ABSTRACT
Objective: To present our method for anterior skull base reconstruction after oncological resections. Methods: One hundred nine patients who had undergone 120 anterior skull base resections of tumors (52 malignant [43%], 68 benign [57%]) via the subcranial approach were studied. Limited dural defects were closed primarily or reconstructed using a temporalis fascia. Large anterior skull base defects were reconstructed by a double-layer fascia lata graft. A split calvarial bone graft, posterior frontal sinus wall, or three-dimensional titanium mesh were used when the tumor involved the frontal, nasal, or orbital bones. A temporalis muscle flap was used to cover the orbital socket for cases of eye globe exenteration, and a rectus abdominis free flap was used for subcranial-orbitomaxillary resection. Pericranial flap wrapping of the frontonaso-orbital segment was performed to prevent osteoradionecrosis if perioperative radiotherapy was planned. Results: The incidence of cerebrospinal fluid (CSF) leak, intracranial infection, and tension pneumocephalus was 5%. Histopathological and immunohistochemical analysis of fascia lata grafts in reoperated patients (n = 7) revealed integration of vascularized fibrous tissue to the graft and local proliferation of a newly formed vascular layer embedding the fascial sheath. Conclusion: A double-layer fascial graft alone was adequate for preventing CSF leak, meningitis, tension pneumocephalus, and brain herniation. We describe a simple and effective method of anterior skull base reconstruction after resections of both malignant and benign tumors.
Keywords: Craniofacial, CSF leak, pneumocephalus, neoplasms, cranial base, malignant tumors
Considerable progress has been made during the last decade in our understanding of the complex anatomy of the anterior skull base. Anatomical and clinical studies have contributed extensively to the development of new surgical approaches, while new imaging tools have significantly increased the accuracy of preoperative evaluation and postoperative follow-up of our patients. Finally, the concept of cooperation between multidisciplinary teams was adopted for the treatment of these tumors allowing complete eradication of large tumors involving the skull base and craniofacial regions.
When tumors arising in the anterior skull base invade both soft and hard tissues of the skull base, tumor resection may create extensive skull base defects and produce a free conduit between the paranasal sinuses and the intracranial space. Following tumor extirpation, skull base cranial base defects require precise and durable reconstruction to (1) form a watertight dural seal, (2) provide a barrier between the contaminated sinonasal space and the sterile subdural compartment, (3) prevent airflow into the intracranial space, (4) maintain a functional sinonasal system, and (5) provide a good cosmetic outcome.
A variety of approaches have been developed to accomplish these goals. A split calvarial bone graft, hydroxyapatite paste, or titanium mesh may be utilized for bony reconstruction. Autologous flaps (pericranial or galeal flaps) and artificial substitutions are often used for reconstruction of the skull base. Unfortunately, these methods bear significant disadvantages. Local flaps are often insufficient in size for reliable restoration of extensive anterior skull base defects.1 Synthetic substitutions of dura and bone can induce chronic inflammation, carry high risk of infection, and are inferior to biological sources in terms of strength and sealing quality.2
Recent progress in microvascular and surgical techniques has enabled the development and implementation of free tissue transfer. Following skull base resection, free flaps may be used for massive defects, with excellent surgical results and low complication rates.1,3,4 Free tissue transfer is, however, a relatively complex surgical procedure that requires high technical qualifications. Another drawback of this method is the bulk of the muscular free flap, which may mask local recurrence and make radiological follow-up more difficult.5
Auto grafts, such as fascia lata and temporalis fascia, had been used in the past for skull base reconstruction, but they were usually covered with a vascularized flap (i.e., free muscular flaps, pericranial or galeal flaps), assuming that an overlying vascular tissue is essential to preserve long-term viability of the fascial graft.6
In any situation, failure to create adequate reconstruction harbors significant complications, among them cerebrospinal fluid (CSF) leak, meningitis, brain herniation, and tension pneumocephalus.7 Although the subcranial or craniofacial approaches have become established procedures for management of anterior skull base tumors,8 surgeons commonly use combinations of methods to accomplish satisfactory anterior skull base reconstruction. Thus, there is no single gold standard technique that is both simple and reliable for reducing the morbidity and mortality associated with anterior cranial base operations.
Here we present a large series of patients who had undergone extirpation of tumors via the subcranial approach followed by reconstruction of anterior skull base defects. We present a reliable and reproducible algorithm for cranial base reconstruction, with low rates of intra- and extracranial complications. We describe the healing process of the double-layer free fascial grafts and provide evidence to show that long-term viability of the fascia is achievable without an overlying vascularized flap.
MATERIAL AND METHODS
This retrospective study was based on a review of the hospital charts and the outpatient clinical and radiological data of 120 consecutive cases operated between 1994 and 2005. All the resections and reconstructions were performed by means of the subcranial approach to the anterior skull base.
Patient Population
Sixty-four males and 56 females aged 2 to 81 years (mean 42 years) were enrolled into this study. All patients were evaluated preoperatively by a head and neck surgeon, a plastic surgeon, and a neurosurgeon. Radiological evaluation included axial and coronal computed tomography (CT) and magnetic resonance imaging (MRI). Neuroangiographic evaluations were also performed when indicated.
Broad-spectrum antibiotics consisting of a combination of cefuroxime and metronidazole were instituted perioperatively. All the patients were operated under general anesthesia, in the supine position. and without shaving the hair at the surgical site. No tracheostomy was performed. A lumbar spine catheter was inserted for a period of 5 days for CSF drainage to facilitate frontal lobe retraction and to reduce the risk of postoperative CSF leak. The mean hospitalization period was 13 days.
Surgical Technique
The surgical technique of the subcranial approach has been described in detail elsewhere.9,10,11,12 Briefly, following anesthesia, the skin is incised above the hairline and a bicoronal flap is created in a supraperiosteal plane. A flap is elevated anteriorly beyond the supraorbital ridges and laterally superficial to the temporalis fascia. The pericranial flap is elevated up to the periorbits, and the supraorbital nerves and vessels are carefully separated from the supraorbital notch. The lateral and medial walls of the orbits are then exposed, and the anterior ethmoidal arteries are clipped or ligated. The pericranium is elevated above the nasal bones, and the flap is rotated forward and held over the face throughout the rest of the procedure. Titanium miniplates are applied to the frontal bones and removed before the osteotomies to ensure exact repositioning of the bony segments at the end of the operation. An osteotomy of the anterior or the anterior and posterior frontal sinus walls, together with the nasal bony frame, part of the medial wall of the orbit, and a segment of the superoposterior nasal septum, is then performed. For a type A osteotomy, the anterior frontal sinus wall as well as the nasal frame are osteotomized and removed in one block. If a type B osteotomy is planned, burr holes are made and the posterior frontal sinus wall is resected after the dura has been detached from the frontal, orbital, and ethmoidal roofs. A part of the distal nasal bone is preserved to support the nasal valve. In cases of lateral invasion of a tumor, the osteotomy lines can be extended to include segments of the orbital roofs. After the nasofronto-orbital (NFO) bone segment is osteotomized, it is stored in saline until the reconstructive procedure. A bilateral ethmoidectomy and a sphenoidotomy are then performed: this approach enables the exposure and assessment of the tumor in its circumference. The tumor is extirpated at this stage and the dura or brain parenchymas are resected when involved by tumor. A medial maxillectomy is performed from above if indicated. Frozen sections are taken during surgery to ensure tumor-free margins. One or both sides of the cribriform plate and olfactory filaments are preserved whenever possible.
In cases of a massive involvement of the lower or lateral segments of the maxilla, the pterygopalatine fossa, or the orbitofrontal segment, we perform a combination of the subcranial approach with the transfacial approach, the facial translocation approach, the transorbital approach, or the midfacial degloving approach.
Reconstruction
The reconstruction technique is tailored to the type and size of the cranial defect, based on radiological and intraoperative calculations. Table 1 summarizes our algorithm for skull base reconstruction, according to the type of craniobasal defect.
Table 1.
Reconstruction Modalities following Anterior Skull Base Tumor Resection
| Type of Defect | Reconstructive Modality |
|---|---|
| DCR, dacryorhinocystostomy; XRT, radiotherapy. | |
| Minimal dural tear | Primary closure |
| Small dural defect | Temporalis fascia |
| Moderate to large dural defect | Fascia lata |
| Bony defect: orbital wall, nasal, and frontal bones | Posterior frontal sinus wall graft Split calvarial bone graft Titanium mesh |
| Posterior sinus wall, intact | Frontal sinus obliteration with abdominal fat |
| Posterior sinus wall, involved | Frontal sinus cranialization |
| Orbital exenteration | Temporalis muscle flap |
| Orbitomaxillary resection | Rectus abdominus free flap and obturator |
| Orbital wall resection | Titanium mesh Fascia lata sling Septal cartilage |
| Orbital wall resection or medial maxillectomy | DCR |
| Perioperative XRT | Pericranial wrapping of all osteotomized bony segments or titanium mesh |
DURAL RECONSTRUCTION
Primary closure of the dura is performed whenever possible. A graft of temporalis fascia is used if the defect is small. If tumor resection results in an extensive skull base defect, a second surgical team simultaneously harvests a large fascia lata sheath (20 × 10 cm). The size of the fascia used for reconstruction is tailored to the dimension of the dural and skull base defects. The fascia is tacked under the edges of the dura and carefully sutured in place. The dural repair is then covered with a second layer of fascia that is applied against the entire undersurface of the ethmoidal roof, the sella, and the sphenoidal area (Fig. 1). Fibrin glue is used to provide additional protection against CSF leak. Following dural repair, Vaseline gauze is applied below the dura and into the paranasal cavity to provide additional support against pulsation.
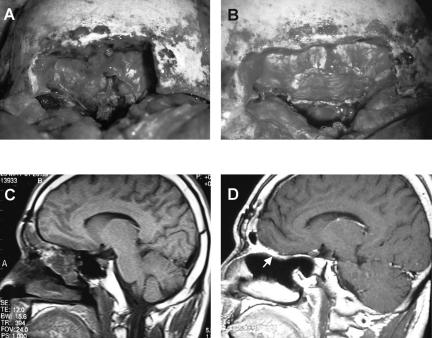
Figure 1.
The fascia lata technique for anterior craniobasal reconstruction in a 45-year-old male with olfactory groove meningioma (T4AN0M0). (A) An intraoperative view of the craniobasal defect following tumor removal. (B) The fascia lata sheath has been applied to the defect. (C) Preoperative sagittal T1 MRI of the anterior cranial fossa. (D) Postoperative sagittal T1 MRI of the anterior cranial fossa 24 months following surgery. The arrow indicates the enhancement of the fascia.
FRONTAL SINUS RECONSTRUCTION
A type A osteotomy is performed for a limited, benign frontal sinus lesion. We obliterate the frontal sinus with abdominal fat in such cases. Alternatively, for malignant tumors, posterior sinus wall violations or major dural tears, we routinely perform a type B osteotomy, with frontal sinus cranialization ex vivo. After removing all the mucosa from the sinus, the earlier osteotomized segment is repositioned in its original anatomical place and fixed with prebent titanium plates.
BONY RECONSTRUCTION
Hydroxyapatite paste (BoneSource®) or 3D titanium mesh can be used for small defects of the calvarium following removal of the frontal sinus outer table. Alternatively, biological materials, such as a split calvarial bone graft or a posterior frontal sinus wall graft, are used for the same purpose. A split calvarial bone graft or posterior frontal sinus wall can be used for reconstruction when the tumor involves the nasal bone or an adjacent fronto-orbital segment. A bone graft can be also used for dorsal nasal support if the nasal septum has been violated. We use a Medpor® biometrical implant (Porex® Corp., Fairburn, GA) in cases of a large frontal or temporal bone defect and only when postoperative radiotherapy is not planned. The same material is used for cosmetic reconstruction of large temporal defects following rotation of a temporalis muscle flap.
ORBITAL RECONSTRUCTION
Reconstruction of the medial orbital wall is performed only in cases in which a total removal of this segment is necessary or if the periorbit is excised. In these situations, we use a split calvarial bone graft, fascia lata sling, or 3D titanium mesh covered with pericranium (Fig. 2). Septal cartilage can be used for reconstruction of a limited defect of the inferior orbital wall. If eye globe exenteration is required, we use a temporalis muscle flap and a split-thickness skin graft to cover the orbital socket. For a radical maxillectomy with or without orbital exenteration, we use a lateral thigh free flap or a rectus abdominis musculocutaneous free flap to obliterate this large defect and to support the obturator from above (Fig. 3). A dacryorhinocystostomy (DCR) is performed on all patients undergoing orbital wall resection or medial maxillectomy.
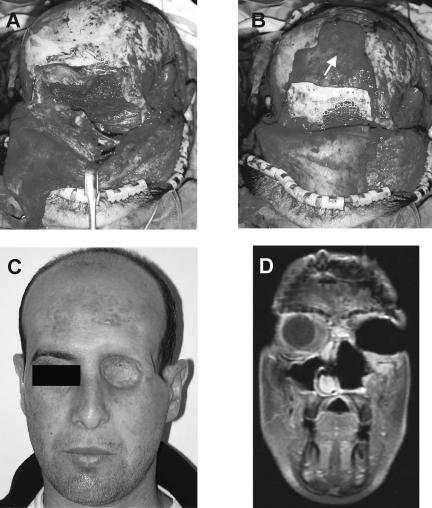
Figure 2.
Orbital reconstruction following subcranial resection in a 28-year-old patient with T4AN0M0 squamous cell carcinoma. (A) The orbita was reconstructed with temporalis muscle rotational flap. The skull base was reconstructed with a double-layer fascia lata. (B) The periorbit was reconstructed with the nasofronto-orbital bone segment and with titanium mesh. Wrapping of the frontonaso-orbital segment was accomplished with a pericranial flap (arrow). (C) A picture of the patient 12 months after surgery. (D) A postoperative T1 MRI shows the area of reconstruction.
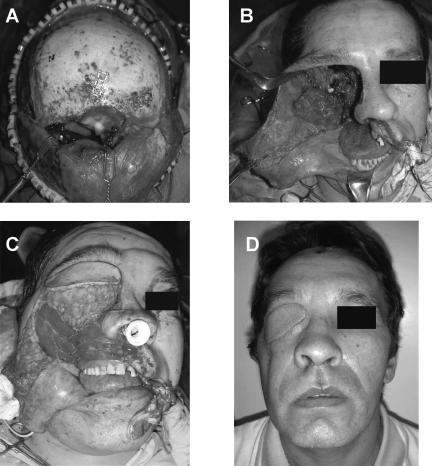
Figure 3.
Reconstruction following subcranial resection, orbital exenteration, and radical maxillectomy in a patient with T4AN0M0 squamous cell carcinoma. (A) The surgical defect after resection of the superior part of the tumor via the subcranial approach. (B) The surgical defect after resection of the inferior part of the tumor via an extended Weber-Fergusson incision. (C) Reconstruction of the orbita and maxilla was achieved using a rectus abdominus free flap and obturator. The anterior skull base was reconstructed with fascia lata. (D) Postoperative results 30 days after surgery.
Following tumor resection and reconstruction, a centripetal compression of both globes is performed to reduce the telecanthus. This method involves guiding two threads through the medial canthal ligament and driving them underneath the NFO segment. The threads are tightened and fixed to the contralateral frontal plates to enable medial compression and alignment, thereby avoiding the telecanthus altogether.
PERICRANIAL WRAPPING
For patients undergoing extirpation of malignant tumors and for whom adjuvant radiation therapy is planned, we wrap the NFO segment with a pericranial flap to prevent osteoradionecrosis.13 A pericranial flap is also used in cases of orbital wall reconstruction to cover the bone-graft segments or the titanium mesh, and we wrap the NFO segment bilaterally with a rotational temporoparietal fascia (Fig. 2).
After surgery, the patients are immediately transferred to the critical care unit for 24 hours. The lumbar drain is removed 3 to 5 days after surgery, and the nasal packing is removed on the eighth postoperative day. All the patients in the current study were followed on a regular basis for a period of 3 to 50 months after discharge (an average follow-up period of 26 months).
Histopathological and Immunohistochemical Analysis
We were able to investigate the healing process of fascia lata grafts in seven patients who had undergone a second surgery. Fragments of fascia lata that had previously been used for skull base reconstruction and had not been involved with the tumor were excised and submitted for histological examination. Each segment was fixed in 10% buffered formalin, the specimen was embedded in paraffin and serially sliced (4-μm thickness), and the microsections were stained with hematoxylin and eosin (H & E). The degree of vascular proliferation and fibrotic reaction was evaluated by a senior pathologist. Confirmation of viable microvascular proliferation within the fascia lata flap was obtained in four cases by immunocytochemical staining for endothelial cells with CD31 and factor VIII antibodies.
RESULTS
The surgical outcomes of 109 patients who had undergone 120 procedures of anterior skull base tumor resections were analyzed. They included 52 cases of malignant tumors (43%) and 68 of benign tumors (57%). There was no significant difference in age or sex between the two groups. The most common malignant tumors were squamous cell carcinoma (n = 12) and esthesioneuroblastoma (n = 12). Forty-one patients had T4 disease and 10 had T3 disease. One patient was operated because of a single lung metastasis to the frontal bone. The most common benign pathology was meningioma (n = 16). Table 2 summarizes the underlying pathologies of the study patients. Fifty-two patients (48%) had undergone at least one previous operation. Thirty-nine patients (36%) had undergone perioperative radiation therapy (14 patients preoperative and 25 patients postoperative), of whom 20 (18%) had also undergone at least one previous operation. The subcranial approach was used as a single procedure in 91/120 (76%) cases. It was combined with a midfacial degloving procedure in 14 cases, with a pterional approach in 5 and with a transorbital approach in 4.
Table 2.
Histopathology of Anterior Skull Base Tumors
| Pathology | N |
|---|---|
| Meningioma | 16 |
| Osteoma | 12 |
| Mucocele | 9 |
| Meningoencephalocele/encephalocele | 7 |
| Inverted papilloma | 8 |
| Angiofibroma | 3 |
| Paraganglioma | 2 |
| Neurofibromatosis 1 | 2 |
| Arteriovenous malformation | 2 |
| Fibrous dysplasia | 2 |
| Chordoma | 3 |
| Hemangioma | 1 |
| Pituitary adenoma | 1 |
| Epidermoid cyst | 1 |
| Squamous cell carcinoma | 12 |
| Esthesioneuroblastoma | 12 |
| Sarcoma | 7 |
| Adenocarcinoma | 4 |
| Hemangiopericytoma | 3 |
| Malignant schwannoma | 3 |
| Plasmocytoma | 2 |
| Melanoma | 2 |
| Sinonasal undifferentiated carcinoma | 2 |
| Adenoid cystic carcinoma | 1 |
| Malignant paraganglioma | 1 |
| Metastatic lung carcinoma | 1 |
| Lymphoma | 1 |
| Total | 120 |
The principal skull base reconstruction procedure was performed by means of a double layer fascia lata (n = 97). The tumor extended to the dura mater in 79 of these cases. The remaining cases included 8 that involved limited defects of the dura which were reconstructed with the use of a temporalis fascia, and 15 which required no dural reconstruction.
We used the temporalis muscle flap in the six cases of orbital exenteration and employed the same method of double-layer fascial flap for anterior skull base reconstruction.
A craniofacial reconstruction was required if tumor resection produced a significant bony defect of the orbital walls, nasal bone, or anterior frontal sinus wall. This was achieved by applying a split calvarial bone graft or posterior sinus wall (10 and 6 cases, respectively). A titanium mesh covered with a pericranial flap was utilized for reconstruction of the medial orbital walls and to cover extensive calvarial defects in 10 cases. We used a hydroxyapatite paste to fill defects in the calvarium after harvesting the outer table for reconstruction in 6 other cases.
We used a rectus abdominis free flap for reconstruction of large defects following subcranial resection and radical maxillectomy in five patients.
The overall complication rate was 38%, but the incidence of CSF leak, intracranial infection, and tension pneumocephalus was 5%. One patient who was operated on for a pituitary adenoma and who suffered from meningitis died 42 days after surgery. The overall postoperative complications associated with skull base and cranial reconstructions are listed in Table 3. Seven of the eight patients who had osteoradionecrosis with fistula had undergone perioperative radiotherapy. Because of the risk for osteoradionecrosis of the NFO segment in patients who undergo perioperative radiation therapy (Fig. 4), we utilized a new method for wrapping the frontal bone segment with pericranial flap.13 There were no cases of bone flap necrosis among the 26 patients who underwent this procedure. Two cases of wound dehiscence associated with temporalis muscle transfer following orbital exenteration were successfully treated with local flaps several days after surgery. The complication rates of the patients with benign and malignant tumors were similar.
Table 3.
Complications of Skull Base Reconstruction Procedures
| Complication | N | Percentage |
|---|---|---|
| CSF, cerebrospinal fluid. | ||
| Telecanthus | 9 | 7.5 |
| Osteoradionecrosis and fistula | 8 | 6.6 |
| Epiphora | 7 | 5.8 |
| Wound infection | 6 | 5 |
| Deep vein thrombosis | 6 | 5 |
| Meningitis | 4 | 3.3 |
| Pneumocephalus | 4 | 3.3 |
| Ptosis | 4 | 3.3 |
| Mucocele | 2 | 1.6 |
| Intracranial hematoma | 2 | 1.6 |
| Facial nerve paralysis | 2 | 1.6 |
| Pneumonia | 2 | 1.6 |
| CSF leak | 1 | 0.8 |
| Serous otitis media | 1 | 0.8 |
| Total | 56 | |
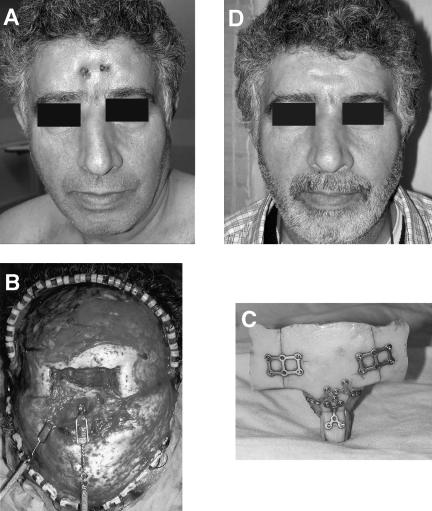
Figure 4.
Reconstruction of a patient with osteoradionecrosis of the frontonaso-orbital segment, performed 24 months after excision of squamous cell carcinoma. (A) Preoperative picture of the patient showing extrusion of the titanium plates through the skin. (B) Reconstruction of the bony segment was performed with a split calvarial bone graft. (C) The surgical defect following removal of the necrotic bone. (D) Postoperative results 6 months after surgery.
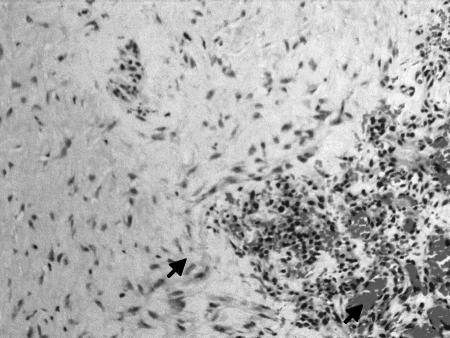
We were able to investigate the healing process of fascia lata grafts in seven patients who had undergone a second surgery. Fragments of the fascia lata that were previously used for skull base reconstruction and were not involved with the index tumor were excised and submitted for histopathological evaluation (H & E). Microscopic examination revealed that the entire graft was composed of viable dense fibrocollagenous tissue (Fig. 5). The bipolar, wavy-shaped nuclei of the fibroblasts were embedded in a collagenous stroma in large areas. Immunohistochemical staining for endothelial cells with CD31 and factor VIII showed proliferation of neovascular channels lined by plumped endothelial cells in the specimens of four cases. The histological findings demonstrated an almost complete fibrous replacement of the fascia lata allograft.
Figure 5.
Neovascularization of the fascia lata graft 12 months after surgery in a case with recurrent tumor (H & E staining). Microscopical examination shows fibroblasts embedded in a dense collagenous stroma (higher arrow). Note the presence of neovascularized channels lined by endothelial cells (lower arrow). The graft has been replaced with a viable dense fibrocollagenous tissue. (20 × magnification.)
DISCUSSION
Reconstruction of the anterior skull base is technically challenging and may be further complicated by several factors. First, there is a paucity of local tissue that is available for transfer into the defect. Second, previous radiation treatment significantly reduces tissue perfusion and so delays normal wound healing. Finally, many of these patients have undergone earlier surgeries prior to the index operation, thus increasing its complexity and decreasing tissue perfusion, secondary to scar tissue formation.
In this article, we propose a comprehensive anterior skull base reconstruction algorithm designed to accommodate various surgical and medical conditions such as those described above. The suitability of each reconstructive approach as listed in Table 1 is based on our experience derived from the 120 surgical procedures presented in this series.
Regional flaps have been shown to be a reasonable option for dural reconstruction.1 This technique is often sufficient to form a barrier separating the dura from the nasopharynx, and the pericranial and galeal flaps are the most common regional flaps chosen for this purpose.14 They are readily available, easily harvested, and can be used to repair various dural defects. Galeal flaps may not, however, always be fit for use, particularly after radiation therapy or previous operations. They can also cause functional and cosmetic complications, including sensory motor loss and regional alopecia.15 Furthermore, galeal or pericranial flaps may be too small to cover defects of the frontal bone (e.g., burr holes and craniotomy bone cuts). Loss of galea and frontalis muscle from the undersurface of the frontal scalp means that there will be a very thin and poorly vascularized flap of skin for covering the bone flap, and therefore its use after radiation therapy could be problematic. As noted in our algorithm, we use the pericranium only for wrapping of bony segments (i.e., NFO or split calvarial bone graft) to provide vascularization to these components in patients scheduled for postoperative radiation therapy. The main goal of this method is to decrease the risk of osteoradionecrosis.13 We had speculated that the pericranium can offer an excellent blood and nutrients supply to the osteotomized bone. To date, we have performed 26 subcranial operations for resection of malignant tumors of the anterior skull base using this technique and none of these patients developed bone flap necrosis or osteomyelitis following radiotherapy. Moreover, the rate of osteoradionecrosis was significantly reduced in patients undergoing malignant subcranial tumor resection with the use of pericranial wrapping.
In the current study, the double-layer fascia lata served as the standard material for anterior skull base reconstruction. In a previous report,16 we described a simple technique for harvesting large fascia lata sheaths, which affords a low complication rate and low donor limb morbidity. The thin and low mass properties of the fascia lata enable the surgeon to cover large dural defects with a single fascial sheath. Furthermore, the flexibility of the fascia lata enables coating of extensive cranial defects, including parts of the orbit and paranasal sinuses. Large cranial base defects and prior surgery and radiotherapy (previously considered indications for free flap reconstruction17) were managed by fascia alone, and free flaps, autologous fat, or skin grafts were not necessary to achieve a reliable anterior skull base reconstruction with excellent surgical results. Several authors believe that bony reconstruction of the skull base is necessary to support the newly reconstructed skull base and to prevent herniation of the cranial content.18 Our current results show that reconstruction with a double-layer fascia does not require a rigid support of bone or synthetic materials: not one brain herniation occurred in our series of 120 procedures. Furthermore, this procedure does not require the high technical qualifications which are essential for free muscle transfer. Finally, the overall duration of the reconstructive procedure described herein is not longer than that of a pericranial flap,19 and takes considerably less time than free tissue transfer procedures.20
Routine postoperative radiological follow-up of patients after extirpation of malignant anterior skull base tumors is required in all cases. The relatively low bulk of the fascia lata facilitates radiological follow-up in patients with an increased risk of local recurrence.
The use of fascial flaps does not require the utilization of skin grafts or muscle flaps for covering the nasopharyngeal space. Transnasal endoscopic examination of our patients several weeks postoperatively revealed that the exposed nasal surface of the fascial flap had been completely covered by the neighboring sinonasal mucosa.
We also studied the histopathology of the reconstructed fascia lata to investigate the healing process of the reconstructed area in patients who had undergone a second operation. Our histological analysis of previously harvested human fascial flaps showed evidence of integration of a vascularized fibrous tissue into the fascial graft. The fascial flap was uniformly coated by fibrous tissue, and invasion of blood vessels was achieved without the presence of an overlying vascularized flap in all fascial flaps that were examined. Using an animal model, Tachibana and colleagues21 demonstrated a tight connection between the fascial graft and the dura within 1 week after surgery, in agreement with our findings. They reported that the fascial graft had been completely replaced by durable fibrous tissue 2 weeks following the surgical repair, and speculated that fibroblast growth factor b plays a significant role in the healing process of free fascial grafts.21,22 Thus, our current work and this animal model confirm that excellent skull base reconstruction can be achieved without the need for blood supply from an overlying regional flap or free muscular flap. Moreover, the rapid healing process of the fascial reconstruction provides a robust physiological barrier between the nasopharynx and the intracranial space within days following surgery, an important advantage of its use.
We used free flaps for reconstruction in the current series only in cases which required anterior skull base tumor resection and radical maxillectomy with or without orbital exenteration. The rectus abdominis is the most commonly used free flap in this anatomical area, followed by the lateral thigh, radial forearm, and latissimus dorsi flaps. Free tissue transfer promises flexibility in flap content and design and provides the opportunity to introduce a large quantity of well-vascularized tissue to the reconstructed area in a single-stage operation. In our study, the rectus abdominis or lateral thigh free flaps also allowed adequate support to the obturator, with good cosmesis and functional outcome.
Bony reconstruction was our choice for orbital, nasal, and anterior frontal sinus wall reconstruction. This can be achieved with the use of biological tissue, such as split calvarial bone graft or posterior wall of frontal sinus, or with artificial materials, such as 3D titanium mesh. These materials are wrapped with a pericranial flap if postoperative radiation is planned. Obliteration of the frontal sinus is indicated following extirpation of small benign tumors confined to the frontal sinus and can be easily achieved with free abdominal fatty tissue. On the other hand, a frontal sinus cranialization is mandatory if the tumor invades the posterior sinus wall or the intracranial space. In this case, the outer layer of the fascia lata serves as the roof of the paranasal cavity.
In conclusion, we reviewed our experience of skull base reconstruction after extirpation of tumors via the subcranial approach and propose a comprehensive approach to complex defects in the anterior craniobasal compartment. Our “workhorse” for dural reconstruction is the double-layer fascia lata, which provides a simple and versatile means of skull base reconstruction after resections of advanced tumors. The other reconstruction methods we use according to need are bone grafts, titanium mesh, temporalis muscle flap, free flaps, and pericranial wrapping. The incidence and severity of perioperative complications associated with our double layer fascia lata technique are low compared with other reconstructive methods. The findings of our study also indicate that free fascial grafts survive via local proliferation of a newly formed vascular layer embedded within the fascial sheath. Future developments of biomedical materials will probably bring with them continuing improvement of current techniques for skull base reconstruction with fewer complications and the promise of a better quality of life for patients.
ACKNOWLEDGMENTS
The authors thank Esther Eshkol for editorial assistance.
REFERENCES
- Neligan P C, Mulholland S, Irish J, et al. Flap selection in cranial base reconstruction. Plast Reconstr Surg. 1996;98:1159–1166. doi: 10.1097/00006534-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Simpson D, Robson A. Recurrent subarachnoid bleeding in association with dural substitute. Report of three cases. J Neurosurg. 1984;60:408–409. doi: 10.3171/jns.1984.60.2.0408. [DOI] [PubMed] [Google Scholar]
- Clayman G L, DeMonte F, Jaffe D M, et al. Outcome and complications of extended cranial-base resection requiring microvascular free-tissue transfer. Arch Otolaryngol Head Neck Surg. 1995;121:1253–1257. doi: 10.1001/archotol.1995.01890110031006. [DOI] [PubMed] [Google Scholar]
- Califano J, Cordeiro P G, Disa J J, et al. Anterior cranial base reconstruction using free tissue transfer: changing trends. Head Neck. 2003;25:89–96. doi: 10.1002/hed.10179. [DOI] [PubMed] [Google Scholar]
- Kiyokawa K, Tai Y, Inoue Y, et al. Efficacy of temporal musculopericranial flap for reconstruction of the anterior base of the skull. Scand J Plast Reconstr Surg Hand Surg. 2000;34:43–53. doi: 10.1080/02844310050160169. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Torii S, Fukuta K, Saito K. Reconstruction of the anterior cranial base with the galeal frontalis myofascial flap and the vascularized outer table calvarial bone graft. Neurosurgery. 1995;36:725–731. doi: 10.1227/00006123-199504000-00013. [DOI] [PubMed] [Google Scholar]
- Boyle J O, Shah K C, Shah J P. Craniofacial resection for malignant neoplasms of the skull base: an overview. J Surg Oncol. 1998;69:275–284. doi: 10.1002/(sici)1096-9098(199812)69:4<275::aid-jso13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Fliss D M, Zucker G, Amir A, et al. The subcranial approach for anterior skull base tumors. Oper Tech Otolaryngol Head Neck Surg. 2000;11:238–253. [Google Scholar]
- Raveh J. Gesichtsschadelverletzungen: Eigene Erfahrungen und modificationen. Aktuel Probl ORL. 1979;3:145–154. [Google Scholar]
- Raveh J, Laedrach K, Lizuka T, et al. In: Donald PJ, editor. Surgery of the Skull Base. New York, NY: Lippincott-Raven; 1998. Subcranial extended anterior approach for skull base tumors: surgical procedure and reconstruction. pp. 239–261.
- Raveh J, Laedrach K, Speiser M, et al. The subcranial approach for fronto-orbital and anteroposterior skull-base tumors. Arch Otolaryngol Head Neck Surg. 1993;119:385–393. doi: 10.1001/archotol.1993.01880160029006. [DOI] [PubMed] [Google Scholar]
- Raveh J, Turk J B, Ladrach K, et al. Extended anterior subcranial approach for skull base tumors: long-term results. J Neurosurg. 1995;82:1002–1010. doi: 10.3171/jns.1995.82.6.1002. [DOI] [PubMed] [Google Scholar]
- Gil Z, Fliss D M. Pericranial wrapping of the frontal bone after anterior skull base tumor resection. Plast Reconstr Surg. 2005;116:395–398. doi: 10.1097/01.prs.0000172761.65844.d0. [DOI] [PubMed] [Google Scholar]
- Fukuta K, Potparic Z, Sugihara T, Rachmiel A, Forte R A, Jackson I T. A cadaver investigation of the blood supply of the galeal frontalis flap. Plast Reconstr Surg. 1994;94:794–800. doi: 10.1097/00006534-199411000-00007. [DOI] [PubMed] [Google Scholar]
- Snyderman C H, Janecka I P, Sekhar L N, Sen C N, Eibling D E. Anterior cranial base reconstruction: role of galeal and pericranial flaps. Laryngoscope. 1990;100:607–614. doi: 10.1288/00005537-199006000-00011. [DOI] [PubMed] [Google Scholar]
- Amir A, Gatot A, Zucker G, et al. Harvesting of fascia lata sheaths: a rational approach. Skull Base. 2000;10:29–34. [PMC free article] [PubMed] [Google Scholar]
- McCutcheon I E, Blacklock J B, Weber R S, et al. Anterior transcranial (craniofacial) resection of tumors of the paranasal sinuses: surgical technique and results. Neurosurgery. 1996;38:471–479. doi: 10.1097/00006123-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Derome P. In: Schmidek H, Sweet W, editor. Current Techniques in Operative Neurosurgery. New York, NY: Grune and Stratton; 1977. The transbasal approach to tumors invading the base of the skull. pp. 223–245.
- Sundaresan N, Shah J P. Craniofacial resection for anterior skull base tumors. Head Neck Surg. 1988;10:219–224. doi: 10.1002/j.1930-2398.1988.tb00002.x. [DOI] [PubMed] [Google Scholar]
- Nibu K, Sasaki T, Kawahara N, Sugasawa M, Nakatsuka T, Yamada A. Complications of craniofacial surgery for tumors involving the anterior cranial base. Neurosurgery. 1998;42:455–461. doi: 10.1097/00006123-199803000-00003. [DOI] [PubMed] [Google Scholar]
- Tachibana E, Saito K, Fukuta K, Yoshida J. Evaluation of the healing process after dural reconstruction achieved using a free fascial graft. J Neurosurg. 2002;96:280–286. doi: 10.3171/jns.2002.96.2.0280. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]