Abstract
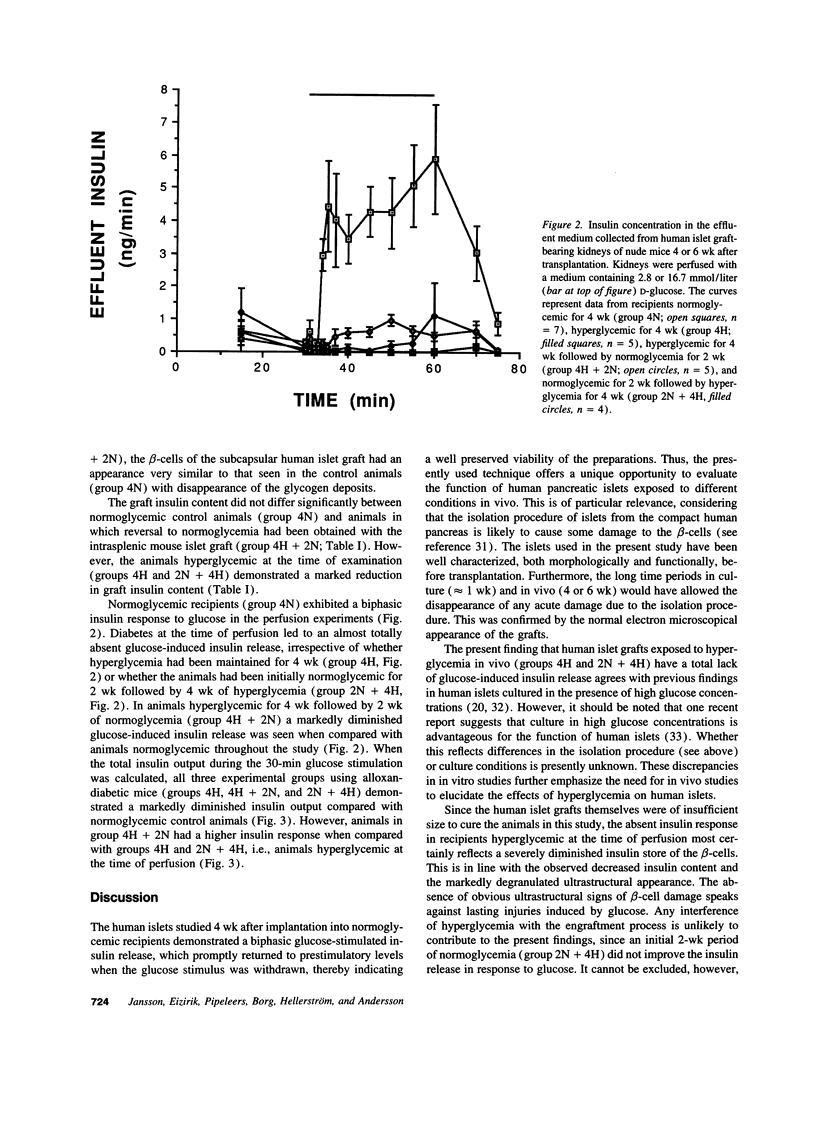
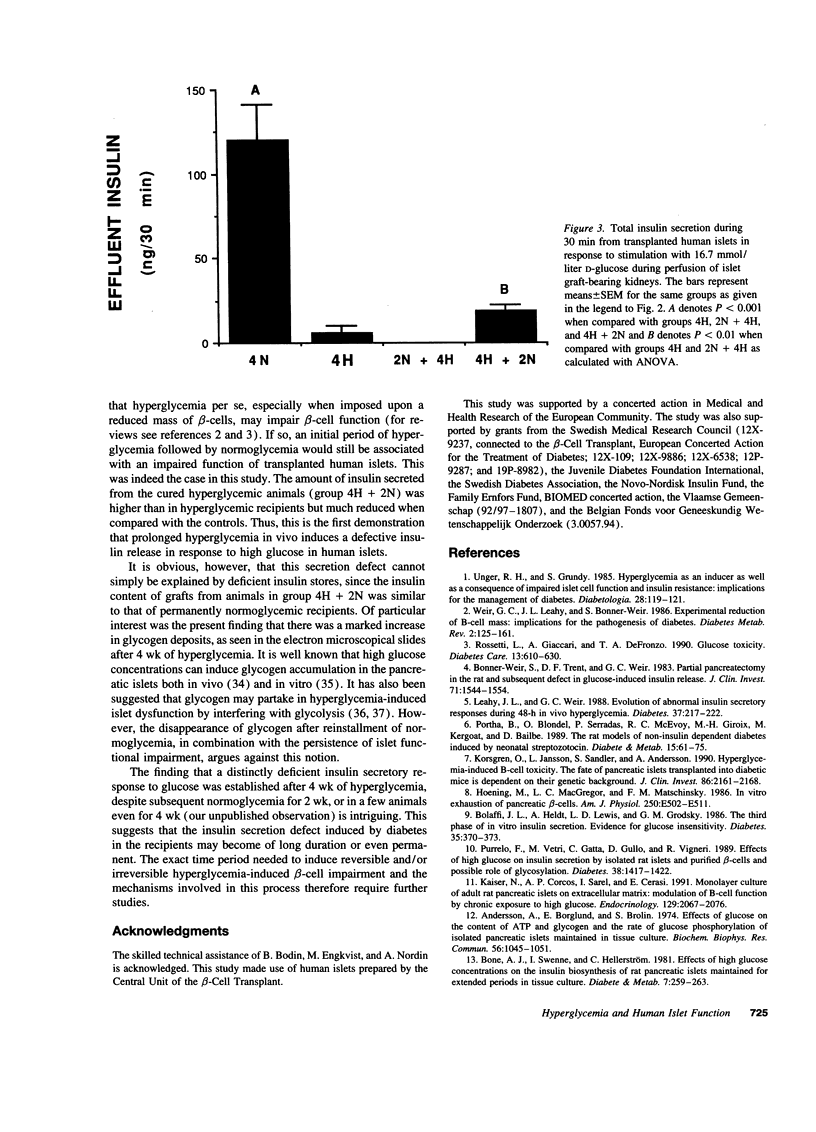
Hyperglycemia-induced beta-cell dysfunction may be an important component in the pathogenesis of non-insulin-dependent diabetes mellitus. However, most available data in this field were obtained from rodent islets. To investigate the relevance of this hypothesis for human beta-cells in vivo, human pancreatic islets were transplanted under the renal capsule of nude mice. Experimental groups were chosen so that grafted islets were exposed to either hyper- or normoglycemia or combinations of these for 4 or 6 wk. Grafts of normoglycemic recipients responded with an increased insulin release to a glucose stimulus during perfusion, whereas grafts of hyperglycemic recipients failed to respond to glucose. The insulin content of the grafts in the latter groups was only 10% of those observed in controls. Recipients initially hyperglycemic (4 wk), followed by 2 wk of normoglycemia regained a normal graft insulin content, but a decreased insulin response to glucose remained. No ultrastructural signs of beta-cell damage were observed, with the exception of increased glycogen deposits in animals hyperglycemic at the time of killing. It is concluded that prolonged exposure to a diabetic environment induces a long-term secretory defect in human beta-cells, which is not dependent on the size of the islet insulin stores.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson A., Borglund E., Brolin S. Effects of glucose on the content of ATP and glycogen and the rate of glucose phosphorylation of isolated pancreatic islets maintained in tissue culture. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1045–1051. doi: 10.1016/s0006-291x(74)80294-9. [DOI] [PubMed] [Google Scholar]
- Andersson A., Eriksson U., Petersson B., Reibring L., Swenne I. Failure of successful intrasplenic transplantation of islets from lean mice to cure obese-hyperglycaemic mice, despite islet growth. Diabetologia. 1981 Mar;20(3):237–241. doi: 10.1007/BF00252634. [DOI] [PubMed] [Google Scholar]
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978 Jun;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- Andersson A., Westman J., Hellerström C. Effects of glucose on the ultrastructure and insulin biosynthesis of isolated mouse pancreatic islets maintained in tissue culture. Diabetologia. 1974 Dec;10(6):743–753. doi: 10.1007/BF01219536. [DOI] [PubMed] [Google Scholar]
- Bolaffi J. L., Heldt A., Lewis L. D., Grodsky G. M. The third phase of in vitro insulin secretion. Evidence for glucose insensitivity. Diabetes. 1986 Mar;35(3):370–373. doi: 10.2337/diab.35.3.370. [DOI] [PubMed] [Google Scholar]
- Bone A. J., Swenne I., Hellerström C. Effects of high glucose concentrations on the insulin biosynthesis of rat pancreatic islets maintained for extended periods in tissue culture. Diabete Metab. 1981 Dec;7(4):259–263. [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget I., Sarri Y., Novials A., Casamitjana R., Vives M., Gomis R. Functional properties of isolated human pancreatic islets beneficial effects of culture and exposure to high glucose concentrations. Diabete Metab. 1994 Mar-Apr;20(2):99–107. [PubMed] [Google Scholar]
- Davalli A. M., Ricordi C., Socci C., Braghi S., Bertuzzi F., Fattor B., Di Carlo V., Pontiroli A. E., Pozza G. Abnormal sensitivity to glucose of human islets cultured in a high glucose medium: partial reversibility after an additional culture in a normal glucose medium. J Clin Endocrinol Metab. 1991 Jan;72(1):202–208. doi: 10.1210/jcem-72-1-202. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Korbutt G. S., Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992 Oct;90(4):1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik D. L., Pipeleers D. G., Ling Z., Welsh N., Hellerström C., Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9253–9256. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik D. L., Strandell E., Sandler S. Culture of mouse pancreatic islets in different glucose concentrations modifies B cell sensitivity to streptozotocin. Diabetologia. 1988 Mar;31(3):168–174. doi: 10.1007/BF00276851. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Hoenig M., MacGregor L. C., Matschinsky F. M. In vitro exhaustion of pancreatic beta-cells. Am J Physiol. 1986 May;250(5 Pt 1):E502–E511. doi: 10.1152/ajpendo.1986.250.5.E502. [DOI] [PubMed] [Google Scholar]
- Kaiser N., Corcos A. P., Sarel I., Cerasi E. Monolayer culture of adult rat pancreatic islets on extracellular matrix: modulation of B-cell function by chronic exposure to high glucose. Endocrinology. 1991 Oct;129(4):2067–2076. doi: 10.1210/endo-129-4-2067. [DOI] [PubMed] [Google Scholar]
- Korsgren O., Jansson L., Andersson A. Effects of hyperglycemia on function of isolated mouse pancreatic islets transplanted under kidney capsule. Diabetes. 1989 Apr;38(4):510–515. doi: 10.2337/diab.38.4.510. [DOI] [PubMed] [Google Scholar]
- Korsgren O., Jansson L., Eizirik D., Andersson A. Functional and morphological differentiation of fetal porcine islet-like cell clusters after transplantation into nude mice. Diabetologia. 1991 Jun;34(6):379–386. doi: 10.1007/BF00403174. [DOI] [PubMed] [Google Scholar]
- Korsgren O., Jansson L., Sandler S., Andersson A. Hyperglycemia-induced B cell toxicity. The fate of pancreatic islets transplanted into diabetic mice is dependent on their genetic background. J Clin Invest. 1990 Dec;86(6):2161–2168. doi: 10.1172/JCI114955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J. L., Weir G. C. Evolution of abnormal insulin secretory responses during 48-h in vivo hyperglycemia. Diabetes. 1988 Feb;37(2):217–222. doi: 10.2337/diab.37.2.217. [DOI] [PubMed] [Google Scholar]
- Madsbad S., Krarup T., Faber O. K., Binder C., Regeur L. The transient effect of strict glycaemic control on B cell function in newly diagnosed type 1 (insulin-dependent) diabetic patients. Diabetologia. 1982 Jan;22(1):16–20. doi: 10.1007/BF00253863. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Sener A., Malaisse W. J. Can desensitization of the B-cell to D-glucose be simulated in cultured pancreatic islets? Acta Diabetol Lat. 1987 Jan-Mar;24(1):17–25. doi: 10.1007/BF02732049. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Maggetto C., Leclercq-Meyer V., Sener A. Interference of glycogenolysis with glycolysis in pancreatic islets from glucose-infused rats. J Clin Invest. 1993 Feb;91(2):432–436. doi: 10.1172/JCI116219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze J., Selam J. L., Pham T. C., Mendoza E., Orsetti A. Sustained insulin-induced remissions of juvenile diabetes by means of an external artificial pancreas. Diabetologia. 1978 Apr;14(4):223–227. doi: 10.1007/BF01219420. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., in't Veld P. A., Van de Winkel M., Maes E., Schuit F. C., Gepts W. A new in vitro model for the study of pancreatic A and B cells. Endocrinology. 1985 Sep;117(3):806–816. doi: 10.1210/endo-117-3-806. [DOI] [PubMed] [Google Scholar]
- Portha B., Blondel O., Serradas P., McEvoy R., Giroix M. H., Kergoat M., Bailbe D. The rat models of non-insulin dependent diabetes induced by neonatal streptozotocin. Diabete Metab. 1989 Mar-Apr;15(2):61–75. [PubMed] [Google Scholar]
- Purrello F., Vetri M., Gatta C., Gullo D., Vigneri R. Effects of high glucose on insulin secretion by isolated rat islets and purified beta-cells and possible role of glycosylation. Diabetes. 1989 Nov;38(11):1417–1422. doi: 10.2337/diab.38.11.1417. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Lacy P. E., Finke E. H., Olack B. J., Scharp D. W. Automated method for isolation of human pancreatic islets. Diabetes. 1988 Apr;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A., DeFronzo R. A. Glucose toxicity. Diabetes Care. 1990 Jun;13(6):610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- Schnell A. H., Swenne I., Borg L. A. Lysosomes and pancreatic islet function. A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell Tissue Res. 1988 Apr;252(1):9–15. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- Shah S. C., Malone J. I., Simpson N. E. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989 Mar 2;320(9):550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- Svensson C., Hellerström C. Long-term effects of a high glucose concentration in vitro on the oxidative metabolism and insulin production of isolated rat pancreatic islets. Metabolism. 1991 May;40(5):513–518. doi: 10.1016/0026-0495(91)90233-m. [DOI] [PubMed] [Google Scholar]
- TORESON W. E. Glycogen infiltration (so-called hydropic degeneration) in the pancreas in human and experimental diabetes mellitus. Am J Pathol. 1951 Mar-Apr;27(2):327–347. [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Leahy J. L., Bonner-Weir S. Experimental reduction of B-cell mass: implications for the pathogenesis of diabetes. Diabetes Metab Rev. 1986;2(1-2):125–161. doi: 10.1002/dmr.5610020108. [DOI] [PubMed] [Google Scholar]
- Westermark P., Eizirik D. L., Pipeleers D. G., Hellerström C., Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995 May;38(5):543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]






