Abstract
Rapid repetitive activities arising from pulmonary veins may initiate atrial fibrillation. The basis of these rapid repetitive activities remains unclear, but recent evidence suggests that the autonomic nervous system plays an important role in their formation. Pulmonary veins and the adjoining left atrium are highly innervated structures. This review summarizes recent developments in the understanding of the anatomy of autonomic nerves in and around pulmonary veins and their implications for atrial fibrillation.
Keywords: Pulmonary vein, Atrial fibrillation, Left atrium, Superior vena cava, Inferior vena cava, Vein of Marshall
Introduction
Haissaguerre et al1 first reported that atrial fibrillation (AF) can be initiated spontaneously by rapid repetitive activities arising from pulmonary veins (PVs). The PV musculature is highly anisotropic, with abrupt fiber orientation changes, muscular breaks, and fibrous encapsulation of muscle bundles.2–5 These changes may predispose to reentry formation.6 Few data regarding the autonomic innervation of PVs and the adjoining LA are available. Emerging basic and clinical reports strongly suggest an important mechanistic role of the autonomic nervous system in the genesis of AF. Recently, these reports have formed the basis of novel therapeutic strategies for catheter ablation of AF.7,8
Role of the autonomic nervous system in AF
In 1978 Coumel et al9 wrote that either limb of the autonomic nervous system may generate AF. Subsequent work from Elvan et al10 supported this assertion. They found that radiofrequency (RF) ablation of the atria eliminated pacing-induced sustained AF but also reduced the corrected sinus node recovery time, mean heart rate response to isoproterenol, and intrinsic heart rate after atropine. Thus, autonomic tone modulation may be partly responsible for eliminating AF. Schauerte et al11 performed high-frequency electrical stimulation of autonomic nerves during atrial refractoriness and provoked rapid ectopic beats arising from the PV and superior vena cava (SVC), which in turn initiated AF. During focal catheter ablation of AF foci within human PVs, Hsieh et al12 found transient alterations of heart rate variability, suggesting that PVs have significant autonomic innervation. Subsequently, Pappone et al7 demonstrated that patients in whom autonomic denervation was documented had a reduced recurrence of AF, compared with those in whom denervation was not documented. However, the specific contribution of each arm of the autonomic nervous system to this favorable outcome remains unclear. Coumel13 have suggested that there were two discrete forms of AF. Sympathetic AF tends to occur in patients with organic heart disease, and vagal AF occurs in healthy young patients. However, emerging evidence suggests a synergism between both arms of the autonomic nervous system on atrial arrhythmogenesis. Sharifov et al14 infused isoproterenol and acetylcholine into the sinus node artery and found that isoproterenol infusion increased the likelihood and ease of AF induction with acetylcholine compared with acetylcholine alone. Similarly, Pattersen et al15 found that injections of acetylcholine and norepinephrine into PV ganglionic “fat pads” adjacent to canine PVs led to pause-dependent induction of triggered activity and arrhythmias arising from PVs in vitro.
Anatomy of atrial and PV autonomic nerves
Pappone et al7 hypothesized that induction of bradycardia was due to vagal nerve stimulation, whereas abolition of bradycardia with continued RF application suggested vagal denervation. However, the distributions of adrenergic and cholinergic nerves in this region were not delineated, so whether sympathetic nerves also were eliminated during RF application is unclear. Numerous investigators have studied the macroscopic and microscopic anatomy of cardiac autonomic nerves within the atria. Among those who focused on PV autonomic nerves, Armour et al16 provided a detailed map of autonomic nerve distributions in human hearts. They found that autonomic nerves were concentrated in “ganglionic plexi” around great vessels such as the PVs. Chiou et al17 determined that these nerves converged functionally onto fat pads located around the SVC–aortic junction, and that catheter ablation of this fat pad effectively denervated many regions of the atria but preserved innervation of the ventricle. On a more microscopic scale, Chevalier et al18 discovered several gradients of PV autonomic innervation, with nerves more abundant in the proximal PV than the distal PV and more abundant in epicardium than endocardium. However, these studies did not distinguish specifically between adrenergic and cholinergic nerves. Therefore, we performed immunostaining of 192 PV–atrial segments harvested from 32 veins from eight human autopsied hearts using anti-tyrosine hydroxylase antibodies to label adrenergic nerves and anti-choline acetyltransferase antibodies to label cholinergic nerves.4 We analyzed nerve densities along the longitudinal and circumferential axes of the PV–atrial junction. Longitudinally (Figure 1A), adrenergic and cholinergic nerve densities were highest in the LA within 5 mm from the PV–LA junction than further distally in the PV or more proximally in the LA proper. Circumferentially (Figure 2), both nerve densities were higher in the superior aspect of the left superior PV, anterosuperior aspect of the right superior PV, and inferior aspects of both inferior PVs than diametrically opposite, and were higher in the epicardial than the endocardial half of the tissue (Figure 1B). Significantly, we could not find areas of discrete adrenergic or cholinergic predominance. Rather, both nerve types have similar macroscopic distributions in and around PVs (Figure 2). Additionally, we found at cellular levels that up to 25% of all nerve fiber bundles contained both adrenergic and cholinergic nerves, and greater than 90% of ganglia contained both adrenergic and cholinergic elements within the same ganglion. These data indicate that adrenergic and cholinergic nerves are highly co-located not only at tissue but also at cellular levels. This anatomic co-localization of adrenergic and cholinergic innervation implies that selectively eliminating only sympathetic or parasympathetic nerves during catheter ablation of AF is virtually impossible.
Figure 1.

Distributions of adrenergic [tyrosine hydroxylase (TH)] and cholinergic [choline acetyltransferase [ChAT]) nerves along the longitudinal (A) and transmural (B) axes.
(Reproduced with permission from Tan et al.4)
Figure 2.
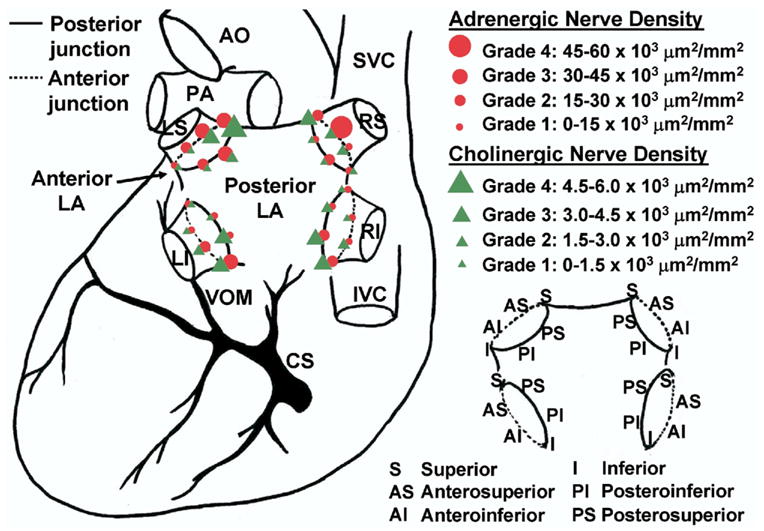
Circumferential distributions of adrenergic and cholinergic nerves around pulmonary vein orifices. AO = aorta; CS = coronary sinus; IVC = inferior vena cava; LA = left atrium; LI = left inferior pulmonary vein; LS = left superior pulmonary vein; PA = pulmonary artery; RI = right inferior pulmonary vein; RS = right superior pulmonary vein; SVC = superior vena cava; VOM = vein of marshall.
(Reproduced with permission from Tan et al.4)
Implications of PV neural anatomy
If both sympathetic and parasympathetic nerves are co-stimulated/ablated, why is bradycardia the dominant response elicited during ganglionic stimulation/ablation rather than tachycardia? We propose several explanations. First, complex extracardiac neural pathways17,19 involved in the generation of bradycardic reflexes during stimulation/ablation around PVs project to vagal nuclei centrally but generally do not involve sympathetic tracts.19 Second, a paracrine mechanism might be in operation as ganglion cells are predominantly cholinergic4 and would release mostly acetylcholine when stimulated/ablated. Third, adrenergic nerves are more widely distributed than are cholinergic nerves.4,20 Hence, RF ablation may eliminate a greater proportion of cholinergic nerves than adrenergic nerves, disrupting sympathovagal balance. However, induction of bradycardic reflexes does not imply that intracardiac sympathetic nerves within ganglionic plexi were not eliminated simultaneously with vagal nerves. Our study also indicates that the PV antrum within 5 mm of the PV–LA junction rather than further away in the atria or distally into the PV is the most densely innervated and therefore the optimal location for autonomic nerve modulation procedures. Clinical reports show that most autonomic reflexes are most commonly elicited within approximately 1 cm of the PV–LA junction.7,21 These reports are consistent with our anatomic data when allowed for dimensional changes in tissue associated with tissue processing for histology.
Neural modulation as a potential therapeutic strategy
The effectiveness of autonomic modulation as an adjunctive therapeutic strategy to catheter ablation of AF has been inconsistent. Although Pappone et al7 and Nakagawa et al21 obtained favorable results, others have found no beneficial22 or deleterious23 outcomes between patients with denervation compared with those without, a finding also underlined by Hirose et al24 in animal studies where partial vagal denervation of the high right atrium was found to increase inducibility of AF. These conflicting studies suggest that interactions between the autonomic nervous system and AF are more complex than is currently understood. Perhaps a degree of individual variability accounts for these discrepancies, with some patients having more pronounced autonomic triggers than others. As an illustration, Scanavacca et al8 recently found in a small number of patients with “autonomic” paroxysmal AF that denervation alone without substrate modification in the atria was effective in preventing AF recurrence in two of 11 patients. These two patients had the most pronounced and persistent changes in heart rate variability. In addition, effects of denervation may be transitory. In the study by Pappone et al7 of patients undergoing PV denervation, the effect on heart rate variability was transient, returning to baseline at 6 months. In animal experiments, autonomic changes associated with elimination of cardiac fat pads returned to normal in 6 weeks.25 Therefore, whether permanent autonomic denervation can be achieved is still uncertain. The evidence to date suggests that autonomic modulation does have an adjunctive role in catheter AF ablation, especially when applied selectively. Further mechanistic and clinical studies are awaited before a wider application can be recommended.
Autonomic innervation of other thoracic veins
Thoracic veins are the veins in the thorax that drain into the heart. They include the SVC, inferior vena cava, PVs, azygos vein, and vein of Marshall. In addition to the PVs, other thoracic veins contain abundant autonomic nerves. Kim et al26 found that the vein of Marshall had abundant sympathetic nerves. Doshi et al27 performed high-density in vitro mapping and found that the vein of Marshall became a frequent source of ectopic beats and arrhythmia during adrenergic stimuli using isoproterenol. Schauerte et al11 were able to induce ectopic beats coming not only from the PVs but also from the SVC during autonomic nerve stimulation. Chiou et al17 determined that the functional pathways of atrial autonomic nerves converged on three major fat pads, one located adjacent to the SVC. These studies suggest that autonomic nerves concentrate not only around PVs but also around the roots of major thoracic veins. The distribution of autonomic nerves may play a role in atrial arrhythmogenesis.
Conclusion
PVs and other thoracic veins are highly innervated structures. Anatomically, sympathetic and vagal nerves are highly co-located at both tissue and cellular levels. This anatomic co-location may form a basis for physiologic synergism between sympathetic and vagal activations on AF but also indicates that selective elimination of either type during catheter ablation of AF is unlikely.
Acknowledgments
We thank Dr. C. Thomas Peter for support.
Footnotes
This study was supported by Grants R01HL71140, R01HL78932, P01HL78931, and R01HL66389 from the National Institutes of Health; the Cardiac Arrhythmia Research Enhancement Support Group Inc. (CARES); Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology; Pauline and Harold Price Endowment; and Piansky Family Trust. Honorarium received for lecture at meeting and financial support for lecture on this subject.
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Saito T, Waki K, Becker AE. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol. 2000;11:888–894. doi: 10.1111/j.1540-8167.2000.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamabe A, Okuyama Y, Miyauchi Y, Zhou S, Pak HN, Karagueuzian HS, Fishbein MC, Chen PS. Correlation between anatomy and electrical activation in canine pulmonary veins. Circulation. 2003;107:1550–1555. doi: 10.1161/01.cir.0000056765.97013.5e. [DOI] [PubMed] [Google Scholar]
- 4.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen P-S, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 5.Ho SY, Cabrera JA, Tran VH, Farre J, Anderson RH, Sanchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart. 2001;86:265–270. doi: 10.1136/heart.86.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou C-C, Nihei M, Zhou S, Tan AY, Kawase A, Macias ES, Fishbein MC, Lin S-F, Chen P-S. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889 –2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 7.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 8.Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrao CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 9.Coumel P, Attuel P, Lavallee J, Flammang D, Leclercq JF, Slama R. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978;71:645– 656. [PubMed] [Google Scholar]
- 10.Elvan A, Pride HP, Eble JN, Zipes DP. Radiofrequency catheter ablation of the atria reduces inducibility and duration of atrial fibrillation in dogs. Circulation. 1995;91:2235–2244. doi: 10.1161/01.cir.91.8.2235. [DOI] [PubMed] [Google Scholar]
- 11.Schauerte P, Scherlag BJ, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, Lazzara R, Jackman WM. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol. 2001;12:592–599. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh MH, Chiou CW, Wen ZC, Wu CH, Tai CT, Tsai CF, Ding YA, Chang MS, Chen SA. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation. 1999;100:2237–2243. doi: 10.1161/01.cir.100.22.2237. [DOI] [PubMed] [Google Scholar]
- 13.Coumel P. [Paroxysmal atrial fibrillation: role of autonomic nervous system] Arch Mal Coeur Vaiss. 1994;87:55–62. Spec No 3. [PubMed] [Google Scholar]
- 14.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Chiou C-W, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes—the third fat pad. Circulation. 1997;95:2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 18.Chevalier P, Tabib A, Meyronnet D, Chalabreysse L, Restier L, Ludman V, Alies A, Adeleine P, Thivolet F, Burri H, Loire R, Francois L, Fanton L. Quantitative study of nerves of the human left atrium. Heart Rhythm. 2005;2:518–522. doi: 10.1016/j.hrthm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Aviado DM, Guevara AD. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci. 2001;940:48–58. [PubMed] [Google Scholar]
- 20.Marron K, Wharton J, Sheppard MN, Fagan D, Royston D, Kuhn DM, de Leval MR, Whitehead BF, Anderson RH, Polak JM. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation. 1995;92:2343–2451. doi: 10.1161/01.cir.92.8.2343. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa H, Scherlag BJ, Wu R, Po S, Lockwood D, Yokoyama K, Herring L, Lazzara R, Jackman WM. Addition of selective ablation of autonomic ganglia to pulmonary vein antrum isolation for treatment of paroxysmal and persistent atrial fibrillation (Abstract) Circulation. 2006;110:III-459. [Google Scholar]
- 22.Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:387–396. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Cummings JE, Gill I, Akhrass R, Dery M, Biblo LA, Quan KJ. Preservation of the anterior fat pad paradoxically decreases the incidence of postoperative atrial fibrillation in humans. J Am Coll Cardiol. 2004;43:994–1000. doi: 10.1016/j.jacc.2003.07.055. [DOI] [PubMed] [Google Scholar]
- 24.Hirose M, Leatmanoratn Z, Laurita KR, Carlson MD. Partial vagal denervation increases vulnerability to vagally induced atrial fibrillation. J Cardiovasc Electrophysiol. 2002;13:1272–1279. doi: 10.1046/j.1540-8167.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- 25.Oh S, Zhang Y, Bibevski S, Marrouche NF, Natale A, Mazgalev TN. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006;3:701–708. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen P-S, Fishbein MC. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 27.Doshi RN, Wu T-J, Yashima M, Kim Y-H, Ong JJC, Cao J-M, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen P-S. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]


