Abstract
Astroctyes express a set of three connexins (Cx26, Cx30, and Cx43) that are contained in astrocyte-to-astrocyte (A/A) gap junctions; oligodendrocytes express a different set of three connexins (Cx29, Cx32, and Cx47) that are contained in the oligodendrocyte side of necessarily heterotypic astrocyte-to-oligodendrocyte (A/O) gap junctions, and there is little ultrastructural evidence for gap junction formation between individual oligodendrocytes. In addition, primarily Cx29 and Cx32 are contained deeper in myelin sheaths, where they form autologous gap junctions at sites of uncompacted myelin. The presence of six connexins in macroglial cell populations has revealed unprecedented complexity of potential connexin coupling partners, and with restricted deployment of gap junctional intercellular communication (GJIC) within the “pan-glial” syncytium. New implications for the organization and regulation of spatial buffering mediated by glial GJIC are derived from recent observations of the existence of separate astrocyte anatomical domains, with only narrow regions of overlap between astrocyte processes at domain borders. Thus, widespread spatial buffering in the CNS may occur not successively through a multitude of processes arising from different astrocytes, but rather in a more orderly fashion from one astrocyte domain to another via intercellular coupling that occurs only at restricted regions of overlap between astrocyte domains, augmented by autocellular coupling that occurs within each domain.
Keywords: Astrocyte domains, connexins, gap junctions, glia, spatial buffering
INTRODUCTION
Astrocytes have been described as forming astrocyte-to-astrocyte (A/A) gap junctions at a variety of sites in brain parenchyma. It has been estimated that each individual astrocyte forms, on average, a staggering 30,000 gap junction per cell and that these junctions link the processes of different astrocytes, as well as different processes of the same astrocyte forming what are defined as autologous junctions (1). In addition, abundant gap junctions are formed between adjacent lamellar processes of astrocytes that ensheath synaptic glomeruli, between processes that establish end-feet on blood vessels and that occur at nodes of Ranvier along myelinated fibers (2).
Astrocytes also form numerous gap junctions with oligodendrocyte somata and their initial processes, and with the outer turn of their uncompacted myelinating processes along axons (A/O gap junctions), thus incorporating oligodendrocytes into the panglial syncytium. However, oligodendrocytes have not been seen forming gap junctions with each other, leaving them to be what has been described as “blind alleys” of the syncytium (3, 4). Nevertheless, oligodendrocytes may still communicate with each other via the syncytium through a series of oligodendrocyte-astrocyte-oligodendrocyte (O/A/O) gap junctions, where astrocytes serve as intermediary conduits for gap junctional flow of ions and metabolites. Although in need of further investigation, such O/A/O-mediated junctional passage of substances in the absence of O/O gap junctions, may explain observations of dye-coupling between oligodendrocytes in vivo (5).
Intensive efforts to identify connexins expressed by macroglial cells have been ongoing for a little over a decade, and may be nearing completion with current knowledge of what appears to be the entire connexin family of gap junction forming proteins. Here, we provide a summary of those efforts in the context of implications that recent findings on the anatomical organization of astrocyte have on processes related to gap junction-mediated spatial buffering.
MATERIALS AND METHODS
Monoclonal and polyclonal anti-Cx26, Cx29, Cx30, Cx32, Cx43, and Cx47 antibodies generated against peptides corresponding to non-conserved, unique sequences within these connexins were obtained from Zymed Laboratories (South San Francisco, CA; www.zymed.com). As previously reported (5–7), the specificity characteristics of each of these antibodies have been demonstrated by western blotting of brain tissue and connexin-transfected HeLa cells, as well as by immunohistochemical labeling of gap junctions in tissues and cultured cells. For LM or EM analysis of connexins in the CNS, mice and rats were perfused transcardially with aldehyde fixatives (0.16 M NaPO4 buffer, pH 7.6, 0.2% picric acid and 1–4% paraformaldehyde), consisting of a range of fixation strengths and post-fixation times determined to be optimal for immunolabeling of each connexin. Cryostat sections of brain and spinal cord (10 μm thick) were processed for immunofluorescence labeling with primary antibodies at concentration of 1–3 μg/ml.
For double immunofluorescence labeling, sections were incubated simultaneously with monoclonal and polyclonal primary antibodies, followed by incubation simultaneously with appropriate secondary FITC-conjugated anti-mouse and Cy3-conjugated anti-rabbit antibodies. To obtain an estimate of the proportion of oligodendrocytes that express Cx29, Cx32, and Cx47, the percentage of these cells immunopositive for both the oligodendrocyte marker CNPase and each of these connexins in various brain regions was determined. Immunohistochemical control procedures to demonstrate antibody specificity involved processing of sections with pre-immune serum, with secondary antibody after omission of primary antibody, or with antibody that has been pre-adsorbed against the peptide used to generate the antibody. In some instances, positive controls included immunodetection of connexins in connexin-transfected, but not in non-transfected control HeLa cells.
Where possible, additional confirmation of antibody specificity was obtained by demonstrations of identical cellular and subcellular immunolocalization patterns with two different anti-connexin antibodies directed against different sequences within the same connexin. In addition, specificity of Cx32 detection in brain was established by comparisons of robust immunolabeling of Cx32 in wildtype mice with an absence of labeling in Cx32 knockout mice. Fluorescence was examined on a Zeiss Axio-scop2 microscope and an Olympus Fluoview IX70 confocal microscope.
RESULTS AND DISCUSSION
Cellular localization of Cx29, Cx32, and Cx47 in brain are summarized in Figure 1, along with our earlier observations concerning Cx26, Cx30, and Cx43. As reviewed in detail elsewhere (2, 6, 8), Cx43 is a well-established component of A/A gap junctions, and Cx30 was shown to be co-localized at the vast majority of these junctions (9, 10). Several reports have also described the presence of Cx26 in adult brain, but differ on descriptions of its cellular localization (11). Results of our studies indicate that Cx26 also is expressed by astrocytes, and that it is largely co-localized with the other two astrocytic connexins. Thus, astrocytes produce three connexins, each of which appears to be incorporated into individual A/A gap junctions.
Figure 1.
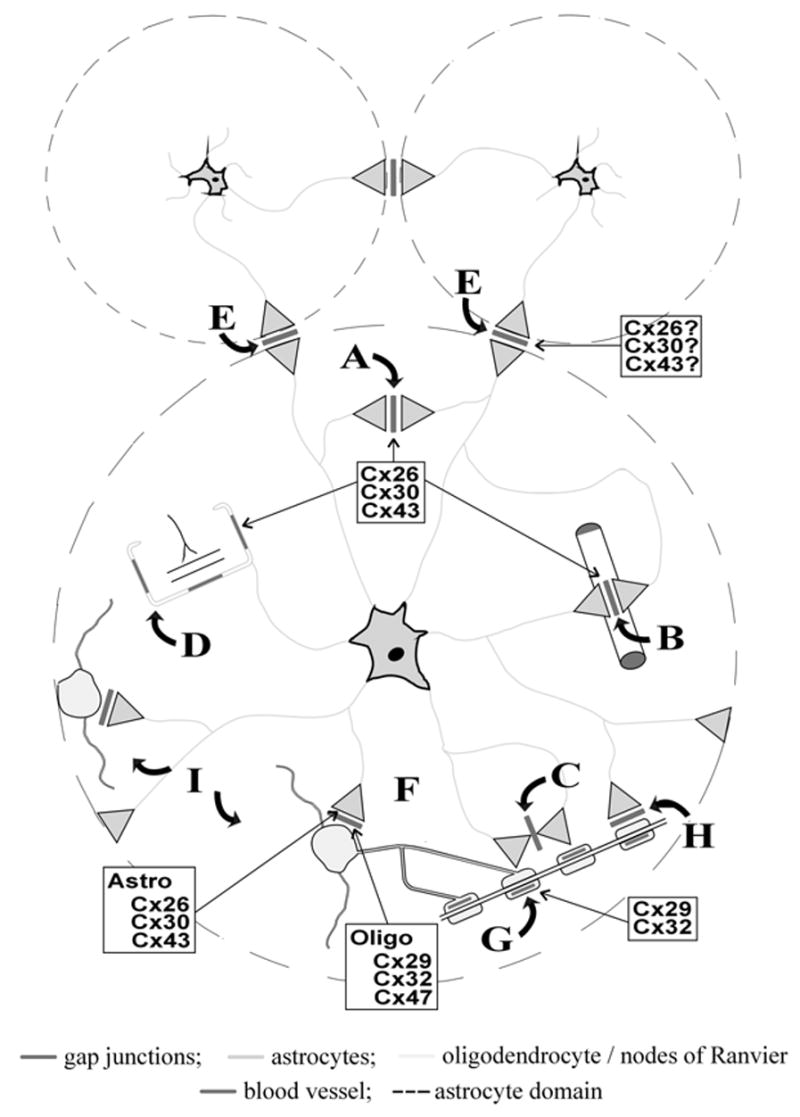
Organization of gap junctions within the glial syncytium based on identification of separate astrocyte anatomical domains. Shown are three minimally overlapping astrocyte domains with one enlarged to illustrate: Intra-domain autologous A/A gap junctions in neuropil (A), at endfeet on blood vessels (B), at nodes of Ranvier (C), and at glial sheaths around glomeruli (D); inter-domain homologous A/A gap junctions between adjacent astrocyte domains (E); intra-domain heterologous A/O gap junctions between astrocyte and oligodendrocyte (F); connexins in myelin sheaths (G). Heterologous A/O gap junctions along myelin sheaths (H); and astrocyte mediation of O/A/O junctional coupling (I). Connexins present in gap junctions at various locations are indicated, and those contained at the very narrow inter-domain A/A gap junctions may contain all three or only a subset of astrocytic connexins. Astro, astrocyte connexins; Oligo, oligodendrocyte connexins. (See Color Plate XVIII).
However, the relative ratios of Cx26, Cx30, and Cx43 in these junctions vary considerably because each of these connexins exhibits a highly heterogeneous but dissimilar distribution in brain. For example, A/A junctions in some CNS regions may contain a far greater abundance of Cx43, whereas those in others may contain equal levels of the three connexins. The significance of this uneven distribution is not clear, although a relationship between levels of astrocyte connexin expression and tonic neuronal activity in particular CNS structures has been considered (11).
None of the three astrocytic connexins has been observed in oligodendrocytes. Ultrastructural examination by thin section immunoEM or freeze-fracture replica immunogold labeling (FRIL) has revealed asymmetric labeling of A/O gap junctions with Cx26, Cx30, and/or Cx43 located only on the astrocyte side of these junctions (6). Oligodendrocytes express entirely different connexins than those contained in astrocytes. The first of these to be described was Cx32 (12), which was reported to be localized not only at the oligodendrocyte side of A/O gap junctions, but also richly distributed along myelinated fibers (13).
More recently, the two newest members of the gap junction forming family of proteins to be identified, Cx29 and Cx47, were both documented to be expressed by oligodendrocytes (5, 7, 14–16), correcting an earlier report suggesting Cx47 expression by neurons (17).
In comparative studies, it was reported that mutually exclusive subpopulations of oligodendrocytes contain Cx29 and Cx32 (14). In our analyses of greater than nine thousand cells in various brain regions of adult mouse brain double-labeled by immunofluorescence for the oligodendrocyte marker CNPase and either Cx29, Cx32, or Cx47, it was found that, on average, greater than 95% of CNPase-positive oligodendrocyte somata contained each of these connexins, although Cx29 was clearly less abundant than the other two connexins. A high degree of Cx29/Cx32 co-localization was also found along myelinated fibers (7). Further, results indicate that while Cx29 and Cx32 are highly concentrated along myelinated fibers, Cx47 is largely though not exclusively restricted to oligodendrocyte somata, indicating differential and selective targeting of connexins to oligodendrocyte subcellular compartments.
The gap junctional pathways for various routes of ion and metabolite flow within the glial syncytium (summarized in Figure 1) are determined by cell-type selectivity of gap junction formation, specificity of connexin expression, permissiveness of functional coupling between the various glial connexins, permselectivity of the permissive connexin coupling partners, and regulation of channel conductance from open to closed states. With three connexins (Cx26, Cx30, Cx43) forming junctions between astrocytes (A/A junctions) and a set of three different connexins (Cx29, Cx32, Cx47) in their oligodendrocyte coupling partners, and potentially six connexins at astrocytes-to-oligodendrocyte gap junctions (A/O junctions), the glial syncytium is uniquely complex among mammalian systems with respect to its complement of connexins and gap junction dispersement.
Astrocytes contribute to K+ and Na+ homeostasis by spatial buffering, which involves gap junction-mediated cell-to-cell movement of ions through the glial syncytium. There is considerable evidence that astrocytic GJIC increases following neuronal activity, and is under dynamic control by mediators released from neurons (18) during constantly changing physiological conditions. It has been shown, for example, that electrical stimulation of deep layers of the cerebral cortex results in glial uptake of the K+ that is released from active neurons, followed by movement of K+ to superficial cortical layers through the astrocytic syncytium (19).
Since K+ movement cannot occur efficiently through the narrow extracellular space, its uptake by glia produces osmotic gradients that are accompanied by water redistribution, resulting in shrinkage of the extracellular space at the site of active neurons and enlargement of this space at sites of inactivity (19). These events were prevented following blockade of gap junctions (20), demonstrating the importance of the gap junction-coupled astrocytic glial syncytium during neural activity.
The “contact spacing model” of astrocytes in the CNS predicted that spatial buffering occurs through extensively interdigitated laminar processes arising from many astrocyte somata that are well separated from each other (21), resulting in minimal volume occupied by a single astrocyte. However, it was recently reported (22) that the majority of astrocyte processes occupy exclusive domains of neuropil, with only limited overlap at the tips of neighboring astrocyte processes occurring along a narrow band at the periphery of the volume occupied by each astrocyte (Figure 1). Astrocyte somata are roughly 8 μm in diameter, their domains occupy a volume about 65,900 μm3, and inter-domain boundaries appear to be roughly 2 μm in width estimated from data reported by Bushong et al. (22). Thus, assuming a relatively uniform distribution of A/A junctions, the majority (75%) of the astrocyte gap junctions appear to be autologous (defined as intra-domain A/A junctions), consistent with earlier findings of the preponderance of such junctions (1). These autologous gap junctions appear to contain all three astrocytic connexins based on our observations. Although it is likely that inter-astrocyte junctions (defined as inter-domain A/A junctions) also contain each of these connexins, the smaller percentage of these junctions and the lack of their identification at inter-astrocyte domain boundries in our documentation of connexins at A/A junctions, leaves uncertain the connexin composition of inter-domain A/A gap junctions.
Because inter-domain A/A gap junctions would (by definition) be crucial to intercellular homeostatic processes, the existence of astrocyte domains implies that GJIC within the glial syncytium occurs in a more orderly fashion than previously envisioned, namely, from astrocyte-to-astrocyte domain and only at restricted borders between adjacent domains. Further, all the attendant functions associated with the gap junctional circuitry (e.g., spatial buffering) at various critical locations within a domain may be dominated by the functional state of single astrocyte domains, and individual astrocytes may maintain regulatory control of GJIC with neighboring domains in a fashion determined by neuronal activity within their domain.
References
- 1.Wolburg H, Rohlmann A. Structure-function relationships in gap junctions. Int Rev Cytol. 1995;157:315–373. doi: 10.1016/s0074-7696(08)62161-0. [DOI] [PubMed] [Google Scholar]
- 2.Nagy JI, Dermietzel R. Gap junctions and connexins in the mammalian central nervous system. In: Hertzberg EL, editor. Advances in Molecular and Cell Biology. Vol. 30. JAI Press Inc; New York: 2000. pp. 323–396. [Google Scholar]
- 3.Mugnaini E. Cell junctions of astrocytes ependyma and related cells in the mammalian central nervous system, with emphasis on the hypothesis of a generalized functional syncytium of supporting cells. In: Fedoroff S, Vernadakis A, editors. Astrocytes. Vol. 1. Academic Press; New York: 1986. pp. 329–371. [Google Scholar]
- 4.Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J Comp Neurol. 1997;388:265–292. doi: 10.1002/(sici)1096-9861(19971117)388:2<265::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Nagy JI, Ionescu AV, Lynn BD, Rash JE. Connexin composition of gap junctions between astrocytes and oligodendrocytes in normal and connexin32 knockout mice. Glia. 2003;44:205–218. doi: 10.1002/glia.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Rev. 2003 doi: 10.1016/j.brainresrev.2004.05.005. In press. [DOI] [PubMed] [Google Scholar]
- 7.Nagy JI, Ionescu AV, Lynn BD, Rash JE. Connexin29 and connexin32 at oligodendrocyte/astrocyte gap junctions and in myelin of mouse CNS. J Comp Neurol. 2003;464:356–370. doi: 10.1002/cne.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 9.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins, and evidence for restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy JI, Patel D, Ochalski PAY, Stelmack GL. Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, colocalization with connexin43 at gap junctions and late developmental appearence. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- 11.Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Yasumura T, Rash JE, Staines WA. Connexin26 in adult rodent central nervous system: Demonstration at astrocyte gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- 12.Scherer SS, Deschenes SM, Xu Y-T, Grinspan JB, Fischbeck KH, Paul DL. Connexin32 is a myelin-related protein in the PNS and CNS. J Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Hertzberg EL, Nagy JI. Connexin32 in oligodendrocytes and association with myelinated fibers in mouse and rat brain. J Comp Neurol. 1997;379:571–591. doi: 10.1002/(sici)1096-9861(19970324)379:4<571::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central peripheral nervous systems. J Neurosci. 2002;22:6458–6470. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teubner B, Odermatt B, Guldenagel M, Sohl G, Degen J, Bukauskas FF, Kronengold J, Verselis VK, Jung YT, Kosak CA, Schilling K, Willecke K. Functional expression of the new gap junction gene connexin47 transcribed in mouse brain and spinal cord neurons. J Neurosci. 2001;21:1117–1126. doi: 10.1523/JNEUROSCI.21-04-01117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, Giaume C. Biol Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 19.Niermann H, Amiry-Moghaddam M, Holthoff K, Witte OW, Ottersen OP. A novel role of vasopressin in the brain: Modulation of activity-dependent water flux in the neocortex. J Neurosci. 2001;21:3045–3051. doi: 10.1523/JNEUROSCI.21-09-03045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holthoff K, Witte OW. Directed spatial potassium redistribution in rat neocortex. Glia. 2000;29:288–292. doi: 10.1002/(sici)1098-1136(20000201)29:3<288::aid-glia10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Distler C, Dreher Z, Stone J. Contact spacing among astrocytes in the central nervous system: An hypothesis of their structural role. Glia. 1991;4:484–494. doi: 10.1002/glia.440040508. [DOI] [PubMed] [Google Scholar]
- 22.Bushong EA, Martone ME, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


