b. [Google Scholar]
- Sachs F, Morris CE. Mechanosensitive ion channels in non specialized cells. In: Blaustein MP, Greger R, Grunicke H, Jahn R, Mendell LM, Miyajima A, Pette D, Schultz G, Schweiger M, editors. Reviews of Physiology and Biochemistry and Pharmacology. Springer; Berlin: 1998a. pp. 1–78. [DOI] [PubMed] [Google Scholar]
- Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. [Review] [231 refs] Reviews of Physiology Biochemistry & Pharmacology. 1998b;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- Sheetz MP, Painter RG, Singer SJ. Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J Cell Biol. 1976;70:193–203. doi: 10.1083/jcb.70.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug- erythrocyte interactions. Proc Natl Acad Sci U S A. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M, Sachs F, Jing Z. Quantitative video microscopy of patch clamped membranes - stress, strain, capacitance and stretch channel activation. Biophysical J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Voltage-dependent ion channels in glial cells. [Review] GLIA. 1994;11:156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- Suchyna T, Sachs F. Dynamic Regulation of Mechanosensitive Channels; Capacitance Used to Monitor Patch Tension in Real Time. Physical Biology. 2004;1:1–18. doi: 10.1088/1478-3967/1/1/001. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000d;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000c;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000a;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000b;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Anishkin A. Mechanosensitive channels: what can we learn from ’simple’ model systems? Trends Neurosci. 2004;27:345–351. doi: 10.1016/j.tins.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Sigurdson W, Kung C, Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J Gen Physiol. 1999;113:525–539. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Juranka P, Morris CE. Membrane stretch affects gating modes of a skeletal muscle sodium channel. Biophys J. 1999;77:758–774. doi: 10.1016/S0006-3495(99)76930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QY, Qi Z, Naruse K, Sokabe M. Characterization of a functionally expressed stretch-activated BKca channel cloned from chick ventricular myocytes. J Membr Biol. 2003;196:185–200. doi: 10.1007/s00232-003-0637-8. [DOI] [PubMed] [Google Scholar]
- Vandorpe DH, Small DL, Dabrowski AR, Morris CE. FMRFamide and membrane stretch as activators of the Aplysia S- channel. Biophysical J. 1994;66:46–58. doi: 10.1016/S0006-3495(94)80749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz R, Sarmento A, Borges N, Cruz C, Azevedo I. Effect of mechanogated membrane ion channel blockers on experimental traumatic brain oedema. Acta Neurochirurgica. 1998;140:371–375. doi: 10.1007/s007010050111. [DOI] [PubMed] [Google Scholar]
- Vles F. Les tensions de surface et led deformations de l’oeuf d’Oursin. Arch d Physique Biol T. 1926;4:263. [Google Scholar]
- Walker RG. A Drosophila Mechanosensory Transduction Channel. [Article] Science The Drosophila Genome. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang JM, Roh SH, Kim S, Lee CW, Kim JI, Swartz KJ. Molecular surface of tarantula toxins interacting with voltage sensors in kv channels. J Gen Physiol. 2004;123:455–467. doi: 10.1085/jgp.200309005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SH, Wimley WC, Ladokhin AS, Hristova K. Protein folding in membranes: determining energetics of peptide-bilayer interactions. Methods Enzymol. 1998;295:62–87. doi: 10.1016/s0076-6879(98)95035-2. [DOI] [PubMed] [Google Scholar]
- Wiggins P, Phillips R. Analytic models for mechanotransduction: Gating a mechanosensitive channel. PNAS. 2004;101:4071–4076. doi: 10.1073/pnas.0307804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalter M, Helfrich W. Effect of Surface Charge on the Curvature Elasticity of Membranes. J Phys Chem. 1988;92:6865–6867. [Google Scholar]
- Yanagisawa M, Inoue A, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene: possible regulation by endothelial phosphoinositide turnover signaling. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S13–S17. [PubMed] [Google Scholar]
- Yeung EW, Head SI, Allen DG. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibers. J Physiol (Lond) 2003a;552.2:449–458. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EW, Head SI, Allen DG. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibres. J Physiol. 2003b;552:449–458. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. Journal Of Physiology-London. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao F, Popov VL, Wen JW, Hamill OP. Mechanically gated channel activity in cytoskeleton-deficient plasma membrane blebs and vesicles from Xenopus oocytes. J Physiol (Lond) 2000;523(Pt 1):117–130. doi: 10.1111/j.1469-7793.2000.t01-1-00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


