Abstract
Our goal in the current report was to design a new fMRI task to probe the role of the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) in processing of salient symptom-related cues during the simultaneous performance of an unrelated task in drug addicted individuals. We used a novel functional magnetic resonance imaging color-word drug Stroop task in 14 individuals with cocaine use disorders; subjects had to press for color of drug vs. matched neutral words. Although there were no accuracy or speed differences between the drug and neutral conditions in the current sample of subjects, drug words were more negatively valenced than the matched neutral words. Further, consistent with prior reports in individuals with other psychopathologies using different Stroop fMRI paradigms, our more classical color-word Stroop design revealed bilateral activations in the caudal-dorsal ACC (cdACC) and hypoactivations in the rostro-ventral ACC/medial OFC (rACC/mOFC). A trend for larger rACC/mOFC hypoactivations to the drug than neutral words did not survive whole-brain corrections. Nevertheless, correlation analyses indicated that (1) the more the cdACC drug-related activation, the more negative the valence attributed to the drug words (r=-0.86, p < 0.0001) but not neutral words; and (2) the more the rACC/mOFC hypoactivation to drug minus neutral words, the more the errors committed specifically to the drug minus neutral words (r=0.85, p < 0.0001). Taken together, results suggest that this newly developed drug Stroop fMRI task may be a sensitive biobehavioral assay of the functions recruited for the regulation of responses to salient symptom-related stimuli in drug addicted individuals.
Keywords: emotional Stroop, interference, inhibitory control, cognitive conflict, attention bias, cocaine addiction, drug addiction, fMRI BOLD, neuropsychology
INTRODUCTION
A dysregulated function of the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) has been previously suggested to mediate core characteristics in drug addiction; these encompass Impairments in Response Inhibition and Salience Attribution (I-RISA) where dyscontrol in a drug context and salience attributed to drug cues are hypothesized to be more pronounced than for non-drug-related stimuli (Goldstein and Volkow, 2002). However, to date, neuroimaging evidence for the role of the OFC and ACC in I-RISA in drug addiction has relied mostly on separate studies of salience attribution/reward processing (e.g., with a decision-making task (Bechara et al., 2002) or a cocaine-related film (Garavan et al., 2000)) vs. inhibitory control (e.g., with neutral go/no-go tasks (Kaufman et al., 2003)). Thus, in drug addicted individuals a unified functional magnetic resonance imaging (fMRI) task is yet to be developed to probe the role of the OFC and ACC in processing of salient symptom-related cues during the simultaneous performance of an unrelated and possibly conflicting task. We currently report results of such a single well-validated paradigm (Whalen et al., 1998; Bush et al., 2000), newly modified (using color-word and not counting responses) for the in-depth analysis of cue-related brain-behavioral interrelationships within drug addicted individuals.
Thus, although closely aligned with the extensive literature in cocaine addicted individuals on neural processes underlying both cocaine-related (i.e., emotional) cue responses (Grant et al., 1996; Childress et al., 1999; Garavan et al., 2000; Kilts et al., 2001; Wexler et al., 2001; Bonson et al., 2002) and inhibitory control (Kaufman et al., 2003; Bolla et al., 2004; Hester and Garavan, 2004), the novelty of the current task is in the combination of both emotional (i.e., non-neutral) and inhibitory control processes within a single task. This dual-task aspect is necessary for testing how the brain reacts to drug-related cues that are irrelevant to the task, but relevant to the subjects' symptomatology (i.e., drug addiction; for a similar approach in other psychopathological disorders see for example (Shin et al., 2001)). To the authors' best knowledge, this is the first time that a color-word emotional Stroop task is used in the fMRI environment to study context-specific processing (drug vs. matched neutral words) in drug addicted individuals.
EXPERIMENTAL PROCEDURES
There were 14 subjects with cocaine use disorders (5 female; 9 African-American, 3 Caucasian, and 2 Hispanic), 43.9±5.6 years old with 12.9±2.2 years of education (Mean±SD), all healthy as determined by physical, neurological and psychiatric evaluations. All subjects were right-handed, had English as first language, and were not color-blind. General intellectual functioning was average (Matrix Reasoning of the Wechsler Abbreviated Scale of Intelligence scaled score=11±4, percentile=57.1±30.0) and reading level was within the lower average range (Wide Range Achievement Test standard score=85±23, percentile=28.9±26.4). All subjects had a DSM-IV diagnosis for Substance Dependence (cocaine, current N=8; partial or full remission N=5) or Abuse (N=1) as determined with the Structured Clinical Interview for DSM-IV, research version - Patient Edition. Length of last voluntary abstinence from cocaine ranged from 1 to 96 days (median=12 days). All experiments on human subjects were conducted in accordance with the Declaration of Helsinki http://www.wma.net and all procedures were carried out with the adequate understanding and written consent of the subjects. Formal approval to conduct the experiments described has been obtained from the local human subjects review board.
The drug Stroop fMRI task used 40 drug words matched on length, frequency in the English language (Francis and Kučera, 1982), and part of speech (noun, adjective, adverb, verb) with 40 household-related items. Similarly to the counting Stroop task (Whalen et al., 1998), words were presented via MRI-compatible goggles in a blocked on-off or off-on order, pseudorandomized across all subjects (Supplementary Material, Figure 1sB). After a long preparation period (2 sec, Figure 1sD), subjects had to press one of 4 response buttons (yellow, blue, red, green), matching the color of the word they had just read; word color order was pseudorandomized across all sequences. Reaction time and accuracy data were collected across all trials. After completion of the MRI session, all subjects rated all words (Table 1s) on valence (“how negative or positive” a word is, from extremely negative to extremely positive, -5 to +5). Just before the fMRI task, training was conducted outside and inside the scanner, both repeated depending on performance (if <70% accuracy, range 2-5 training sequences, 3.4±1.1). For training we used the same paradigm but with the letter A; words were not used so as not to create subsequent habituation effects (see Figure 1sA for task sequence).
MRI scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. The BOLD responses were measured as a function of time using a T2*-weighted single-shot gradient-echo EPI sequence (TE/TR=20/1600 ms, 4 mm slice thickness, 1 mm gap, typically 33 coronal slices, 20 cm FOV, 64x64 matrix size, 90°-flip angle, 200kHz bandwidth with ramp sampling, 128 time points, 4 dummy scans). Padding was used to minimize subject motion, which was also monitored on-line (Caparelli et al., 2003); accepted threshold was 1.5 mm displacement and 1.5° rotation. A T1-weighted 3D-MDEFT sequence was used for structural imaging performed after fMRI; all MRI images were inspected to rule out gross morphological brain abnormalities.
All time series were converted into statistical parametric mapping (SPM) format and the SPM2 package (Wellcome Department of Cognitive Neurology, London UK) was used for subsequent analyses. A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment. The realigned datasets were normalized to the Talairach frame with a 12-parameters affine transformation (Ashburner et al., 1997), using a voxel size of 3×3×3 mm3. An 8-mm full-width-half-maximum Gaussian kernel was used to smooth the data. A general linear model (Friston et al., 1995) and a box-car design convolved with a canonical hemodynamic response function were used to calculate the activation maps. The time series were band pass filtered with the hemodynamic response function as low pass filter and 1/520 sec cut-off frequency as high-pass filter.
To identify significantly activated brain areas, a voxel-by-voxel statistical analysis was applied to the parameter estimates for each subject; here contrasts were created to represent the activations to the drug or neutral conditions as compared to the fixation baseline. We also created differential contrasts directly comparing the drug to the neutral conditions. Maps of BOLD signals were then averaged using a custom program written in IDL (Research Systems, Boulder, CO) and included in a second-level random-effects voxel-by-voxel t-test to inspect task activations, hypoactivations, and differences between the drug and neutral conditions. Simple regression analyses using “absolute” (drug or neutral > or < baseline) or differential (drug > or < neutral) SPM contrasts were then conducted with the respective measures of behavioral performance (performance during each condition or differential scores between conditions). Note that a similar approach (subtraction between different task conditions and subsequent correlation analyses to dissociate function of corticolimbic brain regions) was previously reported (e.g., (Iidaka et al., 2001)). A threshold of p < 0.05 family-wise corrected was used for the main task activations/hypoactivations (t-tests); p < 0.01 uncorrected was used for all voxel-wise whole brain correlations; minimum cluster size was 50 contiguous voxels, 1.35 cc. Functional regions of interest (ROIs) with a volume of 729 mm3 (cubic, 27 voxels) were defined at signal maxima and the average BOLD responses (% signal change) calculated for the a priori ROIs (rACC/mOFC and cdACC). These ROIs were used to confirm the voxel-based correlations. Clarification of anatomical specificity was corroborated with a co-planar stereotaxic atlas of the human brain (Talairach and Tournoux, 1988).
RESULTS
Post-task valence assignments were more negative for drug as compared to neutral words (-1.3±1.6 vs. 0.72±0.9, paired t(13)=-3.5, p<0.01; Wilcoxon Signed Ranks Test Z=-2.8, p<0.01); the more negative these differential ratings (drug minus neutral), the longer was the last voluntary abstinence from cocaine (r=-0.74, p<0.01). As expected (see Supplementary Material), there were no differences between the drug and neutral words in accuracy (Mean±SD) (percent correct: 82±10.1 vs. 82±9.8; percent errors of omission: 18±10.1 vs. 17±10). Speed differences between the conditions also did not reach significance (reaction time: 248.1±27.3 ms vs. 253.9±20.1 ms). These results remained unchanged when excluding the one subject with drug abuse instead of drug addiction diagnosis. This subject was therefore not excluded from further analyses.
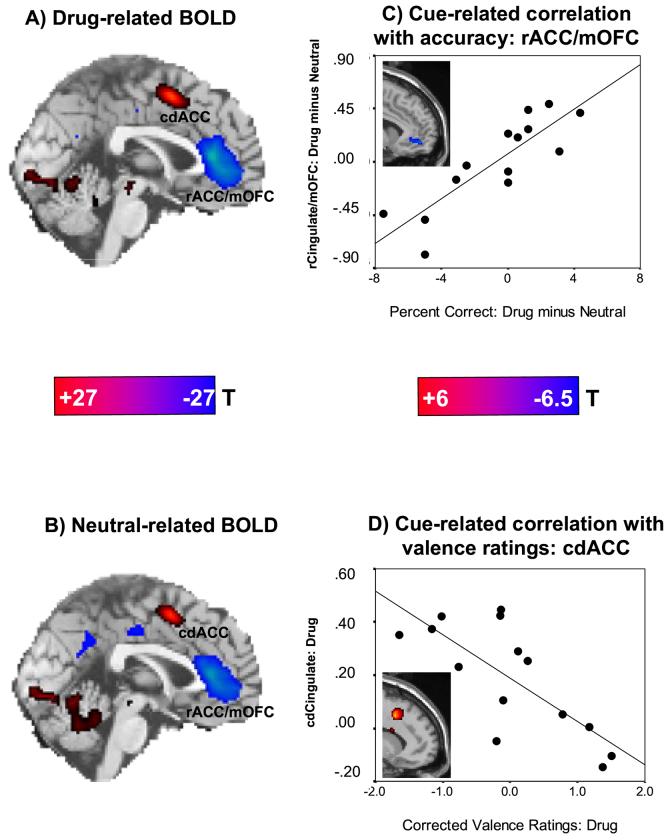
Random-effects voxelwise t-test results revealed that the task produced bilateral activations (task > baseline) in the cdACC, in other frontal regions, parietal lobe (precuneus and inferior parietal lobule), occipital lobe (lingual and fusiform gyrus), and in the caudate, thalamus, and cerebellum. Task hypoactivations (task < baseline) were noted in the rACC extending to the rectal gyrus/medial OFC and ventromedial prefrontal cortex (mOFC), insula, parahippocampal gyrus, and in the cuneus and lingual gyrus, all bilaterally (Figure 1A-B, Table 2s). There were no significant differences in this voxelwise pattern when directly comparing both word conditions for this group of 14 cocaine addicted individuals (see also Figure 2s). Nevertheless, paired t-tests using the ROIs within the rACC/mOFC cluster (x=-6, y=42, Z=-9 and x=-6 y=45 z=-3) revealed significantly larger hypoactivations to the drug (mean=-1.22 and -1.24; SEM=.42 and .31) than neutral (mean=-.62 and -.84; SEM=.33 and .32) words (p<.05). Indeed, the voxelwise analyses displayed a discernable drug minus neutral difference in this region (x=-6, y=42, z=-9; voxels=104; Z=2.8); however, this difference did not reach the nominal significance level (cluster-level corrected) in this study sample.
Figure 1.
Drug Stroop task: neural (BOLD) responses and correlations with behavior in 14 cocaine addicted individuals. A: A sagittal map is shown for cue-related activations (red: drug words > baseline) and hypoactivations (blue: drug words < baseline) (p < 0.05 family-wise corrected; color bar represents t-scores ≥ 7.99). B: A sagittal map is shown for neutral activations (red: neutral words > baseline) and hypoactivations (blue: neutral words < baseline) (p < 0.05 family-wise corrected; color bar represents t-scores ≥ 7.99); note that there were no significant differences in this pattern when directly comparing the word conditions (drug > neutral or drug < neutral; p < 0.01 uncorrected) for this sample (see also Figure 2s). C: The plot shows the association between the BOLD signal change for drug as compared to neutral words (drug > neutral) in the rACG/mOFC as a function of the respective differential performance accuracy and the corresponding linear regression line (r=0.85, p < 0.0001; x=6, y=51, z=-9); the inserted statistical map of brain activation depicts the cluster location corresponding to this correlation (p < 0.01 uncorrected; color bar represents t-scores ≥ 2.68; Table 2sC). D: The plot shows the association between the BOLD signal change for drug words as compared to baseline (drug > baseline) in the cdACG as a fuction of valence ratings for drug words and the corresponding linear regression line (r=-0.86, p < 0.0001; x=12, y=15, z=42); the inserted statistical map of brain activation depicts the cluster location corresponding to this correlation (p < 0.01 uncorrected; color bar represents t-scores ≥ 2.68; Table 2sD). Minimum cluster size was 50 contiguous voxels (1.35 cc) for all voxel-by-voxel analyses. cdACC is caudal-dorsal anterior cingulate cortex; rACC/mOFC is rostral anterior cingulate cortex/medial orbitofrontal cortex.
Although the average performance accuracy or blood-oxygenation level dependent (BOLD) responses for drug vs. neutral words did not differ, at the individual level the drug minus neutral differential accuracy scores (i.e., drug controlling for neutral context) significantly correlated with the drug minus neutral differential BOLD responses in the rACC/mOFC, both in voxel-based analyses (Figure 1C, insert, and Table 2sC) and ROI analyses (Figure 1C, linear regression). Note that these correlations were not significant when separately inspecting the drug or neutral responses, consistent with the increases in sensitivity (i.e., subject classification) when comparing a selected task condition to an active control and not a non-active baseline condition that we previously reported (Zhang et al., 2005). Further, the valence ratings for the drug (and not neutral) words (corrected for general intellectual functioning with a regression analysis) significantly correlated with BOLD responses to the drug (and not neutral) words in the cdACC in both voxel-based analyses (Figure 1D, insert, and Table 2sD) and ROI analyses (Figure 1D, linear regression). These ROI correlations remained significant after controlling (with partial correlations) for numerous possible confounding variables (e.g., demographics and individual characteristics; drug use estimates including severity and duration of use, cigarette smoking, and length of abstinence which correlated with the cdACC, r=.56, p<.05; and task performance including number of training sessions; see Figure 3s for effect on correlations of urine status and cigarette smoking). Voxel-wise correlations with accuracy and valence ratings were also observed for the parahippocampal gyrus (Table 2sC) and cerebellum (Table 2sD), respectively. These correlations were in the reverse direction to the respective rACC/mOFC and cdACC correlations.
Note that at the significance thresholds that we chose for these analyses, we did not observe similar correlations for reaction time as we did for the accuracy measures. However, we did not expect to find similar correlations between these measures of performance with the BOLD signal. Prior studies from our laboratory, using a different fMRI blocked-design task, showed lack of an association between these performance measures in the cocaine addicted but not control subjects; we attributed these results to impaired perception of inner motivation in drug addiction (Goldstein et al., 2007).
DISCUSSION
Cocaine addicted individuals rated drug-related words as more negative than matched neutral words, challenging the commonly held belief - but rarely tested as an empirical hypothesis in human studies - that drug cues are positively valued by drug addicted individuals. These results are consistent with a recent study where alcohol drinkers rated liking ethanol less than apple juice and water (Hobbs et al., 2005) and implicate in the least ambivalence towards drug cues especially for the cocaine addicted individuals with histories of longer abstinence periods.
Consistent with prior reports (Whalen et al., 1998; Bush et al., 2000), this newly developed color-word drug Stroop fMRI task produced bilateral activations in the cdACC, in other frontal regions, parietal and occipital lobes, and in the caudate, thalamus, and cerebellum. Task hypoactivations were noted bilaterally in the rACC/mOFC, insula, parahippocampal gyrus, and in the cuneus and lingual gyrus. A trend for larger rACC/mOFC hypoactivations was noted for the drug as compared to the matched neutral words in this sample of 14 cocaine addicted individuals. This trend remains to be replicated in studies with larger sample sizes.
Further, two correlations with the prefrontal cortex were unique to the task context in this drug addicted sample: (1) the more the rACC/mOFC hypoactivation specifically to drug vs. neutral words (drug more than neutral), the higher was the number of errors for the drug vs. neutral words (drug more than neutral). Thus, although - by design (see Supplementary Material) - mean performance did not differ between the two task conditions, erroneous performance correlated with hypoactivation in the rACC/mOFC and this effect was context specific (i.e., observed only when subtracting neutral from drug responses) (Figure 1C); and (2) the more the cdACC activation specifically to the drug (and not neutral) words, the more negative were the valence ratings attributed to the drug (and not neutral) words (Figure 1D). Note that our subsequent interpretations are therefore based on these significant brain-behavior correlations, not to be attributed to ‘reverse-inference’ (Poldrack, 2006).
The “affective” rACC has been previously implicated in the assessment of the salience of emotional information and regulation of emotional responses (Bush et al., 2000), and particularly in the suppression of task-irrelevant emotional information (Whalen et al., 1998), consistent with the mOFC role in reward processing (Elliott et al., 2000). The first correlation may therefore be consistent with the effect of emotional context on inhibitory control within the rACC/mOFC (e.g., see (Shafritz et al., 2006)): it is possible that the less the accuracy (more distraction) for the drug as compared to the neutral words, the greater the required rACC/mOFC suppression of an emotional reaction to the drug words in these cocaine addicted individuals. This finding (as is the lack of significant accuracy differences between the word conditions) is also consistent with the previously documented ACC error-related hypoactivity in cocaine users and other drug using groups (Kaufman et al., 2003; Forman et al., 2004; London et al., 2005).
The “cognitive” cdACC has been previously implicated in demanding tasks that involve stimulus-response selection in the face of competing streams of information including Stroop-like tasks (Bush et al., 2000), and particularly in conflict resolution (Carter et al., 1998), encompassing emotionally valenced tasks (Davis et al., 2005). The second correlation may therefore be consistent with the demanding cognitive processes that demarcate drug cues as negatively valenced for drug addicted individuals (e.g., overcoming the emotional load of drug words in order to assign what is perceived to be the subjectively appropriate or socially sanctioned negative valence).
Limitations of the current report include the following: (1) to adapt the emotional color-word Stroop task to fMRI, our design has deliberately removed the opportunity to test whether drug-related stimuli are processed behaviorally in a different way than neutral stimuli (see Supplementary Material for a detailed explanation; in brief, word reading was allowed for a long period and was separated from pressing for its color minimizing the conflict inherent in such a task. Further, display of the response key minimized working memory demands). The adaptation to the MRI environment of this color-word drug Stroop task, achieved here for the first time, took precedence when designing the current task given the previous consistent documentation of the role for attentional bias to drug-related stimuli in drug addicted individuals; in these other word or picture color-naming or button pressing behavioral paradigms, accuracy or response latency differences between drug-related and neutral matched stimuli have been reported in abusers of alcohol (Duka and Townshend, 2004) nicotine (Mogg and Bradley, 2002), heroin (Franken et al., 2000), and cocaine (Hester et al., 2005). Nevertheless, the behavioral documentation of an attentional bias using our own task design is warranted; (2) a related concern is the lack of significant differences between the drug and neutral conditions at the BOLD signal level. We postulate that such differences would be significant with larger sample sizes or when including a non drug addicted control group; in the latter case we would expect to document a significant condition by group interaction effect on the BOLD signal in the selected ROIs such that differences between the task conditions will be observed only in the drug using and not control group (e.g., larger hypoactivation in the rACC/mOFC to the drug than neutral condition in the drug users, as indeed suggested by preliminary paired t-test ROI results in the current study); and (3) use of alternating stimuli (Cox et al., 2001) instead of the current block design may have facilitated our ability to obtain significant behavioral differences between the drug and neutral conditions. This could be tested with further optimizations of the current design (e.g., rendering it event-related or non-verbal).
Overall, using a newly designed color-word drug Stroop fMRI task in 14 individuals with cocaine use disorders, results showed unique (drug-related) brain-behavior associations at the individual level. Thus, the more the cdACC activation and rACC/mOFC hypoactivation, the more negative the valence attributed and the more the errors committed specifically in the drug context (controlling for the neutral context), respectively. The significance of these drug-specific multi-modal associations is in suggesting that the rACC/mOFC and the cdACC may uniquely contribute to different aspects of drug-related responses (distraction/decreased task accuracy) and intrusive negative cue-related thoughts in drug addicted individuals. Taken together, if replicated in future studies with larger sample sizes, these results may model the on-line experience of drug addicted individuals when facing drug cues where, despite considerable amount of cognitive effort (modulated by cdACC), unregulated emotional reactions to these salient cues are paramount and are not successfully suppressed (modulated by rACC/mOFC). The effect on results of severity and duration of drug use, and application to populations with other drug use disorders, remain to be accomplished. Prospective studies also need to explore the relevance of these findings to the progression of drug addiction and prediction of relapse (see for example use of fMRI during a decision-making task to predict relapse in methamphetamine dependent subjects (Paulus et al., 2005)).
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (1K23 DA15517-01 and R03 DA 017070-01); National Alliance for Research on Schizophrenia and Depression Young Investigator Award; Laboratory Directed Research and Development from U.S. Department of Energy (OBER), and General Clinical Research Center (5-MO1-RR-10710). We also would like to thank Sahib S. Khalsa and Steve Berry for help with word selection, matching and ratings.
List of abbreviations
- ACC
anterior cingulate cortex
- BOLD
blood-oxygenation level dependent
- cdACC
caudal-dorsal ACC
- DSM-IV
diagnostic and statistical manual of mental disorders, fourth edition
- EPI
echo-planar imaging
- fMRI
functional magnetic resonance imaging
- I-RISA
Impairments in Response Inhibition and Salience Attribution
- MRI
magnetic resonance imaging
- ROI
region of interest
- OFC
orbitofrontal cortex
- rACC/mOFC
rostro-ventral ACC/medial
- OFC SD
standard deviation
- SPM
statistical parametric mapping
- TE
time of echo
- TR
time of repetition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Editor (Clinical): Dr. David A. Lewis, Department of Psychiatry, University of Pittsburgh, W1652 BST, 3811 O Hara Street, Pittsburgh, PA 15213-2593, USA
REFERENCES
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–352. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- Bechara A,B, Dolan S, Andrea H. Decision-Making and addiction (part II): myopia for the future or hypersensitivity to reward. Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Pothos EM, Johnsen BH, Laberg JC. Methodological issues attached to the alcohol Stroop paradigm: comments on a paper by Sharma, Albery & Cook (2001) Addiction. 2001;96:1261–1265. doi: 10.1046/j.1360-0443.2001.96912615.x. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci. 2005;25:8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Francis NW, Kučera H. Frequency Analysis of English Usage. Houghton Mifflin; Boston: 1982. [Google Scholar]
- Franken IH, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. J Psychopharmacol. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164:1–9. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2005 doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: a test of the incentive-sensitisation theory. Psychopharmacology (Berl) 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J Cogn Neurosci. 2001;13:1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Samaras D, Tomasi D, Alia-Klein N, Cottone L, Leskovjan A, Volkow N, Goldstein R. Exploiting temporal information in functional magnetic resonance imaging brain data. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2005;8:679–687. doi: 10.1007/11566465_84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.