ABSTRACT
Tumors of the skull base are rare in children and adolescents and present a complicated management problem for oncologists and surgeons alike. Surgical resection is an integral component of the management of many pediatric neoplasms, especially those that are benign or, though not frankly malignant, are locally invasive. The general principles of skull base reconstruction following tumor ablation are applicable to nearly all patients; the reconstructive algorithm, however, is particularly complex in the pediatric population and the potential benefits of therapy must be balanced against the cumulative impact on craniofacial growth and maturity and the donor site morbidity. A retrospective analysis of all patients less than 19 years of age who underwent resection of a skull base tumor was performed. Particular emphasis was placed on the 12 patients who required complex reconstruction by the plastic surgical service. This represents approximately a third of the operated patients. Data were recorded on patient age, tumor pathology and location, prior therapies, surgical approach, extent of resection, margin status, defect components, details of reconstructive methods employed, complications, additional procedures or interventions, and the use and timing of adjuvant therapies. Patient outcome at most recent follow-up was recorded. All patients were followed clinically and by MRI and/or CT scan of the skull base. The reconstructive details recorded included flap choice, recipient vessels, and any concomitant procedures performed. The indications for and details of any staged surgical revisions or prosthetics were also noted. Complications recorded included partial or total flap loss, cerebrospinal fluid leakage, meningitis, infection, abscess, hematoma or seroma formation, delayed healing, and donor site dysfunction. The vertical rectus abdominis myocutaneous free flap was the most common means of reconstruction utilized in this series. Three of 12 patients had reconstruction related complications. Delayed reconstructive procedures or prosthetic interventions have been performed in 6 of the 12 patients who underwent complex reconstructions. On the basis of our experience and previous reports in the literature, we offer the following guidelines for the successful multidisciplinary care of children and adolescents undergoing skull base reconstruction after tumor resection: (1) skull base reconstruction may be safely performed in children and adolescents using free tissue transfer or local flaps; (2) larger defects and those involving more than one anatomic region of the skull base should be repaired with soft-tissue free flaps; and (3) because of the versatility and reliability of free flaps, pedicled flaps should be reserved for limited defects. Because of the potentially synergistic effects of multimodality treatment for skull base malignancies on craniofacial growth and development, we advocate soft-tissue reconstruction as the primary technique, reserving bony flaps for definitive procedures in survivors who have reached skeletal maturity.
Keywords: Skull base, pediatric, microvascular, reconstruction
Tumors of the skull base are rare in children and adolescents and present a complicated management problem for oncologists and surgeons alike. Many of the tumors that occur in this age group are chemosensitive and are consequently not referred to the surgeon.1 Conversely, however, surgical resection is an integral component of the management of many pediatric neoplasms, especially those that are benign or, though not frankly malignant, are locally invasive.2,3,4,5,6,7,8
Although the feasibility of various surgical approaches to the skull base has been well established, unique issues remain in the pediatric patient.9,10,11,12,13,14 Similarly, although the general principles of skull base reconstruction following tumor ablation are applicable to nearly all patients, the reconstructive algorithm is particularly complex in the pediatric population and the potential benefits of therapy must be balanced against the cumulative impact on craniofacial growth and maturity and the donor site morbidity.15,16,17,18,19 The potentially significant psychosocial impact on this age group must not be underestimated. Members of a multidisciplinary team consisting of specialists in neurosurgery, head and neck surgery, plastic and reconstructive surgery, dental oncology and prosthodontics, pediatric critical care, and pediatric oncology work in conjunction with child life specialists.
The purpose of this article is to review the experience gained in the reconstructive management of pediatric patients who have undergone resection of skull base tumors at the M.D. Anderson Cancer Center over the past 13 years.
MATERIALS AND METHODS
Databases maintained by the departments of neurosurgery and plastic surgery at the University of Texas M.D. Anderson Cancer Center were queried to identify all patients with tumors of the skull base, who at the time of their surgery were 19 years of age or younger. Thirty-five patients meeting these inclusion criteria were identified over the 13-year interval between November 1992 and November 2005. Of these 35 patients, 12 required complex reconstruction of the ablative defect by the plastic surgical service. Data were recorded on patient age, tumor pathology and location, prior therapies, surgical approach, extent of resection, margin status, defect components, details of reconstructive methods employed, complications, additional procedures or interventions, and the use and timing of adjuvant therapies. Patient outcome at most recent follow-up was recorded. All patients were followed clinically and by MRI and/or CT scan of the skull base. The reconstructive details recorded included flap choice, recipient vessels, and any concomitant procedures performed. The indications for and details of any staged surgical revisions or prosthetics were also noted. Complications recorded included partial or total flap loss, cerebrospinal fluid leakage, meningitis, infection, abscess, hematoma or seroma formation, delayed healing, and donor site dysfunction.
RESULTS
The patient's age at diagnosis ranged from 1 to 19 years with a mean of 12.8 years. The median age of the cohort of patients undergoing complex reconstruction was 11.8 years (range 6.4 to 17.9 yrs). There were 19 males and 16 females. Of these 35 patients, 21 (60%) had been treated with at least one modality of therapy prior to their index surgery. Six patients had undergone tumor-directed surgery, 4 had surgery combined with radiation therapy and chemotherapy, 5 had chemotherapy alone, 4 had radiation and chemotherapy, 1 had surgery and chemotherapy, and 1 had surgery and radiation therapy. All of the patients requiring complex reconstruction had undergone prior treatments (surgery: 5, irradiation: 8, chemotherapy: 10). All but 1 patient with Ollier's disease were free of comorbid medical illnesses. All patients were never-smokers. The tumor pathologies encountered are listed in Table 1, with the most common entities being juvenile nasopharyngeal angiofibroma (6), rhabdomyosarcoma (6), nerve sheath tumors (3), and chondrosarcomas (3). Tumors were predominantly located in the infratemporal fossa in 16 patients (47%). The sinonasal region had some degree of tumor involvement in 21 patients (62%). Several patients had extensive tumors that involved multiple skull base regions.
Table 1.
Tumor Locations, Types, and Surgical Approaches in 35 Patients
| ANATOMICAL REGION | FREQUENCY |
| Infratemporal fossa | 16 |
| Orbit | 7 |
| Temporal bone | 6 |
| Ethmoid sinus | 5 |
| Nasal cavity | 5 |
| Sphenoid sinus | 5 |
| Maxillary sinus | 5 |
| Cavernous sinus | 4 |
| Middle cranial fossa | 4 |
| Anterior cranial fossa | 3 |
| Jugular foramen | 3 |
| Posterior skull base | 3 |
| Cerebellopontine angle | 3 |
| Clivus | 2 |
| PATHOLOGY | |
| Benign | |
| Juvenile nasopharyngeal angiofibroma | 6 |
| Nerve sheath tumor | 3 |
| Glomus vagale | 1 |
| Hemangioma | 1 |
| Neurocristic hamartoma | 1 |
| Teratoma | 1 |
| Malignant | |
| Embryonal rhabdomyosarcoma | 6 |
| Desmoid tumor | 2 |
| Chordoma | 2 |
| Squamous cell carcinoma | 1 |
| Liposarcoma | 1 |
| Ewing's sarcoma | 1 |
| Eosinophilic granuloma | 1 |
| Myofibroblastic sarcoma | 1 |
| Olfactory neuroblastoma | 1 |
| Metastatic mesenchymal chondrosarcoma | 1 |
| Mucoepidermoid carcinoma | 1 |
| Malignant fibrous histiocytoma | 1 |
| Chondroblastic osteosarcoma | 1 |
| Chondrosarcoma | 1 |
| Mesenchymal chondrosarcoma | 1 |
| OPERATIVE APPROACHES | |
| Cranio-orbitozygomatic and transfacial | 11 |
| Cranio-orbitozygomatic | 7 |
| Transtemporal | 7 |
| Transfacial | 6* |
| Subtemporal-infratemporal fossa | 6† |
| Bifrontal craniotomy | 3‡ |
| Suboccipital craniotomy | 1 |
Includes 2 transorbital, 2 transmandibular, and 1 trans-sphenoidal approach.
Includes 1 transzygomatic approach.
With lateral rhinotomy in 1 patient.
Forty-one operations were performed in 35 patients. Most patients only required a single surgery but 4 patients had two operations, and 1 patient had three tumor-directed surgeries. Surgical approaches included the cranio-orbitozygomatic approach and its variations in 7 patients, transfacial approaches in 6, combined cranio-orbitozygomatic and transfacial approaches in 11, transtemporal procedures in 7, a subtemporal-infratemporal fossa approach in 6, bifrontal craniotomy in 3, and a suboccipital craniotomy in 1 patient (Table 1). All patients had bony reconstruction with rigid microplate fixation. Of the patients undergoing complex reconstruction, through-and-through defects involving both the mucosa of the upper aerodigestive tract and the facial skin (excluding the orbital contents) were created in 4 patients, and isolated mucosal defects were present in 3 patients. The dura was exposed but intact in 10 patients, exposed and repaired primarily in 1, and repaired with an allograft in 1. See Table 2.
Table 2.
Defect Location and Dural Status
| Characteristic | Number of Patients |
|---|---|
| Location of postsurgical defect* | |
| Region I | 3 |
| Region II | 4 |
| Region III | 2 |
| Combined regions I + II | 3 |
| Dural status | |
| Intact and exposed | 10 |
| Primary repair | 1 |
| Graft repair | 1 |
Based on the classification system of Irish et al.35
A gross total resection was achieved in 32 surgical procedures. A subtotal resection was achieved in 7 procedures. One patient was operated upon twice to obtain a biopsy of a petrous apex mass and was subsequently treated with chemotherapy and radiation therapy (Ewing's sarcoma). All patients undergoing complex reconstruction had a gross total resection.
METHODS OF DEFECT REPAIR
The majority of patients underwent reconstruction using simple rotational flaps by the neurosurgical or head and neck team. Rotational pericranial flaps and split temporalis muscle flaps were the most commonly employed means of reconstruction.
When complex reconstruction by the plastic surgery team was required, the surgical defects resulting from tumor ablation were closed with free flaps in nine patients and regional flaps in three patients. Eleven of the flap procedures were performed concomitant with tumor extirpation; one patient underwent delayed reconstruction of the temporal and infratemporal fossa after primary wound healing because of a poor cosmetic result from dramatic temporal hollowing.
The vertical rectus abdominis myocutaneous (VRAM) flap, employed in six cases, was the most common flap used for immediate reconstruction. The single delayed reconstruction was also performed using a VRAM flap. Other flaps used for immediate reconstruction were a latissimus dorsi myofascial free flap, two temporalis muscle flaps, and one serratus anterior myofascial free flap. The temporalis muscle flaps were used in patients with extended orbital exenteration who had minimal dural exposure and no volume loss in the maxilla. One patient with a defect of the posterior skull base underwent reconstruction with multiple rotational muscle flaps including the trapezius, splenius capitis, and semispinalis capitis.
Eight of the 12 patients required ancillary reconstructive procedures at the time of definitive reconstruction. In 4 patients, split-thickness skin grafts were applied to exposed muscle flaps in the orbit. One of these 4 patients also underwent reconstruction of the orbital rim and floor with a split calvarial bone graft following radical orbital exenteration. One patient had a surgical obturator placed in conjunction with a VRAM flap for repair of a radical orbitomaxillectomy defect. In 1 patient, following anterior craniofacial resection, a pericranial flap was also used to separate the intradural and extradural contents. One patient underwent primary placement of a 2.4-mm reconstruction plate wrapped with a VRAM flap to maintain mandibular contour after hemimandibulectomy. One patient who underwent reconstruction with a temporalis flap required augmentation of the donor defect in the temporal fossa with methylmethacrylate.
In the nine patients who underwent reconstruction using free flaps, all vessels, both flap and recipient, were at least 1.5 mm in diameter. In all nine patients, arterial anastomoses were performed to the external carotid artery or its branches. In all nine patients, venous anastomoses were performed to the internal jugular vein or its branches, with single venous anastomoses employed in eight patients and a second vein used in only one.
The choice of anastomotic technique varied according to the preference of the reconstructive surgeon. There was an equal distribution of end-to-end and end-to-side anastomoses. Either 9–0 or 10–0 nylon was used for all vessels, and there were equal numbers of running and interrupted suture patterns. No vein grafts were required. Patients were not routinely treated with anticoagulation, although heparinized saline was used intraoperatively for irrigation in all cases.
COMPLICATIONS AFTER RECONSTRUCTION
Of the patients undergoing simple reconstruction by the ablative service (n = 23), five potentially reconstruction-related complications were noted (22%). One patient developed a cerebrospinal fluid leak following a left transtemporal approach for resection of a nerve sheath tumor of the facial nerve. Lumbar subarachnoid drainage over 4 days led to resolution of the leak. Closure had been accomplished with a split temporalis rotational flap. Both patients with clival chordomas who required transpalatal exposure developed small palatal fistulas, a recognized risk of this procedure.20 In both patients spontaneous closure was achieved using palatal splints. Delayed revision was required in one patient following a temporalis muscle rotational flap due to marked temporal scalloping. A temporal implant was placed to improve contour. One 18-month-old patient developed jaw deviation and inadequate zygomatic growth following an orbitozygomatic approach to an infratemporal fossa desmoid tumor.
Four of the 12 patients requiring complex reconstruction developed postoperative complications, 3 of which were potentially related to the reconstruction. (Table 3) There was 1 flap loss, due to thrombosis of the artery and vein on postoperative day 5 after latissimus dorsi flap repair of an orbitomaxillectomy defect. The flap could not be salvaged, and it was replaced with a VRAM free flap. The patient was treated with systemic heparin therapy but developed an arterial thrombosis on postoperative day 3 after VRAM flap reconstruction. Although the VRAM flap was successfully salvaged, continued systemic anticoagulation resulted in the development of hematomas at the latissimus dorsi donor site and in the neck that required evacuation. The patient was ultimately identified as having an antithrombin III deficiency necessitating long-term anticoagulation with low-molecular-weight heparin. There was 1 case of cellulitis of the neck that resolved with intravenous antibiotics during the first postoperative week. The 10-year-old child who required multiple rotational flaps for a posterior skull base defect developed postoperative torticollis that slowly resolved over several weeks. Other delayed complications include ectropion in 1 patient and 2 cases of partial loss of the split-thickness skin graft overlying muscle in patients who had undergone orbital reconstruction. In both of these patients, the wound healed by secondary intention. No patient developed symptomatic donor site weakness and several have been active in sports and school activities. Two patients required intervention by child psychology and psychiatry for depression.
Table 3.
Complications
| Complication | Number of Patients (%) |
|---|---|
| Acute | |
| Flap loss | 1 (8%) |
| Pedicle thrombosis | 1 (8%)* |
| Infection | |
| Cellulitis | 1 (8%) |
| Abscess | 0 |
| Fistula | 0 |
| Hematoma | 1 (8%) |
| Donor site morbidity | |
| Hematoma | 1 (8%) |
| Seroma | 0 |
| Torticollis | 1 (8%) |
| Late | |
| Delayed healing | 2 (17%) |
| Infection | 0 |
| Ectropion | 1 (8%) |
| Meningitis | 1 (8%)† |
One patient experienced three separate acute complications, including flap loss due to thrombosis of the artery and vein. The flap was successfully salvaged with a VRAM flap that developed an arterial thrombus on the third postoperative day. The thrombus was evacuated, and the flap was salvaged.
The patient with meningitis succumbed to nadir sepsis 5 weeks postoperatively after restarting chemotherapy.
OTHER COMPLICATIONS
One patient developed meningitis (Pseudomonas aeroginosa) more than 30 days after left orbitocranial craniotomy for resection of an orbital rhabdomyosarcoma and free flap reconstruction and ultimately succumbed to nadir sepsis. This patient had an uncomplicated postoperative course, achieving primary wound healing, and had begun adjuvant chemotherapy 3 weeks after reconstruction. She became profoundly neutropenic as a result of the adjuvant therapy prior to the development of meningitis. The postoperative course of the patient with an endocrinologically active multifocal glomus tumor was complicated by episodes of hypertension and dysphagia that significantly improved within 7 days after the surgery. Transient neuropathies of the maxillary division of the trigeminal nerve (n = 2) and of the facial nerve (n = 1) cleared within the first postoperative week. One patient had transient postoperative diabetes insipidus. Unilateral sensorineural hearing loss in one patient, a new hypoglossal neuropathy in a second patient, and new ophthalmic and maxillary neuropathies in the patient with a cavernous sinus hemangioma occurred. One patient developed spontaneous thrombosis of the sigmoid sinus-jugular bulb-jugular vein complex requiring 6 months of anticoagulation. Although not classified as complications, the cranial nerves of two patients with tumor-infiltrated facial nerves and two patients with tumor-infiltrated maxillary nerves were resected. Complete facial and maxillary neuropathies were present, as expected, postoperatively.
OUTCOME
Recurrence developed in 11 patients after a median of 6.97 years from the surgery at M.D. Anderson Cancer Center. The tumor recurred locally in 7 patients, locally and regionally in 1, regionally in 1, at a second site in 1 and distantly in 1. One patient developed acute myeloblastic leukemia (AML). The patient with distant metastases (ERMS) did not receive further treatment. The 2 patients with locally recurrent chordoma underwent irradiation (1 with protons and 1 with intensity modulated radiation therapy [IMRT]) and the patient with a locally and regionally recurrent glomus tumor had both the temporal bone and neck irradiated. One of the patients with chordoma subsequently progressed. Four of the 6 patients with local recurrence (ERMS, mesenchymal chondrosarcoma, desmoid, teratoma), the 1 patient with a regional recurrence (liposarcoma), and the 1 patient with tumor at a second site (squamous cell carcinoma) underwent reoperation. A gross total resection was accomplished in each. Subsequent to this second resection, the patient with the squamous cell carcinoma was radiated and 1 patient received combination chemotherapy and radiation therapy (ERMS). A second recurrence was noted in 3 patients at a mean interval of 6.3 months. Two recurrences were local (teratoma, liposarcoma) and 1 was local and distant (mesenchymal chondrosarcoma). The 2 patients with local recurrence underwent a second gross total resection with postoperative radiation added in 1 (liposarcoma) and chemotherapy with cis-retinoic acid in the other (teratoma).
At the time of our review, with a median follow-up period of 35.5 months (range 1 to 144 months), 24 patients had no evidence of tumor, while another 7 patients were alive with residual disease (6 of 7 with stable disease, 1 with progressive disease). Four patients died during the follow-up period. One patient died within 30 days of surgery secondary to septicemia and meningitis (see above). Two patients died 8 and 13 months after their surgery, respectively, due to systemic metastases of their tumors. One patient died 37 months after surgery of AML. All patients with benign tumors of the skull base were still alive at the time of this review, whereas 4 of 22 patients with malignancy have died. The overall 1-year and 5-year actuarial survival for this series of 35 patients with tumors of the skull base was 91% and 86%, respectively. Overall survival for those patients with benign tumors was 100% at both 1 year and 5 years, while those patients with malignant tumors had 1-year and 5-year overall survival rates of 85% and 78%, respectively. The median progression-free survival was 6.97 years (95% CI, 1.17 to 12.77 yrs). Progression-free survival for those patients with benign tumors was 92% at 1 year and 82% at 5 years. Patients with malignancies fared less well, with progression-free survivals of 81% at 1 year and 40% at 5 years.
Delayed reconstructive procedures or prosthetic interventions have been performed in 6 of the 12 patients who underwent complex reconstructions. One patient developed a relative enophthalmos because of excessive volume restoration achieved in the temporal fossa following VRAM flap reconstruction. This was corrected with a radial forearm osteocutaneous free flap that provided support to the orbit and additional bulk in the malar region. A second patient, after undergoing a successful extirpation of bulky neurofibroma from the infratemporal fossa and repair of the resulting defect, underwent a second-stage procedure to resect the superficial extension of this lesion into her midface. The resultant defect was closed with another free latissimus dorsi flap, and a static sling was performed because nearly all the caudal muscles of facial expression had been ablated. Eventually, this patient was evaluated for an orbitofacial prosthesis, as she had undergone a prior orbitectomy.
Two patients have been fit with palatal obturators after undergoing radical orbitomaxillectomies. One patient required debulking of the intraoral component of his flap so that a palatal obturator could be affixed to his remaining dentition to promote correction of hypernasality. Although one patient initially wore a mandibular partial prosthesis, it was unstable and difficult to wear. Therefore, her 2.4-mm plate was replaced with a free fibula osteocutaneous flap to improve the contour of the mandibular angle and provide a stable, vascularized base for dental implants without removal of the previously placed VRAM flap.
CASE ILLUSTRATION
At age 9½ years this boy was diagnosed with a right maxillary sinus rhabdomyosarcoma following biopsy of an expanding cheek mass. He was treated with vincristine, doxorubicin, topotecan, and cisplatin chemotherapy and received 55 Gy of external beam irradiation with complete radiographic remission. Three years later recurrence was identified with parameningeal involvement. He received irinotecan and vincristine chemotherapy and subsequent cyclophosphamide, doxorubicin, and tirapazamine chemotherapy but tumor progression was seen. The chemotherapy was switched to ifosfamide, carboplatin, and etoposide with stabilization of his disease. He underwent a complete surgical resection of the tumor via a right cranio-orbitozygomatic and transfacial approach with complete orbitomaxillectomy, posterior mandibulectomy, and middle fossa floor resection. The dura was exposed but intact (Fig. 1). Soft-tissue reconstruction was accomplished with a free VRAM flap with skin paddles for both the intraoral defect (marked with dot) and the orbital defect (Figs. 2–4). The vein was anastamosed to the facial vein and the artery to the superior thyroid artery, both in an end-to-end fashion. Pre- and postoperative MRIs and CTs are shown in Figure 5. Intraoral debulking of the flap was subsequently needed to place a palatal obturator to correct hypernasality. Following surgery the patient was treated with two further courses of chemotherapy using ifosfamide, carboplatin, and etoposide. He has remained without evidence of disease over these past 2 years.
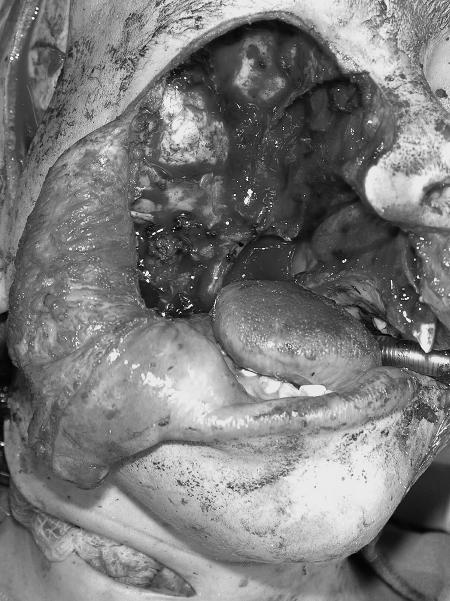
Figure 1.
Intraoperative photograph following resection of parameningeal recurrent rhabdomyosarcoma. Note the exposed temporal dura in continuity with the oropharynx. (Figure is property of The Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center and is used with permission.)
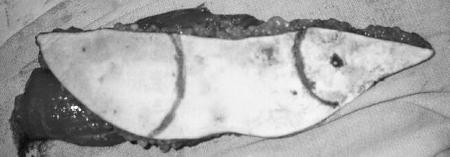
Figure 2.
Intraoperative photograph of harvested VRAM flap. Two skin paddles have been marked. The skin paddle with the dot was used to reconstruct the palate while the other skin paddle reconstructed the orbitectomy defect. (Figure is property of The Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center and is used with permission.)
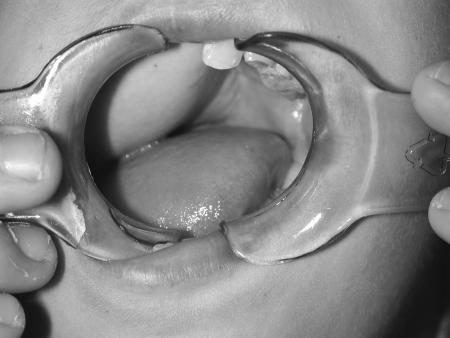
Figure 3.
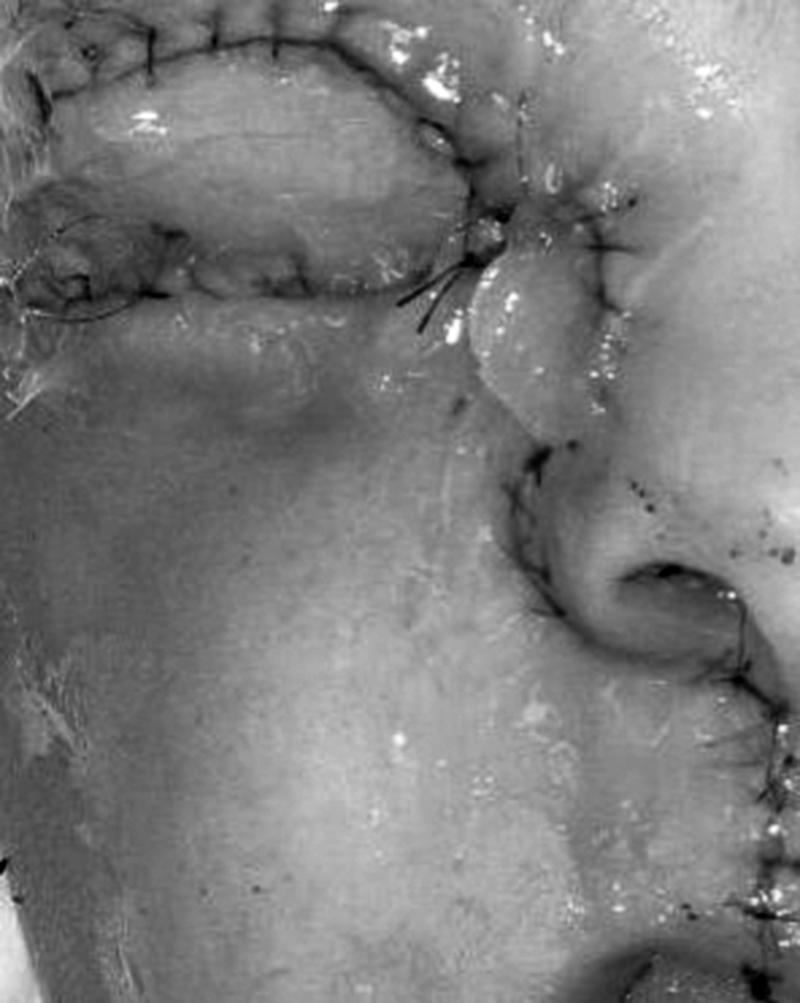
Intraoperative photograph of the external appearance of the reconstructed defect. (Figure is property of The Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center and is used with permission.)
Figure 4.
Postoperative photograph of the intraoral reconstruction. Surgical debulking of the intraoral portion of the flap was subsequently performed to allow for placement of an obturator to help correct the patient's hypernasality. (Figure is property of The Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center and is used with permission.)
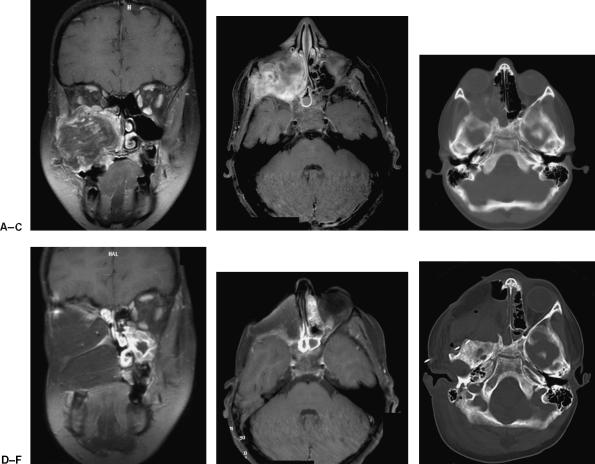
Figure 5.
Preoperative (A) coronal and (B) axial contrast-enhanced MRI as well as preoperative (C) CT reveal a large recurrent rhabdomyosarcoma centered in the maxillary sinus with extension into the orbit, sphenoid wing, and infratemporal fossa. Postoperative (D) coronal and (E) axial contrast-enhanced MRI as well as postoperative (F) CT show the extent of soft-tissue and skull base resection and the subsequent reconstruction using the VRAM flap. (Figure is property of The Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center and is used with permission.)
DISCUSSION
There are important differences in skull base surgery between children and adults. In children, the cranial bone is thinner, prompting a reconsideration of the methods of stabilization during surgery; the floor of the frontal and middle cranial fossa may be flatter; and common surgical landmarks may be absent or altered.9 For instance, the pterion is located more anteriorly in children, and the supraorbital notch or foramen may be absent before the age of 8 years. The indications for skull base surgery and subsequent reconstruction are also different in children, as children are more likely to suffer from sarcomas than adults and tumors are more likely to involve the middle cranial fossa.5 Pediatric skull base surgery and reconstruction comprises only a fraction of the multimodality treatment to which these children are subjected, and the ultimate cosmetic and functional outcome will be determined by the complex interplay between craniofacial development, the effects of chemotherapy and radiation, the extent of surgical resection, and the reconstruction method(s) employed.5,17,21,22,23,24,25,26
We found that approaches utilizing osteotomies of the orbital rims and/or zygoma, usually in concert with a craniotomy, were the most commonly employed means of access to the skull base tumors in our series (18/41). Transfacial approaches, in combination with the cranio-orbitozygomatic approach (11/41), or alone (6/41), were also found to be useful. These osteotomies were not problematic except in 1 patient aged 18 months, in whom zygomatic osteotomies resulted in asymmetric facial growth suggesting that it may be necessary to avoid the use of this approach in very young patients. Several authors have addressed the risk of altering craniofacial growth patterns with facial osteotomies in children. Teo and colleagues14 in their report based on 26 pediatric patients found that no long-term disturbances of facial growth patterns developed following disruption of the skull base. This confirmed results reported by Lang and associates13 a year earlier. Both surgical teams primarily utilized orbital and zygomatic osteotomies in their patient population, although no patient under 4 years of age had zygomatic osteotomies performed in either surgical series. At younger than 4 years of age craniofacial growth has achieved less than 80% of its adult state.25 Taken together, the data suggest that zygomatic osteotomies in children younger than 4 years of age may cause significant alteration in growth, whereas in children over the age of 4 to 5 years such osteotomies may result in only very subtle asymmetries,25,27 and no craniofacial growth asymmetries should be expected in children over 5 years old. In keeping with our data in the adult population, we observed no evidence of disturbed orbital growth among our pediatric patients having orbital osteotomies.28
Although we did not use the Le Fort I osteotomy approach in our pediatric series, it should be noted that Lewark and coworkers29 described the loss of several tooth buds in children younger than 5 due to the placement of the horizontal transmaxillary osteotomy integral to this approach. Although they did not see problems with facial growth, this study supports the age of 4 to 5 years as being critical to increased growth-related complications with skull base approaches.
Approaches through the temporal bone were used in seven patients. Combined middle fossa and transmastoideal (presigmoid/retrolabyrinthine) approaches were used in two patients. The infratemporal fossa approach was used alone in one patient and one patient underwent both a middle fossa approach and a subsequent infratemporal fossa approach. Similar approaches were employed by Jackson et al6 and Cunningham and associates3 in their pediatric patients undergoing neuro-otologic skull base surgery. Complications of cerebrospinal fluid leak, hearing loss, and transient facial neuropathy, as seen in our patient population, were also encountered. In children the mastoid air cells are typically less well developed, making the identification of the labyrinth more difficult. The expertise of an experienced neuro-otologist is required.
Rigid microplate fixation of the cranium, including the orbit and zygoma, was used in all patients without complication. Plate translocation did not occur, nor did any plates become palpable, both reported complications of this technique.30,31 Nevertheless, we agree that use of resorbable microplate fixation is an alternative and that excellent results can be achieved. Their use is probably most indicated in children less than 5 years of age.32,33
In general, pediatric skull base reconstruction follows the principles of adult skull base reconstruction. Free tissue transfer has been demonstrated to be the preferable technique in several large reviews, as it promotes improved primary wound healing of skull base defects compared with regional or pedicled flaps. The goals of skull base reconstruction in children are similar to those in adults: achieve a dural seal, cover and support neural and vascular structures, separate the intracranial contents from the contaminated environment of the oral and nasal cavities, re-establish the oral or nasal cavity while restoring three-dimensional appearance, and maintain function. Furthermore, the need for secondary procedures to improve contour has been noted since the early days of free tissue transfer for skull base defects or deformities.15,34
In our series of 12 children and adolescents with ablative skull base deficits, 11 patients underwent immediate reconstruction, and 1 patient underwent delayed primary reconstruction because of an unsatisfactory cosmetic result shortly after tumor extirpation. These 12 patients requiring primary reconstruction comprise 34% of all children and adolescents undergoing skull base surgery at our comprehensive cancer center over 13 years. Eleven of the 12 reconstructions were performed following resection of malignant neoplasms, with rhabdomyosarcoma representing the most frequently encountered histologic subtype. Despite the predilection for the middle cranial base of skull base tumors in children and adolescents, the resultant surgical defects were equally distributed between regions I and II, and the dura was exposed in all cases.5,35
To optimize chances for primary wound healing, primary reconstruction was performed with free tissue transfer in nine patients; the only patients in whom pedicled flaps were employed had defects limited to the orbit and floor of the anterior cranial fossa and to a lateral occipital defect. The VRAM flap was the most commonly used flap. Adjunctive reconstructive techniques, such as split-thickness skin grafts, were readily employed if clinically indicated. The size of both recipient and flap vessels was noted to be adequate in all cases, and no vein grafts were required to anastomose the flap to the external carotid and internal jugular systems.
It is our practice to repair skull base defects in children with soft-tissue flaps as the initial reconstructive modality. Although we acknowledge that bony reconstruction is an option, it is our belief that soft-tissue reconstruction provides the most reliable way to meet the fundamental goals of skull base reconstruction. For mandibular or midface defects amenable to bony reconstruction, our preference is to bridge the area with a plate and/or soft tissue in the acute setting, reserving definitive bony reconstruction until the patient reaches puberty.17
Although skull base surgery in children has not clearly demonstrated an adverse effect on craniofacial development, nonoperative modalities such as radiation therapy are associated with significant negative consequences.5,14 The effects of radiation on pediatric patients with skull base malignancies have been shown to include retardation of facial bone and soft-tissue growth, short stature, visual or auditory dysfunction, dental abnormalities, and various endocrinopathies, and these sequelae can affect both the donor and recipient sites.22,23,24,36,37,38 With nearly half of pediatric patients with skull base tumors demonstrating some adverse effect of radiation, some groups have advocated more aggressive surgical resections to achieve a gross total resection in an attempt to avoid or delay radiation therapy.1,5,39,40,41 Although a fibula free flap may continue to grow with the adjacent mandible or maxilla, the growth of adjacent recipient tissues may be altered; furthermore, harvest of a fibula free flap prior to skeletal maturity may result in tibial overgrowth or a valgus deformity.16 In all of the patients in our series who received radiation therapy as part of their tumor management, we anticipated asymmetric craniofacial growth and decided to defer bony reconstruction until the disparity was defined and the patient had reached puberty.
Although a definitive psychosocial assessment was not performed, two patients required the services of the child psychology and child psychiatry services for depression and related substance abuse. This is likely a gross underestimation of the mental health issues that these children face, as they must cope with not only their disease process and its prognosis but also the physical and psychological ramifications of their postoperative appearance in the challenging environment of childhood and adolescence. This underscores the importance of having a multidisciplinary team of physicians, surgeons, psychologists, and child life specialists involved in caring for children with skull base tumors.
CONCLUSIONS
Tumors of the cranial base are rare in the pediatric population. Gross total surgical resection is the optimal treatment of benign lesions of the skull base and for most low-grade malignancies. The surgical approach must be individualized based on the age of the patient, the tumor location, and the pathology. The role of resection in the management of high-grade soft-tissue sarcomas is usually reserved for persistent disease following chemotherapy, although total resection, if possible, should be considered as primary therapy to place the patient in a better outcome group. Multidisciplinary care in centers experienced in the management of these complex tumors is of utmost importance.
On the basis of our experience reported here and previous reports in the literature, we offer the following guidelines for the successful multidisciplinary care of children and adolescents undergoing skull base reconstruction after tumor resection:
Skull base reconstruction may be safely performed in children and adolescents using free tissue transfer or local flaps;
Larger defects and those involving more than one anatomic region of the skull base should be repaired with soft-tissue free flaps;
Because of the versatility and reliability of free flaps, pedicled flaps should be reserved for limited defects;
Because of the potentially synergistic effects of multimodality treatment for skull base malignancies on craniofacial growth and development, we advocate soft-tissue reconstruction as the primary technique, reserving bony flaps for definitive procedures in survivors who have reached skeletal maturity.
Regardless of the method of initial reconstruction, surviving patients will require additional interventions, potentially including additional free flaps and prosthodontics. Given the psychological issues that children and adolescents with skull base malignancies face, experts in child psychology or psychiatry should be involved in these patients' care.
REFERENCES
- Raney R B, Anderson J R, Barr F G, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of Intergroup Rhabdomyosarcoma Study Group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001;23:215–220. doi: 10.1097/00043426-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Bales C, Kotapka M, Loevner L A, et al. Craniofacial resection of advanced juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head Neck Surg. 2002;128:1071–1078. doi: 10.1001/archotol.128.9.1071. [DOI] [PubMed] [Google Scholar]
- Cunningham C D, Friedman R A, Brackmann D E, et al. Neurotologic skull base surgery in pediatric patients. Otol Neurotol. 2005;26:231–236. doi: 10.1097/00129492-200503000-00017. [DOI] [PubMed] [Google Scholar]
- Donald P J, Enepikedes D, Boggan J. Giant juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head Neck Surg. 2004;130:882–886. doi: 10.1001/archotol.130.7.882. [DOI] [PubMed] [Google Scholar]
- Hanbali F, Tabrizi P, Lang F F, DeMonte F. Tumors of the skull base in children and adolescents. J Neurosurg. 2004;100(suppl 2):169–178. doi: 10.3171/ped.2004.100.2.0169. [DOI] [PubMed] [Google Scholar]
- Jackson C G, Pappas D G, Jr, Manolidis S, et al. Pediatric neurotologic skull base surgery. Laryngoscope. 1996;106:1205–1209. doi: 10.1097/00005537-199610000-00005. [DOI] [PubMed] [Google Scholar]
- Perez-Cruet M J, Burke J M, Weber R, et al. Aggressive fibromatosis involving the cranial base in children. Neurosurgery. 1998;43:1096–1102. doi: 10.1097/00006123-199811000-00050. [DOI] [PubMed] [Google Scholar]
- Tsai E C, Santoreneos S, Rutka J T. Tumors of the skull base in children: review of tumor types and management strategies. Neurosurg Focus. 2002;12:e1. doi: 10.3171/foc.2002.12.5.2. [DOI] [PubMed] [Google Scholar]
- Brockmeyer D, Gruber D P, Haller J, et al. Pediatric skull base surgery: experience and outcomes in 55 pateints. Pediatr Neurosurg. 2003;38:9–15. doi: 10.1159/000067563. [DOI] [PubMed] [Google Scholar]
- Bruce D A. In: Albright L, Pollack I, Adelson D, editor. Principles and Practice of Pediatric Neurosurgery. New York, NY: Thieme; 1999. Skull base tumors in children. pp. 663–684.
- Holmes A D, Klug G L, Breidahl A F. The surgical management of osseous cranial base tumours in children. Aust NZJ Surg. 1997;67:722–730. doi: 10.1111/j.1445-2197.1997.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Kennedy J D, Haines S J. Review of skull base surgery approaches: with special reference to pediatric patients. J Neurooncol. 1994;20:291–312. doi: 10.1007/BF01053045. [DOI] [PubMed] [Google Scholar]
- Lang D A, Neil-Dwyer G, Evans B T, et al. Craniofacial access in children. Acta Neurochir (Wien) 1998;140:33–40. doi: 10.1007/s007010050054. [DOI] [PubMed] [Google Scholar]
- Teo C, Dornhoffer J, Hanna E, et al. Application of skull base techniques to pediatric neurosurgery. Childs Nerv Syst. 1999;15:103–109. doi: 10.1007/s003810050343. [DOI] [PubMed] [Google Scholar]
- Chang D W, Langstein H N, Gupta A, et al. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107:1346–1355. doi: 10.1097/00006534-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Genden E M, Buchbinder D, Chaplin J M, et al. Reconstruction of the pediatric maxilla and mandible. Arch Otolaryngol Head Neck Surg. 2000;126:293–300. doi: 10.1001/archotol.126.3.293. [DOI] [PubMed] [Google Scholar]
- Moore B A, DeMonte F, Robb G L, Chang D W. Reconstruction of ablative skull base defects in the pediatric population. Plast Reconstr Surg. doi: 10.1097/01.prs.0000270312.84787.d1. In press. [DOI] [PubMed] [Google Scholar]
- Serletti J M, Schingo V A, Deuber M A, et al. Free tissue transfer in pediatric patients. Ann Plast Surg. 1996;36:561–568. doi: 10.1097/00000637-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Uusitalo M, Ibarra M, Fulton L, et al. Reconstruction with rectus abdominis myocutaneous free flap after orbital exenteration in children. Arch Ophthalmol. 2001;119:1705–1709. doi: 10.1001/archopht.119.11.1705. [DOI] [PubMed] [Google Scholar]
- DeMonte F, Diaz E, Jr, Callender D, Suk I. Transmandibular, circumglossal, retropharyngeal approach for chordomas of the clivus and upper cervical spine: technical note. Neurosurg Focus. 2001;10:e10. doi: 10.3171/foc.2001.10.3.11. [DOI] [PubMed] [Google Scholar]
- Kumar M, Fallon R J, Hill J S, et al. Esthesioneuroblastoma in children. J Pediatr Hematol Oncol. 2002;24:482–487. doi: 10.1097/00043426-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Paulino A C, Simon J H, Zhen W, Wen B C. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2000;48:1489–1495. doi: 10.1016/s0360-3016(00)00799-9. [DOI] [PubMed] [Google Scholar]
- Raney R B, Anderson J R, Kollath J, et al. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984–1991. Med Pediatr Oncol. 2000;34:413–420. doi: 10.1002/(sici)1096-911x(200006)34:6<413::aid-mpo6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Raney R B, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and -III. Med Pediatr Oncol. 1999;33:362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Sgouros S, Natarajan K, Hockley A D, et al. Skull base growth in childhood. Pediatr Neurosurg. 1999;31:259–268. doi: 10.1159/000028873. [DOI] [PubMed] [Google Scholar]
- Supance J S, Seid A B. Craniofacial resection for ethmoid carcinoma in children. Int J Pediatr Otorhinolaryngol. 1981;3:185–194. doi: 10.1016/0165-5876(81)90001-x. [DOI] [PubMed] [Google Scholar]
- Farkas L G, Posnick J C, Hreczko T M. Anthropometric growth study of the head. Cleft Palate Craniofac J. 1992;29:303–308. doi: 10.1597/1545-1569_1992_029_0303_agsoth_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- DeMonte F, Tabrizi P, Culpepper S A, et al. Ophthalmological outcome after orbital entry during anterior and anterolateral skull base surgery. J Neurosurg. 2002;97:851–856. doi: 10.3171/jns.2002.97.4.0851. [DOI] [PubMed] [Google Scholar]
- Lewark T M, Allen G C, Chowdhury K, et al. Le Fort I osteotomy and skull base tumors: a pediatric experience. Arch Otolaryngol Head Neck Surg. 2000;126:1004–1008. doi: 10.1001/archotol.126.8.1004. [DOI] [PubMed] [Google Scholar]
- Fearon J A, Munro I R, Bruce D A. Observations on the use of rigid fixation for craniofacial deformities in infants and young children. Plast Reconstr Surg. 1995;95:634–637. [PubMed] [Google Scholar]
- Goldberg D S, Bartlett S P, Yu J C, et al. Critical review of microfixation in pediatric craniofacial surgery. J Craniofac Surg. 1995;6:301–307. doi: 10.1097/00001665-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Eppley B L, Sadove A M, Havlik R J. Resorbable plate fixation in pediatric craniofacial surgery. Plast Reconstr Surg. 1997;100:1–7. doi: 10.1097/00006534-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Imola M J, Hamlar D D, Shao W, et al. Resorbable plate fixation in pediatric craniofacial surgery. Arch Facial Plast Surg. 2001;3:79–80. doi: 10.1001/archfaci.3.2.79. [DOI] [PubMed] [Google Scholar]
- Neligan P C, Mulholland S, Irish J, et al. Flap selection in cranial base reconstruction. Plast Reconstr Surg. 1996;98:1159–1166. doi: 10.1097/00006534-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Irish J C, Gullane P J, Gentili F, et al. Tumors of the skull base: outcome and survival analysis of 77 cases. Head Neck. 1994;16:3–10. doi: 10.1002/hed.2880160103. [DOI] [PubMed] [Google Scholar]
- Benk V, Liebsch N J, Munzenrider J E, et al. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys. 1995;31:577–581. doi: 10.1016/0360-3016(94)00395-2. [DOI] [PubMed] [Google Scholar]
- Guyuron B, Dagys A P, Munro I R, et al. Effect of irradiation on facial growth: a 7- to 25-year follow-up. Ann Plast Surg. 1983;11:423–427. doi: 10.1097/00000637-198311000-00010. [DOI] [PubMed] [Google Scholar]
- Hug E B, Sweeney R A, Nurre P M, et al. Proton radiotherapy in management of pediatric base of skull tumors. Int J Radiat Oncol Biol Phys. 2002;52:1017–1024. doi: 10.1016/s0360-3016(01)02725-0. [DOI] [PubMed] [Google Scholar]
- Blatt J, Snyderman C, Wollman M R, et al. Delayed resection in the management of non-orbital rhabdomyosarcoma of the head and neck in childhood. Med Pediatr Oncol. 1997;28:294–298. doi: 10.1002/(sici)1096-911x(199704)28:4<294::aid-mpo9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Healy G B, Upton J, Black P M, et al. The role of surgery in rhabdomyosarcoma of the head and neck in children. Arch Otolaryngol Head Neck Surg. 1991;117:1185–1188. doi: 10.1001/archotol.1991.01870220133025. [DOI] [PubMed] [Google Scholar]
- Wurm J, Constantinidis J, Grabenbauer G G, Iro H. Rhabdomyosarcomas of the nose and paranasal sinuses: treatment results in 15 cases. Otolaryngol Head Neck Surg. 2005;133:42–50. doi: 10.1016/j.otohns.2005.03.023. [DOI] [PubMed] [Google Scholar]